Grape Seed Proanthocyanidin Extract Improves Growth Performance and Protects Against Hydrogen Peroxide-Induced Oxidative Stress to the Liver and Intestine in Weaned Hyla Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Sampling Procedure
2.3. Determination of Serum Biochemical Indicators and MDA
2.4. Measurements of Intestinal and Liver Morphology
2.5. Determination of Antioxidant Indexes in the Small Intestine and Liver
2.6. Statistical Analysis
3. Results
3.1. Effects of Dietary Supplementation with GSPE on the Growth Performance of Weaned Rabbits (Exp.1)
3.2. Effects of Dietary Supplementation with GSPE on the Slaughter Performance of Weaned Rabbits (Exp.1)
3.3. Effects of Dietary Supplementation with GSPE on the Organ Coefficients of Weaned Rabbits (Exp.1)
3.4. Effects of Dietary Supplementation with GSPE on the Serum Biochemical Indicators of Weaned Rabbits (Exp.1)
3.5. Effects of H2O2 Injection on the Growth Performance of Hyla Rabbits (Exp.2)
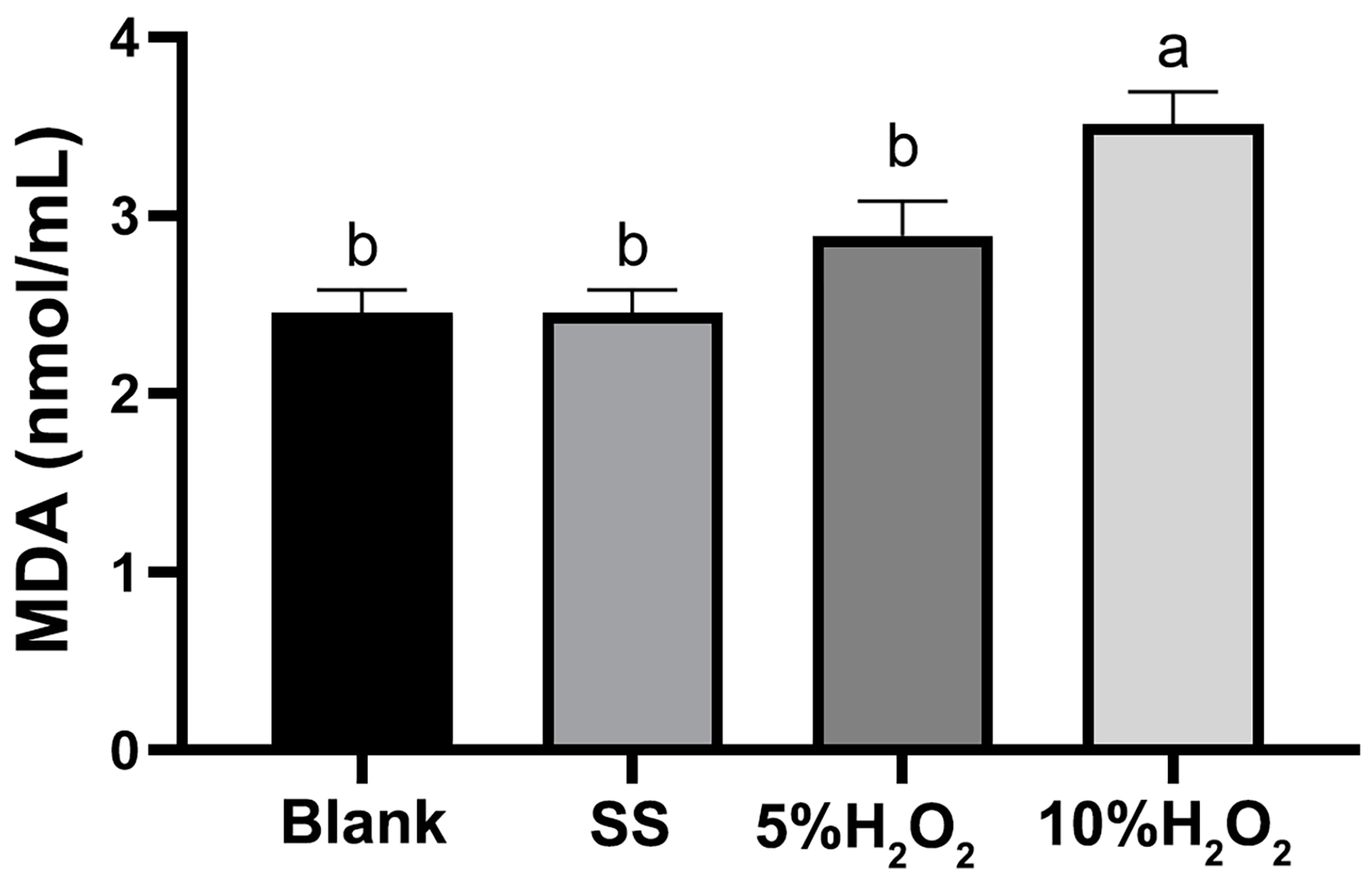
3.6. Effects of H2O2 Injection on the Serum MDA Concentration of Hyla Rabbits (Exp.2)
3.7. Effects of Dietary Supplementation with GSPE on Liver Morphology of Weaned Rabbits Challenged with H2O2 (Exp.3)
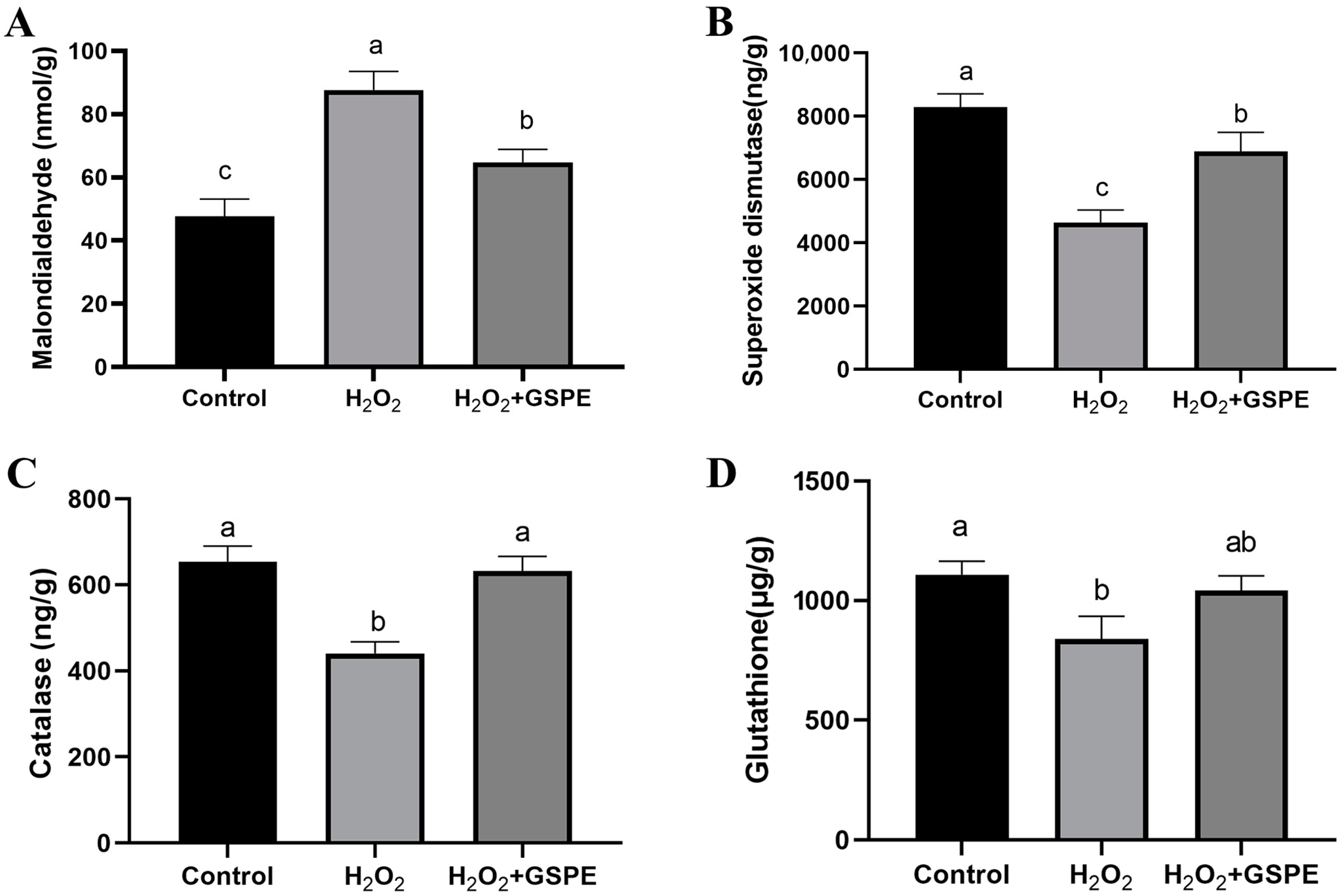
3.8. Effects of Dietary Supplementation with GSPE on Liver Oxidative Capacity of Weaned Rabbits Challenged with H2O2 (Exp.3)
3.9. Effects of Dietary Supplementation with GSPE on Small Intestinal Morphometric Measurements of Weaned Rabbits Challenged with H2O2 (Exp.3)
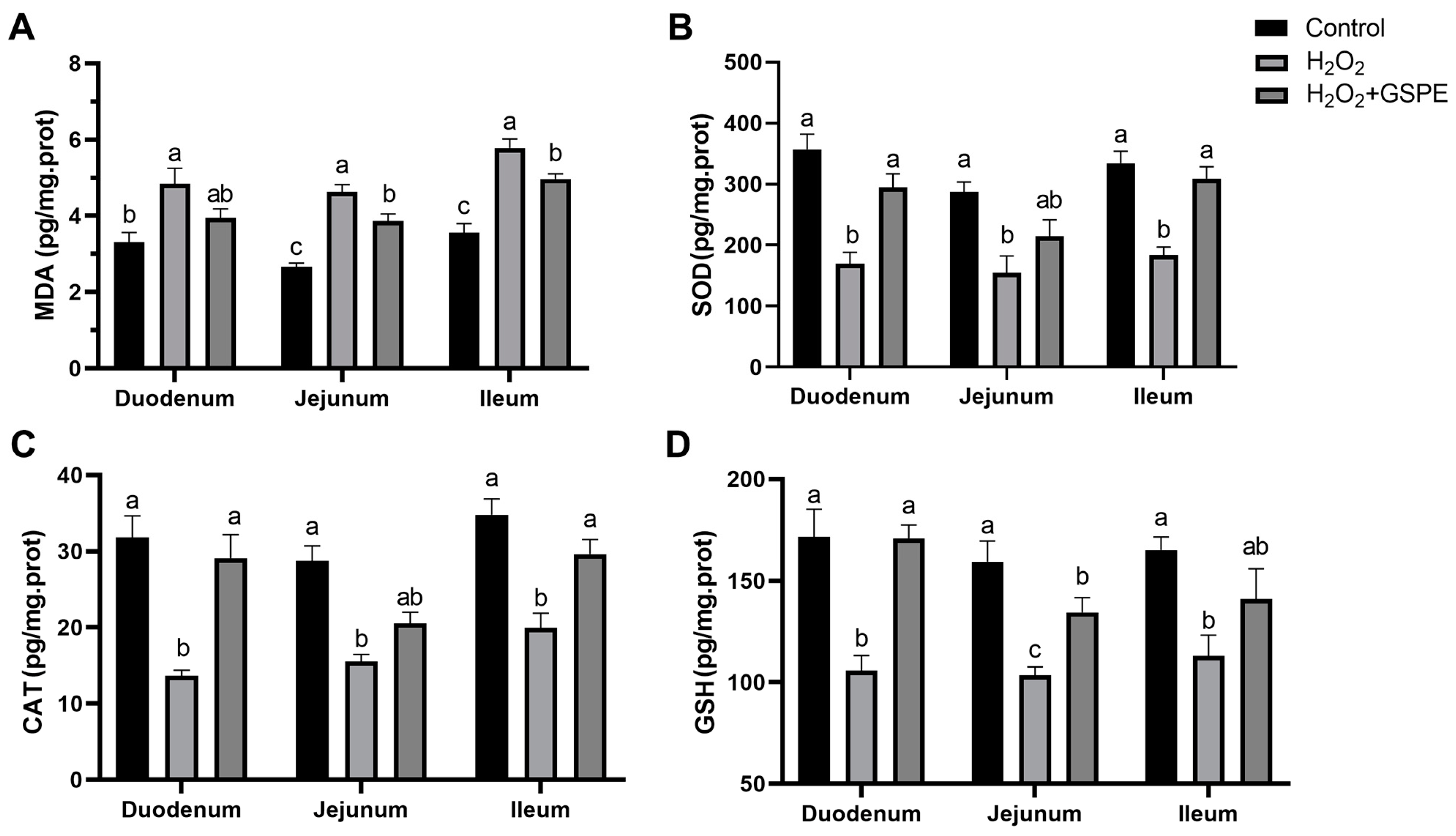
3.10. Effects of Dietary Supplementation with GSPE on Small Intestinal Oxidative Capacity of Weaned Rabbits Challenged with H2O2 (Exp.3)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef]
- Machado, I.F.; Miranda, R.G.; Dorta, D.J.; Rolo, A.P.; Palmeira, C.M. Targeting oxidative stress with polyphenols to fight liver diseases. Antioxidants 2023, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, W.; Liu, J.; Tang, D.; Yang, L.; Chen, X. Oxidative cell death in cancer: Mechanisms and therapeutic opportunities. Cell Death Dis. 2024, 15, 556. [Google Scholar] [CrossRef] [PubMed]
- Figueira, T.R.; Barros, M.H.; Camargo, A.A.; Castilho, R.F.; Ferreira, J.C.; Kowaltowski, A.J.; Sluse, F.E.; Souza-Pinto, N.C.; Vercesi, A.E. Mitochondria as a source of reactive oxygen and nitrogen species: From molecular mechanisms to human health. Antioxid. Redox Signal. 2013, 18, 2029–2074. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.; Li, Y.; Chen, D.; Yu, B.; He, J. Chlorogenic acid attenuates oxidative stress-induced intestinal epithelium injury by co-regulating the PI3K/Akt and IκBα/NF-κB signaling. Antioxidants 2021, 10, 1915. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, Y.; Zhang, L.; Guo, Q. Oxidative stress and liver disease. Hepatol. Res. 2012, 42, 741–749. [Google Scholar] [CrossRef]
- Gharib, H.S.; Abdel-Fattah, A.F.; Mohammed, H.A.; Abdel-Fattah, D.M. Weaning induces changes in behavior and stress indicators in young New Zealand rabbits. J. Adv. Vet. Anim. Res. 2018, 5, 166–172. [Google Scholar] [CrossRef]
- Ji, R.; Chen, J.; Xu, J.; Zhang, L.; Liu, L.; Li, F. Protective effect of chlorogenic acid on liver injury in heat-stressed meat rabbits. J. Anim. Physiol. Anim. Nutr. 2024, 108, 1203–1213. [Google Scholar] [CrossRef]
- Somi, M.H.; Hajipour, B.; Abad, G.D.A.; Hemmati, M.R.; Ghabili, K.; Khodadadi, A.; Vatankhah, A.M. Protective role of lipoic acid on methotrexate induced intestinal damage in rabbit model. Indian J. Gastroenterol. 2011, 30, 38–40. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Hassan, F.A.; Mahrose, K.M.; Basyony, M.M. Effects of grape seed extract as a natural antioxidant on growth performance, carcass characteristics and antioxidant status of rabbits during heat stress. Arch. Anim. Nutr. 2016, 70, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Abd Allah, E.; Abdel Hady, D.E.; Ghodaia, A.E.; Ameen, H.; Eliraqy, E.Z.; Farouk, Z.; Shedeed, S. Impact of Grape Seed Extract (Proanthocyanidins) on Pathophysiological Changes and Antioxidant Capacity in Rabbit Does Expose to Heat Stress. Egypt. J. Vet. Sci. 2024, 55, 2139–2146. [Google Scholar] [CrossRef]
- Rajput, S.A.; Sun, L.; Zhang, N.; Khalil, M.M.; Gao, X.; Ling, Z.; Zhu, L.; Khan, F.A.; Zhang, J.; Qi, D. Ameliorative effects of grape seed proanthocyanidin extract on growth performance, immune function, antioxidant capacity, biochemical constituents, liver histopathology and aflatoxin residues in broilers exposed to aflatoxin B1. Toxins 2017, 9, 371. [Google Scholar] [CrossRef]
- Wei, X.; Li, L.; Yan, H.; Li, Q.; Gao, J.; Hao, R. Grape seed procyanidins improve intestinal health by modulating gut microbiota and enhancing intestinal antioxidant capacity in weaned piglets. Livest. Sci. 2022, 264, 105066. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Yu, B.; Huang, Z.; Luo, Y.; Zheng, P.; Mao, X.; Yu, J.; Tan, H.; Luo, J. Grape seed proanthocyanidins improves growth performance, antioxidative capacity, and intestinal microbiota in growing pigs. Front. Microbiol. 2024, 15, 1501211. [Google Scholar] [CrossRef]
- Feng, W.; Xu, Y.; Su, S.; Yu, F.; Li, J.; Jia, R.; Song, C.; Li, H.; Xu, P.; Tang, Y. Transcriptomic analysis of hydrogen peroxide-induced liver dysfunction in Cyprinus carpio: Insights into protein synthesis and metabolism. Sci. Total Environ. 2024, 917, 170393. [Google Scholar] [CrossRef]
- Yin, J.; Duan, J.; Cui, Z.; Ren, W.; Li, T.; Yin, Y. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. Rsc Adv. 2015, 5, 15479–15486. [Google Scholar] [CrossRef]
- Kong, F.; Wu, F.; Liu, Y.; Lai, N.; Wang, G.; Shen, S.; Han, S.; Li, B.; Zhi, Y.; Chen, S. Effects of enzymolytic soybean meal on the growth performance, digestive enzyme activity, some serum indexes, carcase performance and meat quality of Rex rabbits. Ital. J. Anim. Sci. 2022, 21, 1307–1314. [Google Scholar] [CrossRef]
- Chen, J.; Li, F.; Yang, W.; Jiang, S.; Li, Y. Supplementation with exogenous catalase from Penicillium notatum in the diet ameliorates lipopolysaccharide-induced intestinal oxidative damage through affecting intestinal antioxidant capacity and microbiota in weaned pigs. Microbiol. Spectr. 2021, 9, e00654-21. [Google Scholar] [CrossRef]
- Zhang, P.; Jing, C.; Liang, M.; Jiang, S.; Huang, L.; Jiao, N.; Li, Y.; Yang, W. Zearalenone exposure triggered cecal physical barrier injury through the TGF-β1/Smads signaling pathway in weaned piglets. Toxins 2021, 13, 902. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, X.; Zhang, L.; Zhan, X.; Liu, Z.; Zhuo, Y.; Lin, Y.; Fang, Z.; Che, L.; Feng, B. Effects of a diet supplemented with exogenous catalase from penicillium notatum on intestinal development and microbiota in weaned piglets. Microorganisms 2020, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Zhang, H.J.; Wang, J.; Wu, S.G.; Yue, H.Y.; Jiang, X.R.; Qi, G.H. Effects of dietary grape proanthocyanidins on the growth performance, jejunum morphology and plasma biochemical indices of broiler chicks. Animal 2017, 11, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Jahanbakhshi, A.; Pourmozaffar, S.; Mozanzadeh, M.T.; Adeshina, I.; Zehra, S.; Vega-Heredia, S. Dietary Effect of Grape Seed Proanthocyanidin Extract on Growth Performance, Serum Biochemical Parameters, Skin Mucosal Immune Response, and Antioxidant Capacity in Goldfish (Carassius auratus). Ann. Anim. Sci. 2023, 23, 215–223. [Google Scholar] [CrossRef]
- Mohammadi, Y.; Kamangar, B.B.; Zarei, M.A. Effects of diets containing grape seed proanthocyanidin extract on the growth and oxidative capacity of common carp (Cyprinus carpio). Aquaculture 2021, 540, 736689. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Yu, B.; Luo, Y.; Huang, Z.; Zheng, P.; Mao, X.; Dai, Z.; Yu, J.; Yan, H. Dietary supplementation of grape seed proanthocyanidins improves growth performance, carcass traits, and meat quality in growing-finishing pigs. Anim. Nutr. 2024; in press. [Google Scholar] [CrossRef]
- Kim, J.; Yun, K.; Cho, A.; Kim, D.H.; Lee, Y.K.; Choi, M.J.; Kim, S.-h.; Kim, H.; Yoon, J.W.; Park, H.C. High cortisol levels are associated with oxidative stress and mortality in maintenance hemodialysis patients. BMC Nephrol. 2022, 23, 98. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Yuan, S.; Chen, D.; Zhang, K.; Yu, B. Effects of oxidative stress on growth performance, nutrient digestibilities and activities of antioxidative enzymes of weanling pigs. Asian-Australas. J. Anim. Sci. 2007, 20, 1600–1605. [Google Scholar] [CrossRef]
- Sánchez-Valle, V.; Chavez-Tapia, N.C.; Uribe, M.; Méndez-Sánchez, N. Role of oxidative stress and molecular changes in liver fibrosis: A review. Curr. Med. Chem. 2012, 19, 4850–4860. [Google Scholar] [CrossRef]
- Takahashi, Y. Nonalcoholic fatty liver disease and adult growth hormone deficiency: An under-recognized association? Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101816. [Google Scholar] [CrossRef]
- Yousef, M.; Saad, A.; El-Shennawy, L. Protective effect of grape seed proanthocyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem. Toxicol. 2009, 47, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, E.; Panahi, M.; Ghaffari, M.A.; Ghorbani, A. Effects of grape seed proanthocyanidin extract on oxidative stress induced by diabetes in rat kidney. Iran. Biomed. J. 2011, 15, 100. [Google Scholar]
- Liu, X.; Lin, X.; Mi, Y.; Li, J.; Zhang, C. Grape seed proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in the hens. Oxidative Med. Cell. Longev. 2018, 2018, 9390810. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Yang, S.-H.; Han, J.-X.; Li, P.; Zhang, Y.; Dong, S.; Chen, X.; Guo, J.; Wang, J.; He, J.-B. The protective effect of grape-seed proanthocyanidin extract on oxidative damage induced by zearalenone in Kunming mice liver. Int. J. Mol. Sci. 2016, 17, 808. [Google Scholar] [CrossRef]
- Mu, C.; Yang, W.; Wang, P.; Zhao, J.; Hao, X.; Zhang, J. Effects of high-concentrate diet supplemented with grape seed proanthocyanidins on growth performance, liver function, meat quality, and antioxidant activity in finishing lambs. Anim. Feed Sci. Technol. 2020, 266, 114518. [Google Scholar] [CrossRef]
- Gu, A.; Zhang, T.; Wang, T.; Wu, Z.; Shan, A.; Li, J. Effects of grape seed proanthocyanidins extract on fat deposition and lipid metabolism in rats. Chin. J. Anim. Nutr. 2021, 33, 4133–4144. [Google Scholar]
- Feng, Y.; Chen, X.; Chen, D.; He, J.; Zheng, P.; Luo, Y.; Yu, B.; Huang, Z. Dietary grape seed proanthocyanidin extract supplementation improves antioxidant capacity and lipid metabolism in finishing pigs. Anim. Biotechnol. 2023, 34, 4021–4031. [Google Scholar] [CrossRef]
- Habib, A.; Mihas, A.A.; Abou-Assi, S.G.; Williams, L.M.; Gavis, E.; Pandak, W.M.; Heuman, D.M. High-density lipoprotein cholesterol as an indicator of liver function and prognosis in noncholestatic cirrhotics. Clin. Gastroenterol. Hepatol. 2005, 3, 286–291. [Google Scholar] [CrossRef]
- Shao, Z.-H.; Vanden Hoek, T.L.; Xie, J.; Wojcik, K.; Chan, K.C.; Li, C.-Q.; Hamann, K.; Qin, Y.; Schumacker, P.T.; Becker, L.B. Grape seed proanthocyanidins induce pro-oxidant toxicity in cardiomyocytes. Cardiovasc. Toxicol. 2003, 3, 331–339. [Google Scholar] [CrossRef]
- Xing, T.; Chen, X.; Li, J.; Zhang, L.; Gao, F. Dietary taurine attenuates hydrogen peroxide-impaired growth performance and meat quality of broilers via modulating redox status and cell death signaling. J. Anim. Sci. 2021, 99, skab089. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Aw, T.Y. Molecular and cellular responses to oxidative stress and changes in oxidation-reduction imbalance in the intestine. Am. J. Clin. Nutr. 1999, 70, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hua, Y.; Yang, C.; Liu, S.; Tan, L.; Guo, J.; Li, Y. Polysaccharides extracted from mulberry fruits (Morus nigra L.): Antioxidant effect of ameliorating H2O2-induced liver injury in HepG2 cells. BMC Complement. Med. Ther. 2023, 23, 112. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, H.; Lu, J.; Lv, Y.; Baiyun, R.; Li, S.; Liu, B.; Lv, Z.; Zhang, Z. Dietary grape seed proanthocyanidin extract regulates metabolic disturbance in rat liver exposed to lead associated with PPARα signaling pathway. Environ. Pollut. 2018, 237, 377–387. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. Cell fate specification and differentiation in the adult mammalian intestine. Nat. Rev. Mol. Cell Biol. 2021, 22, 39–53. [Google Scholar] [CrossRef]
- Grandhaye, J.; Douard, V.; Rodriguez-Mateos, A.; Xu, Y.; Cheok, A.; Riva, A.; Guabiraba, R.; Zemb, O.; Philippe, C.; Monnoye, M. Microbiota changes due to grape seed extract diet improved intestinal homeostasis and decreased fatness in parental broiler hens. Microorganisms 2020, 8, 1141. [Google Scholar] [CrossRef]
- Sheng, K.; Zhang, G.; Sun, M.; He, S.; Kong, X.; Wang, J.; Zhu, F.; Zha, X.; Wang, Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020, 11, 7817–7829. [Google Scholar] [CrossRef]


| Ingredients (%) | Content | Nutrient Levels 2 (%) | Content |
|---|---|---|---|
| Corn | 17 | Digestible energy (MJ/kg) | 10.25 |
| Rice husk | 5 | Crude protein | 15.86 |
| Wheat bran | 20 | Crude fiber | 18.28 |
| Soybean meal | 14 | Acid detergent fiber | 19.98 |
| Alfalfa meal | 24 | Neutral detergent fiber | 36.25 |
| Bean straw powder | 16 | Ether extract | 3.71 |
| Premix 1 | 4 | Calcium | 0.78 |
| Total | 100 | Phosphorus | 0.43 |
| Items 1 | Treatment 2 | RMSE | p Value | |||
|---|---|---|---|---|---|---|
| G0 | G200 | G400 | G800 | |||
| IBW (g) | 800 | 797 | 801 | 809 | 21.10 | 0.756 |
| FBW (g) | 1853 | 1863 | 1832 | 1835 | 76.66 | 0.881 |
| ADFI (g/d) | 125.18 | 124.66 | 118.13 | 120.39 | 8.12 | 0.391 |
| ADG (g/d) | 30.10 | 30.47 | 29.45 | 29.31 | 1.98 | 0.715 |
| F/G | 4.16 a | 4.09 ab | 4.01 b | 4.11 ab | 0.08 | 0.043 |
| Survival rate (%) | 79.8 | 79.8 | 91.7 | 83.4 | - | - |
| Items | Treatment 1 | RMSE | p Value | |||
|---|---|---|---|---|---|---|
| G0 | G200 | G400 | G800 | |||
| Full eviscerated weight (g) | 785 | 790 | 799 | 816 | 7.34 | 0.894 |
| Full eviscerated weight ratio (%) | 41.29 | 41.31 | 41.58 | 42.62 | 2.15 | 0.681 |
| Half eviscerated weight (g) | 872 | 871 | 894 | 897 | 8.27 | 0.918 |
| Half eviscerated weight ratio (%) | 45.85 | 45.58 | 46.50 | 46.84 | 2.28 | 0.764 |
| Items | Treatment 1 | RMSE | p Value | |||
|---|---|---|---|---|---|---|
| G0 | G200 | G400 | G800 | |||
| Thymus (g/kg) | 2.56 | 2.31 | 2.48 | 2.26 | 0.24 | 0.117 |
| Spleen (g/kg) | 0.864 | 0.785 | 0.761 | 0.764 | 0.17 | 0.683 |
| Liver (g/kg) | 26.69 b | 28.41 ab | 33.65 a | 29.11 ab | 3.39 | 0.013 |
| Kidney (g/kg) | 7.22 | 6.52 | 7.13 | 6.86 | 0.88 | 0.519 |
| Items 2 | Treatment 1 | RMSE | p Value | |||
|---|---|---|---|---|---|---|
| G0 | G200 | G400 | G800 | |||
| TP (g/L) | 57.15 | 59.28 | 55.75 | 56.42 | 3.82 | 0.429 |
| UREA (g/L) | 8.51 | 9.00 | 7.84 | 7.57 | 1.51 | 0.363 |
| ALB (g/L) | 30.55 | 32.17 | 31.57 | 30.82 | 1.97 | 0.492 |
| TG (mmol/L) | 0.66 | 1.20 | 0.70 | 0.82 | 0.58 | 0.393 |
| TCHO (mmol/L) | 1.93 a | 1.74 ab | 1.49 b | 1.68 ab | 0.20 | 0.012 |
| Ca (mmol/L) | 4.60 | 4.62 | 4.69 | 4.61 | 0.20 | 0.869 |
| P (mmol/L) | 3.06 | 3.28 | 3.11 | 3.13 | 0.50 | 0.738 |
| ALP (U/L) | 116.17 | 125.00 | 132.00 | 111.50 | 24.37 | 0.486 |
| Items 2 | Treatment 1 | RMSE | p Value | |||
|---|---|---|---|---|---|---|
| Blank | SS | 5% H2O2 | 10% H2O2 | |||
| IBW (g) | 1468 | 1474 | 1469 | 1466 | 30.77 | 0.961 |
| FBW (g) | 1681 a | 1688 a | 1656 a | 1612 b | 37.82 | 0.002 |
| ADFI (g/d) | 126.10 a | 125.72 a | 118.64 a | 105.28 b | 13.51 | 0.015 |
| ADG (g/d) | 30.38 a | 30.54 a | 26.73 b | 20.74 c | 3.41 | <0.001 |
| F/G | 4.15 c | 4.12 c | 4.45 b | 5.10 a | 0.17 | <0.001 |
| Survival rate (%) | 100 | 100 | 100 | 100 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, M.; Liu, L.; Li, F.; Chen, J. Grape Seed Proanthocyanidin Extract Improves Growth Performance and Protects Against Hydrogen Peroxide-Induced Oxidative Stress to the Liver and Intestine in Weaned Hyla Rabbits. Animals 2025, 15, 327. https://doi.org/10.3390/ani15030327
Gong M, Liu L, Li F, Chen J. Grape Seed Proanthocyanidin Extract Improves Growth Performance and Protects Against Hydrogen Peroxide-Induced Oxidative Stress to the Liver and Intestine in Weaned Hyla Rabbits. Animals. 2025; 15(3):327. https://doi.org/10.3390/ani15030327
Chicago/Turabian StyleGong, Maohua, Lei Liu, Fuchang Li, and Jiali Chen. 2025. "Grape Seed Proanthocyanidin Extract Improves Growth Performance and Protects Against Hydrogen Peroxide-Induced Oxidative Stress to the Liver and Intestine in Weaned Hyla Rabbits" Animals 15, no. 3: 327. https://doi.org/10.3390/ani15030327
APA StyleGong, M., Liu, L., Li, F., & Chen, J. (2025). Grape Seed Proanthocyanidin Extract Improves Growth Performance and Protects Against Hydrogen Peroxide-Induced Oxidative Stress to the Liver and Intestine in Weaned Hyla Rabbits. Animals, 15(3), 327. https://doi.org/10.3390/ani15030327






