The Invasive Alien Species Callinectes sapidus Threatens the Restoration of Ostrea edulis and Paracentrotus lividus in the Mediterranean Sea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
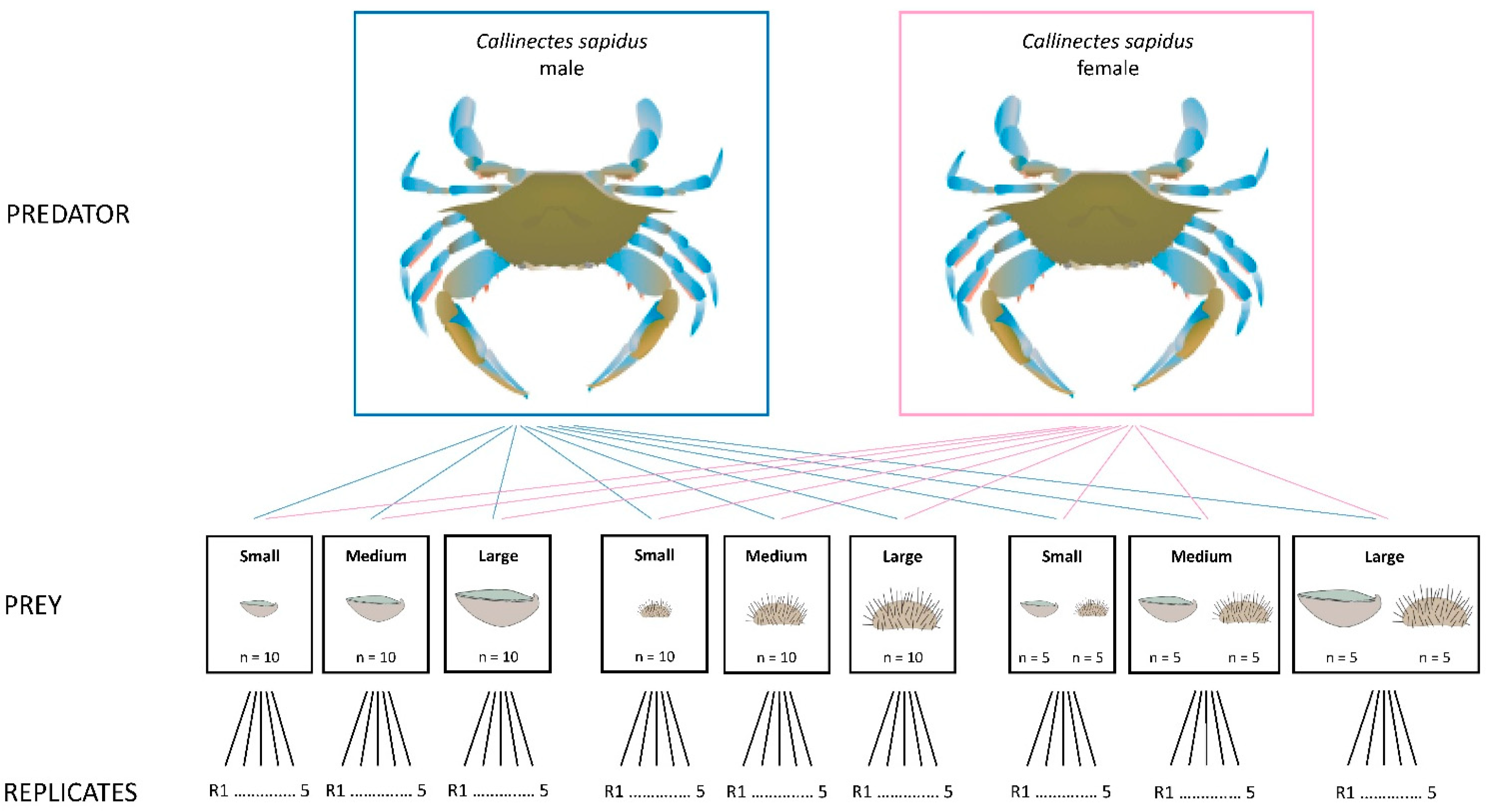
2.1. Experimental Design
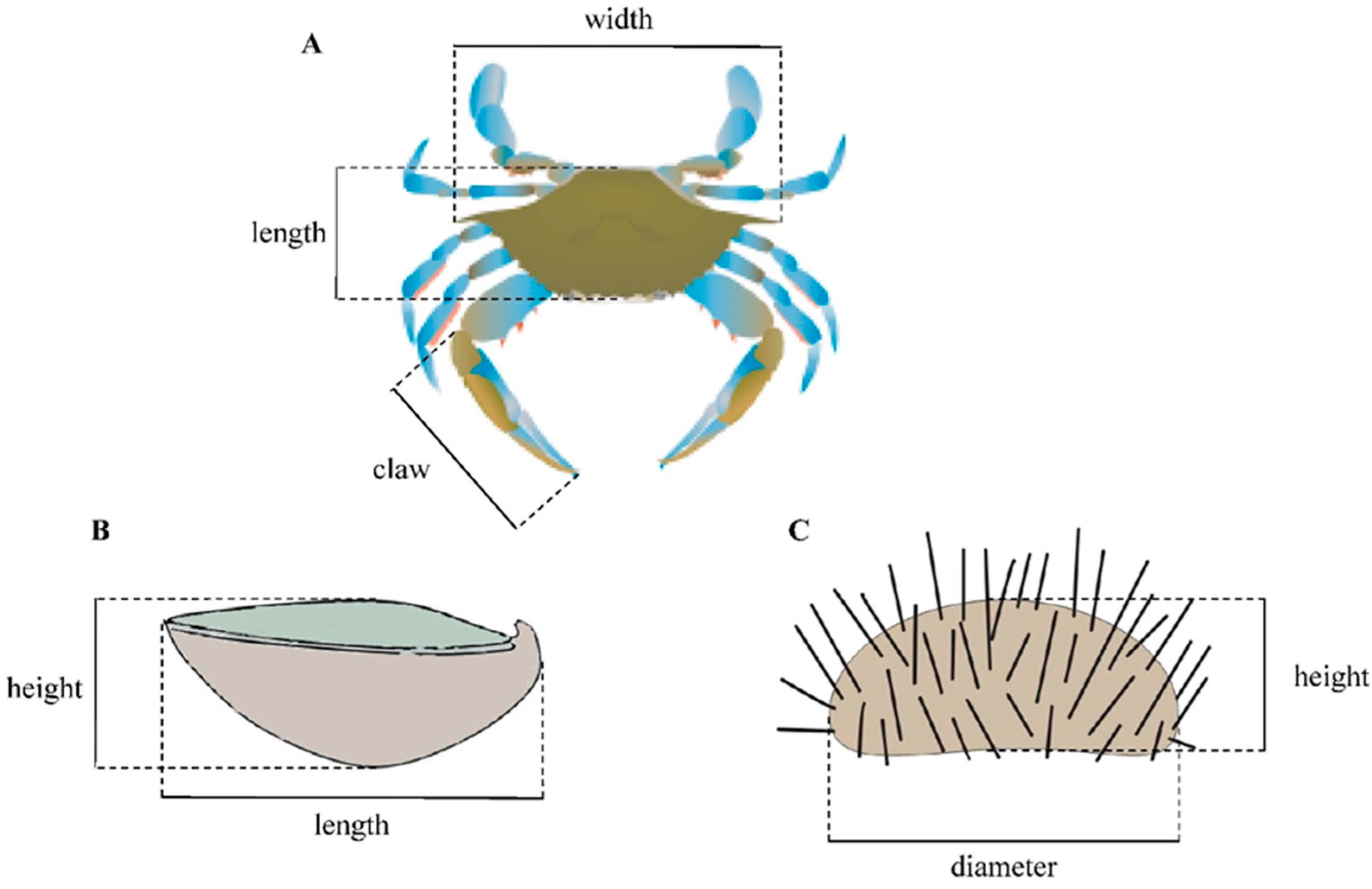
2.2. Predators
2.3. Ostrea edulis Prey
2.4. Paracentrotus lividus Prey
2.5. Compressive Strength
2.6. Data Analysis
3. Results
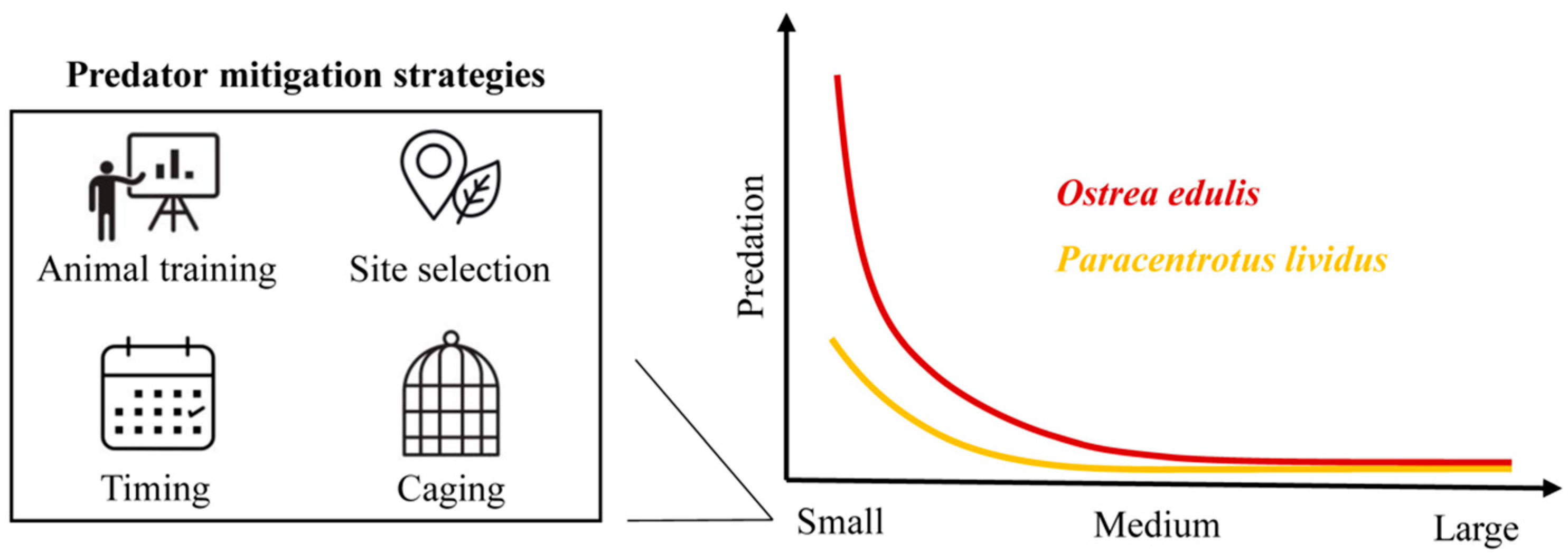
3.1. Predation
3.2. Compressive Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IMC | International Marine Centre |
| N | Newton |
| RAS | Recirculating Aquaculture System |
| S | Small |
| M | Medium |
| L | Large |
| Oe | Ostrea edulis |
| Pl | Paracentrotus lividus |
References
- Colsoul, B.; Pouvreau, S.; Di Poi, C.; Pouil, S.; Merk, V.; Peter, C.; Boersma, M.; Pogoda, B. Addressing critical limitations of oyster (Ostrea edulis) restoration: Identification of nature-based substrates for hatchery production and recruitment in the field. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 2101–2115. [Google Scholar] [CrossRef]
- Andrew, N.L.; Agatsuma, Y.; Ballesteros, E.; Bazhin, A.G.; Creaser, E.P.; Barnes, D.K.A.; Botsford, L.W.; Bradbury, A.; Campbell, A.; Dixon, J.D.; et al. Status and management of world sea urchin fisheries. In Oceanography and Marine Biology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002; 40p. [Google Scholar]
- Thurstan, R.H.; Hawkins, J.P.; Raby, L.; Roberts, C.M. Oyster (Ostrea edulis) extirpation and ecosystem transformation in the Firth of Forth, Scotland. J. Nat. Conserv. 2013, 21, 253–261. [Google Scholar] [CrossRef]
- Baião, L.F.; Moura, A.P.; Rocha, C.; Valente, L.M.P.; Cunha, L.M. Dimensions for the valorisation of sea urchin (Paracentrotus lividus) gonads production through the eyes of experienced chefs. Int. J. Gastron. Food Sci. 2021, 26, 100438. [Google Scholar] [CrossRef]
- Lotze, H.K.; Reise, K.; Worm, B.; van Beusekom, J.; Busch, M.; Ehlers, A.; Heinrich, D.; Hoffmann, R.C.; Holm, P.; Jensen, C.; et al. Human transformations of the Wadden Sea ecosystem through time: A synthesis. Helgol. Mar. Res. 2005, 59, 84–95. [Google Scholar] [CrossRef]
- Duchêne, J.; Bernard, I.; Pouvreau, S. Vers un retour de l’huître indigène en rade de Brest. Espèces 2015, 16, 51–57. [Google Scholar]
- Allain, J.Y. Structure des populations de Paracentrotus lividus (Lamarck) (Echinodermata, Echinoidea) soumises à la pêche sur le côtes Nord de Bretagne. Rev. Trav. Inst. Pêches Marit. 1975, 39, 171–212. [Google Scholar]
- Byrne, M. Annual reproductive cycles of the commercial sea urchin Paracentrotus lividus from an exposed intertidal and a sheltered subtidal habitat on the west coast of Ireland. Mar. Biol. 1990, 104, 275–289. [Google Scholar] [CrossRef]
- Pais, A.; Serra, S.; Meloni, G.; Saba, S.; Ceccherelli, G. Harvesting effects on Paracentrotus lividus population structure: A case study from north western Sardinia, Italy, before and after the fishing season. J. Coastal. Res. 2012, 28, 570–575. Available online: https://www.jstor.org/stable/41508570 (accessed on 6 December 2025).
- OSPAR Commission. Background document for Ostrea edulis and Ostrea edulis beds. OSPAR 2009, 482, 22. [Google Scholar]
- European Parliament. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora; European Parliament: Strasbourg, France, 1992; pp. 47–50.
- European Parliament. Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive); European Parliament: Strasbourg, France, 2008; pp. 19–40.
- Pogoda, B.; Brown, J.; Hancock, B.; Preston, J.; Pouvreau, S.; Kamermans, P.; Sanderson, W.; von Nordheim, H. The Native Oyster Restoration Alliance (NORA) and the Berlin Oyster Recommendation: Bringing back a key ecosystem engineer by developing and supporting best practice in Europe. Aquat. Living Resour. 2019, 32, 13. [Google Scholar] [CrossRef]
- Loi, B.; Guala, I.; da Silva, R.P.; Brundu, G.; Baroli, M.; Farina, S. Hard time to be parents? Sea urchin fishery shifts potential reproductive contribution of population onto the shoulders of the young adults. PeerJ 2017, 5, e3067. [Google Scholar] [CrossRef]
- Beddington, J.R.; Agnew, D.J.; Clark, C.W. Current Problems in the Management of Marine Fisheries. Science 2007, 316, 1713–1716. [Google Scholar] [CrossRef]
- Brondízio, E.S.; Settele, J.; Díaz, S.; Ngo, H.T. Global Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; ISBN 978-3-947851-20-1. [Google Scholar]
- zu Ermgassen, P.S.E.; Strand, Å.; Bakker, N.; Blanco, A.; Bonačić, K.; Boudry, P.; Brundu, G.; Cameron, T.C.; Connellan, I.; da Costa, F.; et al. Overcoming Ostrea edulis seed production limitations to meet ecosystem restoration demands in the UN decade on restoration. Aquat. Living Resour. 2023, 36, 16. [Google Scholar] [CrossRef]
- zu Ermgassen, P.S.E.; Albentosa, M.; Bakker, N.; Blanco, A.; Bonačić, K.; Carboni, S.; Brundu, G.; Colsoul, B.; Piñeiro, N.A.; da Costa, F.; et al. Ten priority questions for increasing the consistency and success in hatchery production of the European flat oyster for habitat restoration. Aquat. Living Resour. 2023, 36, 29. [Google Scholar] [CrossRef]
- Brundu, G.; Farina, S.; Domenici, P. Going back into the wild: The behavioural effects of raising sea urchins in captivity. Conserv. Physiol. 2020, 8, coaa015. [Google Scholar] [CrossRef]
- Cárcamo, P.F. Effects of food type and feeding frequency on the performance of early juveniles of the sea urchin Loxechinus albus (Echinodermata: Echinoidea): Implications for aquaculture and restocking. Aquaculture 2015, 436, 172–178. [Google Scholar] [CrossRef]
- Couvray, S.; Miard, T.; Bunet, R.; Martin, Y.; Grillasca, J.P.; Bonnefont, J.L.; Coupé, S. Experimental release of juvenile sea urchins (Paracentrotus lividus) in exploited sites along the French Mediterranean coast. J. Shellfish Res. 2015, 34, 555–563. [Google Scholar] [CrossRef]
- Hutchison, M.; Butcher, A.; Norris, A.; Kirkwood, J.; Chilcott, K. A Review of Domestication Effects on Stocked Fishes, Strategies to Improve Post Stocking Survival of Fishes and Their Potential Application to Threatened Fish Species Recovery Programs in the Murray–Darling Basin; Queensland Department of Agriculture, Fisheries and Forestry. Murray–Darling Basin Authority: Brisbane, Australia, 2012; 34p.
- Brown, C.; Laland, K. Social learning and life skills training for hatchery reared fish. J. Fish Biol. 2001, 59, 471–493. [Google Scholar] [CrossRef]
- Hughes, A.; zu Ermgassen, P.S.E. European Native Oyster Habitat Restoration Site Selection Checklist; Native Oyster Restoration Alliance: Berlin, Germany, 2021. [Google Scholar]
- Katsanevakis, S.; Olenin, S.; Puntila-Dodd, R.; Rilov, G.; Stæhr, P.A.U.; Teixeira, H.; Tsirintanis, K.; Birchenough, S.N.R.; Jakobsen, H.H.; Knudsen, S.W.; et al. Marine invasive alien species in Europe: 9 years after the IAS Regulation. Front. Mar. Sci. 2023, 10, 1271755. [Google Scholar] [CrossRef]
- Leadley, P.; Krug, C.B.; Obura, D.; Shannon, L.; Gonzalez, A. Transformative Actions on All Drivers of Biodiversity Loss Are Urgently Required to Achieve the Global Goals by 2050. 2020. Available online: https://www.cbd.int/doc/c/5735/c241/efeeac8d7685af2f38d75e4e/sbstta-24-inf-31-en.pdf (accessed on 6 December 2025).
- Serbetis, C. Un nouveau crustacé commestible en mer Egeé Callinectes sapidus Rath. (Decapod brach.). Proc. Gen. Fish. Counc. Medit. 1959, 5, 505–507. [Google Scholar]
- Zenetos, A.; Corsini-Foka, M.; Crocetta, F.; Gerovasileiou, V.; Karachle, P.K.; Simboura, N.; Tsiamis, K.; Pancucci-Papadopoulou, M.A. Deep cleaning of alien and cryptogenic species records in the Greek Seas (2018 update). Manag. Biol. Invasions 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Eggleston, D.B. Functional responses of blue crabs Callinectes sapidus Rathbun feeding on juvenile oysters Crassostrea virginica (Gmelin): Effects of predator sex and size, and prey size. J. Exp. Mar. Biol. Ecol. 1990, 143, 73–90. [Google Scholar] [CrossRef]
- Hill, J.M.; Weissburg, M.J. Habitat complexity and predator size mediate interactions between intraguild blue crab predators and mud crab prey in oyster reefs. Mar. Ecol. Prog. Ser. 2013, 488, 209–219. [Google Scholar] [CrossRef]
- Kampouris, T.E.; Porter, J.S.; Sanderson, W.G. Callinectes sapidus Rathbun, 1896 (Brachyura: Portunidae): An assessment on its diet and foraging behaviour, Thermaikos Gulf, NW Aegean Sea, Greece: Evidence for ecological and economic impacts. Crustac. Res. 2019, 48, 23–37. [Google Scholar] [CrossRef]
- Zenetos, A.; Cinar, M.; Pancucci-Papadopoulou, M.; Harmelin, J.; Furnari, G.; Andaloro, F.; Bellou, N.; Streftaris, N.; Zibrowius, H. Annotated list of marine alien species in the Mediterranean with records of the worst invasive species. Mediterr. Mar. Sci. 2005, 6, 63–118. [Google Scholar] [CrossRef]
- Nehring, S. Invasion history and success of the American Blue Crab Callinectes sapidus in European and adjacent waters. In In the Wrong Place–Alien Marine Crustaceans: Distribution, Biology and Impacts; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 607–624. [Google Scholar]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. The Atlantic blue crab Callinectes sapidus in southern European coastal waters: Distribution, impact and prospective invasion management strategies. Mar. Pollut. Bull. 2017, 119, 5–11. [Google Scholar] [CrossRef]
- Clavero, M.; Franch, N.; Bernardo-Madrid, R.; López, V.; Abelló, P.; Queral, J.M.; Mancinelli, G. Severe, rapid and widespread impacts of an Atlantic blue crab invasion. Mar. Pollut. Bull. 2022, 176, 113479. [Google Scholar] [CrossRef]
- Shauer, M.; Zangaro, F.; Specchia, V.; Pinna, M. Investigating invasion patterns of Callinectes sapidus and the relation with research effort and climate change in the Mediterranean Sea. Sci. Rep. 2025, 15, 34967. [Google Scholar] [CrossRef]
- Gavioli, A.; Mancinelli, G.; Turolla, E.; Lanzoni, M.; Paesanti, V.; Soana, E.; Eggleston, D.B.; Christian, R.R.; Castaldelli, G. Impacts of the invasive blue crab Callinectes sapidus on small-scale fisheries in a Mediterranean lagoon using fishery landing data. Sci. Total Environ. 2025, 974, 179236. [Google Scholar] [CrossRef]
- Albentosa, M.; Akinyemi, M.I.; Vera, M.; Ibarrola, I.; Filgueira, R.; Galimany, E.; da Costa, F.; Pardo, B.G.; Vázquez-Luis, M.; Hernández, A.; et al. Recovery of eutrophized marine ecosystems using the European flat oyster, Ostrea edulis. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 645–660. [Google Scholar] [CrossRef]
- zu Ermgassen, P.S.E.; Gamble, C.; Debney, A.; Colsoul, B.; Fabra, M.; Sanderson, W.G.; Strand, Å.; Preston, J. European Guidelines on Biosecurity in Native Oyster Restoration; The Zoological Society of London, UK: London, UK, 2020. [Google Scholar]
- Prado, P.; Peñas, A.; Ibáñez, C.; Cabanes, P.; Jornet, L.; Álvarez, N.; Caiola, N. Prey size and species preferences in the invasive blue crab, Callinectes sapidus: Potential effects in marine and freshwater ecosystems. Estuar. Coast. Shelf Sci. 2020, 245, 106997. [Google Scholar] [CrossRef]
- Cabiddu, S.; Addis, P.; Palmas, F.; Pusceddu, A.; Solari, P.; Pasquini, V. Feeding behavior and preference of the invasive blue crab (Callinectes sapidus Rathbun, 1896) for Mediterranean native bivalves in mesocosm. Hydrobiologia 2025, 852, 2907–2922. [Google Scholar] [CrossRef]
- Frongia, C.; Pagani, S.; Chindris, A.; Graham, P.; Carboni, S.; Brundu, G. Comparative evaluation of Ostrea edulis (Linnaeus, 1758) and Crassostrea gigas (Thunberg, 1793) biodeposition in Mediterranean lagoons and under controlled conditions. Reg. Stud. Mar. Sci. 2023, 67, 103188. [Google Scholar] [CrossRef]
- Newell, R.I.E.; Kennedy, V.S.; Shaw, K.S. Comparative vulnerability to predators, and induced defense responses, of eastern oysters Crassostrea virginica and non-native Crassostrea ariakensis oysters in Chesapeake Bay. Mar. Biol. 2007, 152, 449–460. [Google Scholar] [CrossRef]
- Maneiro, V.; Santos, Y.; Pazos, A.J.; Silva, A.; Torres-Corral, Y.; Sánchez, J.L.; Pérez-Parallé, M.L. Effects of food ration, water flow rate and bacteriological levels of broodstock on the reproductive conditioning of the European flat oyster (Ostrea edulis, Linnaeus 1758). Aquac. Rep. 2020, 18, 100412. [Google Scholar] [CrossRef]
- Helm, M.M.; Bourne, N.; Lovatelli, A. The Hatchery Culture of Bivalves: A Practical Manual; Fisheries Technical Paper No. 471; FAO: Rome, Italy, 2004; 177p. [Google Scholar]
- Preston, J.; Gamble, C.; Debney, A.; Helmer, L.; Hancock, B.; zu Ermgassen, P.S.E. European Native Oyster Habitat Restoration Handbook; The Zoological Society of London: London, UK, 2020. [Google Scholar]
- Brundu, G.; Pagani, S.; Graham, P. The shell growth of Crassostrea gigas and Ostrea edulis in windy condition: A preliminary evaluation. Aquac. Res. 2021, 52, 6802–6807. [Google Scholar] [CrossRef]
- Brundu, G.; Vallainc, D.; Baroli, M.; Figus, A.M.; Pinna, A.; Carboni, S. Effects of on-demand feeding on sea urchin larvae (Paracentrotus lividus; Lamarck, 1816), development, survival and microalgae utilization. Aquac. Res. 2017, 48, 1550–1560. [Google Scholar] [CrossRef]
- Brundu, G.; Vian Monleón, L.; Vallainc, D.; Carboni, S. Effects of larval diet and metamorphosis cue on survival and growth of sea urchin post-larvae (Paracentrotus lividus; Lamarck, 1816). Aquaculture 2016, 465, 265–270. [Google Scholar] [CrossRef]
- Asnaghi, V.; Chindris, A.; Leggieri, F.; Scolamacchia, M.; Brundu, G.; Guala, I.; Loi, B.; Chiantore, M.; Farina, S. Decreased pH impairs sea urchin resistance to predatory fish: A combined laboratory-field study to understand the fate of top-down processes in future oceans. Mar. Environ. Res. 2020, 162, 105194. [Google Scholar] [CrossRef]
- Lombardi, S.A.; Chon, G.D.; Lee, J.J.-W.; Lane, H.A.; Paynter, K.T. Shell hardness and compressive strength of the eastern oyster, Crassostrea virginica, and the Asian oyster, Crassostrea ariakensis. Biol. Bull. 2013, 225, 175–183. [Google Scholar] [CrossRef]
- Mancinelli, G.; Clamuzina, B.; Petrić, M.; Carrozzo, L.; Glamuzina, L.; Zotti, M.; Raho, D.; Vizzini, S. The trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in the food web of Parila Lagoon (South Eastern Adriatic, Croatia): A first assessment using stable isotopes. Mediterr. Mar. Sci. 2016, 15, 634–643. [Google Scholar] [CrossRef]
- Aslan, H.; Polito, M.J. Trophic ecology of the Atlantic blue crab Callinectes sapidus as an invasive non-native species in the Aegean Sea. Biol. Invasions 2021, 23, 2289–2304. [Google Scholar] [CrossRef]
- De Giorgi, R.; Bardelli, R.; Cilenti, L.; Falco, S.; Fanizzi, F.P.; Guerra, M.T.; Katselis, G.; Kevrekidis, K.; Mancini, F.; Doria, L.; et al. Opportunistic omnivory impairs the use of the Atlantic blue crab Callinectes sapidus as a trace metal biomonitor in invaded Mediterranean coastal waters. Mar. Pollut. Bull. 2024, 206, 116715. [Google Scholar] [CrossRef]
- Weissburg, M.J.; Ferner, M.C.; Pisut, D.P.; Smee, D.L. Ecological consequences of chemically mediated prey perception. J. Chem. Ecol. 2002, 28, 1953–1970. [Google Scholar] [CrossRef]
- Eggleston, D.B. Foraging behavior of the blue-crab, Callinectes sapidus, on juvenile oysters, Crassostrea virginica: Effects of prey density and size. Bull. Mar. Sci. 1990, 46, 62–82. Available online: https://scholarworks.wm.edu/vimsarticles/1545 (accessed on 6 December 2025).
- Bishop, M.J.; Peterson, C.H. When r-selection may not predict introduced-species proliferation: Predation of a nonnative oyster. Ecol. Appl. 2006, 16, 718–730. [Google Scholar] [CrossRef]
- Sayegh, A.F.; Dong, S.B. Analysis of laminated curved beams. J. Eng. Mech. Div. 1970, 96, 471–482. [Google Scholar] [CrossRef]
- Seed, R. Predation of the ribbed mussel Geukensia demissa by the blue crab Callinectes sapidus. Neth. J. Sea Res. 1982, 16, 163–172. [Google Scholar] [CrossRef]
- Hughes, R.N.; Seed, R. Size selection of mussels by the blue crab Callinectes sapidus: Energy maximizer or time minimizer. Mar. Ecol. Prog. Ser. 1981, 6, 83–89. [Google Scholar] [CrossRef]
- Schenk, S.C.; Wainwright, P.C. Dimorphism and the functional basis of claw strength in six brachyuran crabs. J. Zool. 2001, 255, 105–119. [Google Scholar] [CrossRef]
- Ortega--Jiménez, E.; Cuesta, J.A.; Laiz, I.; González--Ortegón, E. Diet of the Invasive Atlantic Blue Crab Callinectes sapidus Rathbun, 1896 (Decapoda, Portunidae) in the Guadalquivir Estuary (Spain). Estuaries Coasts 2024, 47, 1075–1085. [Google Scholar] [CrossRef]
- Colsoul, B.; Boudry, P.; Pérez-Parallé, M.L.; Bratoš Cetinić, A.; Hugh-Jones, T.; Arzul, I.; Mérou, N.; Mathias Wegner, K.; Peter, C.; Merk, V.; et al. Sustainable large-scale production of European flat oyster (Ostrea edulis) seed for ecological restoration and aquaculture: A review. Rev. Aquac. 2021, 13, 1423–1468. [Google Scholar] [CrossRef]
- Strand, Å.; Bakker, N.; Bird, A.; Blanco, A.; Bonačić, K.; Brundu, G.; Colsoul, B.; Connellan, I.; da Costa, F.; Fabra, M.; et al. What Restoration Practitioners Need to Know About the Oyster Production Industry; NORA: Berlin, Germany, 2021. [Google Scholar]
- Olla, B.L.; Davis, M.W.; Ryer, C.H. Behavioural deficits in hatchery reared fish, potential effects on survival following release. Aquac. Fish. Manag. 1994, 25, 19–34. [Google Scholar]
- Boudouresque, C.F.; Verlaque, M. Paracentrotus lividus. In Sea Urchins: Biology and Ecology, 3rd ed.; Lawrence, J.M., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 43, pp. 297–327. [Google Scholar]
- Hines, A.H. Ecology of juvenile and adult blue crabs. In The Blue Crab: Callinectes sapidus; Kennedy, V.S., Cronin, L.E., Eds.; Maryland Sea Grant College: College Park, MD, USA, 2007; pp. 565–650. [Google Scholar]
- Marchessaux, G.; Bosch-Belmar, M.; Cilenti, L.; Lago, N.; Mangano, M.C.; Marsiglia, N.; Sarà, G. The invasive blue crab Callinectes sapidus thermal response: Predicting metabolic suitability maps under future warming Mediterranean scenarios. Front. Mar. Sci. 2022, 9, 1055404. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Dickinson, G.H.; Matoo, O.B.; Bagwe, R.; Dickinson, A.; Beniash, E.; Sokolova, I.M. Interactive effects of elevated temperature and CO2 levels on energy metabolism and biomineralization of marine bivalves Crassostrea virginica and Mercenaria mercenaria. Comp. Biochem. Physiol. 2013, 166, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, G.H.; Ivanina, A.V.; Matoo, O.B.; Pörtner, H.O.; Lannig, G.; Bock, C.; Beniash, E.; Sokolova, I.M. Interactive effects of salinity and elevated CO2 levels on juvenile eastern oysters, Crassostrea virginica. J. Exp. Biol. 2012, 215, 29–43. [Google Scholar] [CrossRef]
- Green, M.A.; Jones, M.E.; Boudreau, C.L.; Moore, R.L.; Westman, B.A. Dissolution mortality of juvenile bivalves in coastal marine deposits. Limnol. Oceanogr. 2004, 49, 727–734. [Google Scholar] [CrossRef]
- Wahltinez, S.J.; Stacy, N.I.; Hadfield, C.A.; Harms, C.A.; Lewbart, G.A.; Newton, A.L.; Nunamaker, E.A. Perspective: Opportunities for advancing aquatic invertebrate welfare. Front. Vet. Sci. 2022, 9, 973376. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.T. KCl induced paralysis facilitates detachment of hatchery reared juvenile green sea urchins, Strongylocentrotus droebachiensis. Aquaculture 2003, 216, 155–164. [Google Scholar] [CrossRef]




| PREDATOR | Biomass (g) | Length (mm) | Width (mm) | Claw (mm) |
| C. sapidus male | 296.5 ± 20.5 | 75.4 ± 1.4 | 152 ± 2.8 | 95.7 ± 1.9 |
| C. sapidus female | 188.8 ± 15.7 | 70.4 ± 2.5 | 145 ± 7.1 | 66.8 ± 1.1 |
| PREY | Biomass (g) | Length (mm) | Diameter (mm) | Height (mm) |
| O. edulis—large | 123.4 ± 2.4 | 82.7 ± 0.7 | 33 ± 0.9 | |
| O. edulis—medium | 31.1 ± 0.5 | 60.6 ± 0.4 | 15.2 ± 0.2 | |
| O. edulis—small | 3.2 ± 0.1 | 32.9 ± 0.3 | 7.1 ± 0.2 | |
| P. lividus—large | 62.2 ± 1.1 | 53.3 ± 0.4 | 33.4 ± 0.4 | |
| P. lividus—medium | 14 ± 0.4 | 31.3 ± 0.3 | 19.1 ± 0.4 | |
| P. lividus—small | 1.1 ± 0.1 | 13.5 ± 0.1 | 7.9 ± 0.1 |
| Callinectes sapidus | F | p | |
| Biomass | 188.800 | 0.0031 | Ma > Fe |
| Length | 70.400 | 0.1155 | Ma = Fe |
| Width | 145.000 | 0.3871 | Ma = Fe |
| Claw | 67.800 | 0.0001 | Ma > Fe |
| Ostrea edulis | H 2, 90 | p | |
| Biomass | 79.12153 | 0.0000 | L > M > S |
| Length | 79.21347 | 0.0000 | L > M > S |
| Height | 79.39078 | 0.0000 | L > M > S |
| Paracentrotus lividus | H 2, 270 | p | |
| Biomass | 239.1183 | 0.000 | L > M > S |
| Diameter | 239.6507 | 0.000 | L > M > S |
| Height | 238.7411 | 0.000 | L > M > S |
| 1 h | 4 h | 8 h | 24 h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | df | MS | F | p | MS | F | p | MS | F | p | MS | F | p |
| Intercept | 1 | 0.0657 | 4.5904 | 0.0373 | 0.5389 | 14.6628 | 0.0004 | 0.5591 | 14.6769 | 0.0004 | 1.5708 | 74.4895 | 0.0001 |
| Sex | 1 | 0.0032 | 0.2215 | 0.6401 | 0.1554 | 4.2290 | 0.0452 | 0.1664 | 4.3672 | 0.0420 | 0.1279 | 6.0639 | 0.0174 |
| Species | 1 | 0.0032 | 0.2215 | 0.6401 | 0.1777 | 4.8341 | 0.0328 | 0.1893 | 4.9704 | 0.0305 | 0.7973 | 37.8073 | 0.0001 |
| Size | 2 | 0.0657 | 4.5904 | 0.0150 | 0.5389 | 14.6628 | 0.0001 | 0.5591 | 14.6769 | 0.0001 | 1.5708 | 74.4895 | 0.0001 |
| Sex-Species | 1 | 0.0657 | 4.5904 | 0.0373 | 0.0067 | 0.1814 | 0.6721 | 0.0091 | 0.2383 | 0.6276 | 0.0001 | 0.0004 | 0.9846 |
| Sex-Size | 2 | 0.0032 | 0.2215 | 0.8022 | 0.1554 | 4.2290 | 0.0203 | 0.1664 | 4.3672 | 0.0181 | 0.1279 | 6.0639 | 0.0045 |
| Species-Size | 2 | 0.0032 | 0.2215 | 0.8022 | 0.1777 | 4.8341 | 0.0122 | 0.1893 | 4.9704 | 0.0109 | 0.7973 | 37.8073 | 0.0001 |
| Sex-Species-Size | 2 | 0.0657 | 4.5904 | 0.0150 | 0.0067 | 0.1814 | 0.8347 | 0.0091 | 0.2383 | 0.7889 | 0.0001 | 0.0004 | 0.9996 |
| Error | 48 | 0.0143 | 0.0368 | 0.0381 | 0.0211 | ||||||||
| Tukey’s HSD | S > M = L | Fe > Ma | Fe > Ma | Fe > Ma | |||||||||
| Oe > Pl | Oe > Pl | Oe > Pl | |||||||||||
| S > M = L | S > M = L | S > M = L | |||||||||||
| S(Fe) > S(Ma) = M = L | S(Fe) > S(Ma) = M = L | S(Fe) > S(Ma) > M = L | |||||||||||
| S(Oe) > S(Pl) = M = L | S(Oe) > S(Pl) = M = L | S(Oe) > S(Pl) = M = L | |||||||||||
| 1 h | 4 h | 8 h | 24 h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect | df | MS | F | p | MS | F | p | MS | F | p | MS | F | p |
| Intercept | 1 | 0.7855 | 28.9739 | 0.0001 | 1.7092 | 75.3869 | 0.0001 | 2.0878 | 74.4937 | 0.0001 | 2.4438 | 131.4860 | 0.0001 |
| Sex | 1 | 0.2914 | 10.7505 | 0.0019 | 0.2226 | 9.8189 | 0.0029 | 0.1460 | 5.2097 | 0.0269 | 0.0903 | 4.8573 | 0.0324 |
| Species | 1 | 0.0212 | 0.7821 | 0.3809 | 0.2809 | 12.3905 | 0.0009 | 0.3841 | 13.7042 | 0.0005 | 0.4919 | 26.4686 | 0.0001 |
| Size | 2 | 0.7855 | 28.9739 | 0.0001 | 1.7092 | 75.3869 | 0.0001 | 2.0878 | 74.4937 | 0.0001 | 2.4438 | 131.4860 | 0.0001 |
| Sex-Species | 1 | 0.0682 | 2.5144 | 0.1194 | 0.0244 | 1.0783 | 0.3043 | 0.0003 | 0.0126 | 0.9110 | 0.0099 | 0.5331 | 0.4689 |
| Sex-Size | 2 | 0.2914 | 10.7505 | 0.0001 | 0.2226 | 9.8189 | 0.0003 | 0.1460 | 5.2097 | 0.0090 | 0.0903 | 4.8573 | 0.0120 |
| Species-Size | 2 | 0.0212 | 0.7821 | 0.4632 | 0.2809 | 12.3905 | 0.0001 | 0.3841 | 13.7042 | 0.0001 | 0.4919 | 26.4686 | 0.0001 |
| Sex-Species-Size | 2 | 0.0682 | 2.5144 | 0.0915 | 0.0244 | 1.0783 | 0.3483 | 0.0003 | 0.0126 | 0.9874 | 0.0099 | 0.5331 | 0.5902 |
| Error | 48 | 0.0271 | 0.0227 | 0.0280 | 0.0186 | ||||||||
| Tukey’s HSD | Ma > Fe | Ma > Fe | Ma > Fe | Ma > Fe | |||||||||
| S > M = L | Oe > Pl | Oe > Pl | Oe > Pl | ||||||||||
| S(Ma) > S(Fe) = M = L | S > M = L | S > M = L | S > M = L | ||||||||||
| S(Ma) > S(Fe) > M = L | S(Ma) > S(Fe) > M = L | S(Ma) > S(Fe) > M = L | |||||||||||
| S(Oe) > S(Pl) > M = L | S(Oe) > S(Pl) > M = L | S(Oe) > S(Pl) > M = L | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brundu, G.; Graham, P.; Corrias, M.; Frongia, C.; Carboni, S. The Invasive Alien Species Callinectes sapidus Threatens the Restoration of Ostrea edulis and Paracentrotus lividus in the Mediterranean Sea. Animals 2025, 15, 3553. https://doi.org/10.3390/ani15243553
Brundu G, Graham P, Corrias M, Frongia C, Carboni S. The Invasive Alien Species Callinectes sapidus Threatens the Restoration of Ostrea edulis and Paracentrotus lividus in the Mediterranean Sea. Animals. 2025; 15(24):3553. https://doi.org/10.3390/ani15243553
Chicago/Turabian StyleBrundu, Gianni, Philip Graham, Mattia Corrias, Cheoma Frongia, and Stefano Carboni. 2025. "The Invasive Alien Species Callinectes sapidus Threatens the Restoration of Ostrea edulis and Paracentrotus lividus in the Mediterranean Sea" Animals 15, no. 24: 3553. https://doi.org/10.3390/ani15243553
APA StyleBrundu, G., Graham, P., Corrias, M., Frongia, C., & Carboni, S. (2025). The Invasive Alien Species Callinectes sapidus Threatens the Restoration of Ostrea edulis and Paracentrotus lividus in the Mediterranean Sea. Animals, 15(24), 3553. https://doi.org/10.3390/ani15243553









