Diagnostic Imaging Features of Mammary Gland Tumors in Dogs and Cats
Simple Summary
Abstract
1. Introduction
2. Imaging Techniques for the Diagnosis and Evaluation of Disease Progression
3. Imaging Features of Primary Lesions
3.1. Ultrasonography
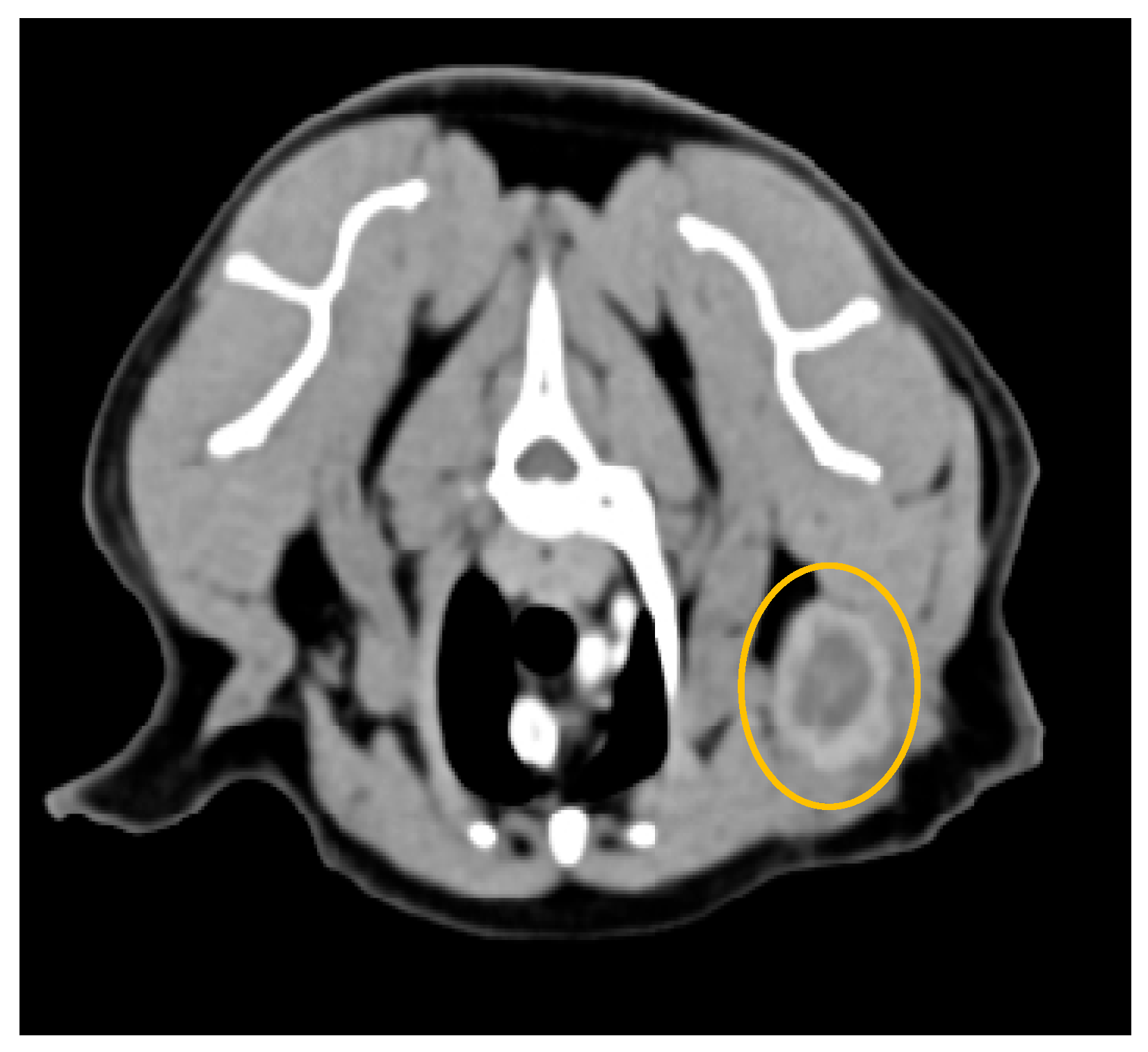
3.2. Computed Tomography
3.3. Positron Emission Tomography
3.4. Magnetic Resonance Imaging
4. Imaging Features of Metastatic Lesions
4.1. Radiography
4.2. Computed Tomography
4.3. Ultrasound
4.4. Nuclear Medicine
5. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARFI | Acoustic radiation force impulse |
| CEUS | Contrast-enhanced ultrasonography |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| MGT | Mammary gland tumors |
| PET | Positron emission tomography |
| RX | Radiology |
| Sci | Scintigraphy |
| US | Ultrasonography |
| 3D | Three-dimensional |
References
- Vazquez, E.; Lipovka, Y.; Cervantes-Arias, A.; Garibay-Escobar, A.; Haby, M.M.; Queiroga, F.L.; Velazquez, C. Canine Mammary Cancer: State of the Art and Future Perspectives. Animals. 2023, 13, 3147. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K. Canine Mammary Gland Tumors. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 573–596. [Google Scholar] [CrossRef] [PubMed]
- Brearley, M.J. Mammary Gland Tumours in the Dog. Practice 1989, 11, 248–253. [Google Scholar] [CrossRef]
- Murphy, S. Mammary Tumours in Dogs and Cats. Practice 2008, 30, 334–339. [Google Scholar] [CrossRef]
- Guirguis, P.; Beggs, D.S. Systematic Review: Does Pre-Pubertal Spaying Reduce the Risk of Canine Mammary Tumours? Animals 2025, 15, 436. [Google Scholar] [CrossRef]
- Kristiansen, V.M.; Peña, L.; Díez Córdova, L.; Illera, J.C.; Skjerve, E.; Breen, A.M.; Cofone, M.A.; Langeland, M.; Teige, J.; Goldschmidt, M.; et al. Effect of Ovariohysterectomy at the Time of Tumor Removal in Dogs with Mammary Carcinomas: A Randomized Controlled Trial. J. Vet. Intern. Med. 2016, 30, 230–241. [Google Scholar] [CrossRef]
- Hart, B.L.; Hart, L.A.; Thigpen, A.P.; Willits, N.H. Assisting Decision-Making on Age of Neutering for 35 Breeds of Dogs: Associated Joint Disorders, Cancers, and Urinary Incontinence. Front. Vet. Sci. 2020, 7, 548304. [Google Scholar] [CrossRef] [PubMed]
- Saba, C.F.; Rogers, K.S.; Newman, S.J.; Mauldin, G.E.; Vail, D.M. Mammary Gland Tumors in Male Dogs. J. Vet. Intern. Med. 2007, 21, 1056–1059. [Google Scholar] [CrossRef]
- Novosad, C.A. Principles of Treatment for Mammary Gland Tumors. Clin. Tech. Small Anim. Pract. 2003, 18, 107–109. [Google Scholar] [CrossRef]
- Skorupski, K.A.; Overley, B.; Shofer, F.S.; Goldschmidt, M.H.; Miller, C.A.; Sørenmo, K.U. Clinical Characteristics of Mammary Carcinoma in Male Cats. J. Vet. Intern. Med. 2005, 19, 52–55. [Google Scholar] [CrossRef]
- Zhelavskyi, M.M.; Dmytriv, O.Y. Mammary Tumors of the Dog and the Cat: Modern Approaches to Classification and Diagnosis (Review). Sci. Messenger LNU Vet. Med. Biotechnol. 2023, 25, 39–44. [Google Scholar] [CrossRef]
- Zappulli, V.; De Zan, G.; Cardazzo, B.; Bargelloni, L.; Castagnaro, M. Feline Mammary Tumours in Comparative Oncology. J. Dairy Res. 2005, 72, 98–106. [Google Scholar] [CrossRef]
- Giménez, F.; Hecht, S.; Craig, L.E.; Legendre, A.M. Early Detection, Aggressive Therapy: Optimizing the Management of Feline Mammary Masses. J. Feline Med. Surg. 2010, 12, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Baumann, D.; Hauser, B.; Hubler, M.; Flückiger, M. Originalarbeit Zeichen von Metastasen Auf Dem Röntgenbild von Hunden Mit Mammatumoren: Eine Retro-Spektive Studie. Signs of Metastatic Disease on Thoracic Radiographs of Dogs Suffering from Mammary Gland Tumours: A Retrospective Study (1990–1998). Band 2004, 146, 431–435. [Google Scholar]
- Baptista, C.S.; Santos, A.A.; Villagrasa, M.H.; Matos, A.J. Diagnostic Imaging Findings of Uncommon Sites for Distant Metastasis in Canine Mammary Tumours—Abstracts from the annual meeting of the european college of veterinary diagnostic imaging and european association of veterinary diagnostic imaging. Vet. Radiol. Ultrasound. 2014, 55, 651–679. [Google Scholar] [CrossRef]
- Domínguez, E.; Anadón, E.; Espada, Y.; Grau-Roma, L.; Majó, N.; Novellas, R. Imaging diagnosis—Ultrasonographic appearance of small bowel metastasis from canine mammary carcinoma. Vet. Radiol. Ultrasound 2014, 55, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Im, K.-S.; Kim, N.-H.; Chon, S.-K.; Doster, A.R.; Sur, J.-H. Inflammatory Mammary Carcinoma with Metastasis to the Brain and Distant Organs in a Spayed Shih Tzu Dog. J. Vet. Diagn. Investig. 2011, 23, 1079–1082. [Google Scholar] [CrossRef]
- Clemente, M.; Pérez-Alenza, M.D.; Peña, L. Metastasis of Canine Inflammatory versus Non-Inflammatory Mammary Tumours. J. Comp. Pathol. 2010, 143, 157–163. [Google Scholar] [CrossRef]
- Aupperle, H.; März, I.; Ellenberger, C.; Buschatz, S.; Reischauer, A.; Schoon, H.-A. Primary and Secondary Heart Tumours in Dogs and Cats. J. Comp. Pathol. 2007, 136, 18–26. [Google Scholar] [CrossRef]
- Alenza, M.D.P.; Tabanera, E.; Peña, L. Inflammatory Mammary Carcinoma in Dogs: 33 Cases (1995–1999). J. Am. Vet. Med. Assoc. 2001, 219, 1110–1114. [Google Scholar] [CrossRef]
- Baptista, C.S.; Villagrasa, M.; Marinho, A.A. Standardised B-Scan and A-Scan Echographic Evaluation of Spontaneous Anterior Uveal Melanomas in the Dog. Vet. J. 2006, 171, 322–330. [Google Scholar] [CrossRef]
- Choi, H.; Na, H.; Lee, S.-K.; Bae, S.; Oh, T.; Lee, K. Cutaneous metastasis of mammary gland tumor in a dog: A case report. Korean J. Vet. Res. 2022, 62, e2. [Google Scholar] [CrossRef]
- Petrucci, G.; Henriques, J.; Gregório, H.; Vicente, G.; Prada, J.; Pires, I.; Lobo, L.; Medeiros, R.; Queiroga, F. Metastatic Feline Mammary Cancer: Prognostic Factors, Outcome and Comparison of Different Treatment Modalities—A Retrospective Multicentre Study. J. Feline Med. Surg. 2021, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Cassali, G.D.; de Campos, C.B.; Bertagnolli, A.C.; Estrela-Lima, A.; Lavalle, G.E.; Damasceno, K.A.; Di Nardi, A.B.; Cogliati, B.; da Costa, F.V.A.; Sobral, R.; et al. Consensus for the Diagnosis, Prognosis and Treatment of Feline Mammary Tumors. Braz J. Vet. Res. Anim. Sci. 2018, 55, e135084. [Google Scholar] [CrossRef]
- Rodrigues-Jesus, J.; Vilhena, H.; Canadas-Sousa, A.; Dias-Pereira, P. Feline Mammary Tumors: A Comprehensive Review of Histological Classification Schemes, Grading Systems, and Prognostic Factors. Vet. Sci. 2025, 12, 736. [Google Scholar] [CrossRef] [PubMed]
- Barbagianni, M.S.; Gouletsou, P.G. Modern Imaging Techniques in the Study and Disease Diagnosis of the Mammary Glands of Animals. Vet. Sci. 2023, 10, 83. [Google Scholar] [CrossRef]
- Sánchez, D.; Romero, L.; López, S.; Campuzano, M.; Ortega, R.; Morales, A.; Guadarrama, M.; Cesarman-Maus, G.; García-Pérez, O.; Lizano, M. 18F-FDG—PET/CT in Canine Mammary Gland Tumors. Front. Vet. Sci. 2019, 6, 280. [Google Scholar] [CrossRef]
- Soultani, C.; Patsikas, M.N.; Mayer, M.; Kazakos, G.M.; Theodoridis, T.D.; Vignoli, M.; Ilia, T.S.M.; Karagiannopoulou, M.; Ilia, G.M.; Tragoulia, I.; et al. Contrast Enhanced Computed Tomography Assessment of Superficial Inguinal Lymph Node Metastasis in Canine Mammary Gland Tumors. Vet. Radiol. Ultrasound 2021, 62, 557–567. [Google Scholar] [CrossRef]
- Lamb, C.R.; Whitlock, J.; Foster-Yeow, A.T.L. Prevalence of Pulmonary Nodules in Dogs with Malignant Neoplasia as Determined by CT. Vet. Radiol. Ultrasound 2019, 60, 300–305. [Google Scholar] [CrossRef]
- Miles, K.G.; Lattimer, J.C.; Jergens, A.E.; Krause, G.F. A retrospective evaluation of the radiographic evidence of pulmonary metastatic disease on initial presentation in the dog. Vet. Radiol. 1990, 31, 79–82. [Google Scholar] [CrossRef]
- Armbrust, L.J.; Biller, D.S.; Bamford, A.; Chun, R.; Garrett, L.D.; Sanderson, M.W. Comparison of Three-View Thoracic Radiography and Computed Tomography for Detection of Pulmonary Nodules in Dogs with Neoplasia. J. Am. Vet. Med. Assoc. 2012, 240, 1088–1094. [Google Scholar] [CrossRef]
- Barthez, P.Y.; Hornof, W.J.; Théon, A.P.; Craychee, T.J.; Morgan, J.P. Receiver Operating Characteristic Curve Analysis of the Performance of Various Radiographic Protocols When Screening Dogs for Pulmonary Metastases. J. Am. Vet. Med. Assoc. 1994, 204, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Klaengkaew, A.; Sutthigran, S.; Thammasiri, N.; Yuwatanakorn, K.; Thanaboonnipat, C.; Ponglowhapan, S.; Choisunirachon, N. The Evaluation of Non-Anesthetic Computed Tomography for Detection of Pulmonary Parenchyma in Feline Mammary Gland Carcinoma: A Preliminary Study. BMC Vet. Res. 2021, 17, 237. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Meomartino, L.; Gnudi, G.; Brunetti, A.; Di Giancamillo, M. Imaging Techniques in Veterinary Medicine. Part II: Computed Tomography, Magnetic Resonance Imaging, Nuclear Medicine. Eur. J. Radiol. Open 2023, 10, 100467. [Google Scholar] [CrossRef]
- Rose, R.J.; Worley, D.R. A Contemporary Retrospective Study of Survival in Dogs with Primary Lung Tumors: 40 Cases (2005–2017). Front. Vet. Sci. 2020, 7, 519703. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.; de Miranda, B.d.S.P.; Aires, L.P.N.; Lima, B.B.; de Oliveira, A.P.L.; Feliciano, G.S.M.; Uscategui, R.A.R. The Importance of Ultrasonography in the Evaluation of Mammary Tumors in Bitches. Animals 2023, 13, 1742. [Google Scholar] [CrossRef]
- Meomartino, L.; Greco, A.; Di Giancamillo, M.; Brunetti, A.; Gnudi, G. Imaging Techniques in Veterinary Medicine. Part I: Radiography and Ultrasonography. Eur. J. Radiol. Open. 2021, 8, 100382. [Google Scholar] [CrossRef] [PubMed]
- Matos da Silva, D.; Inajá Juliani, A.; Lopes Ribeiro, C.; Rodini Engrácia Filho, J.; Domit Guérios, S.; Rodrigues Fróes, T. Abdominal Ultrasonographic Findings in Dogs with Mammary Tumors: Association with Tumor Characteristics and Survival. Turk. J. Vet. Anim. Sci. 2019, 43, 808–816. [Google Scholar] [CrossRef]
- Matos, A.J.F.; Baptista, C.S.; Gärtner, M.F.; Rutteman, G.R. Prognostic Studies of Canine and Feline Mammary Tumours: The Need for Standardized Procedures. Vet. J. 2012, 193, 24–31. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.; Uscategui, R.A.R.; Maronezi, M.C.; Simões, A.P.R.; Silva, P.; Gasser, B.; Pavan, L.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. Ultrasonography Methods for Predicting Malignancy in Canine Mammary Tumors. PLoS ONE 2017, 12, e0178143. [Google Scholar] [CrossRef]
- Vannozzi, I.; Tesi, M.; Zangheri, M.; Innocenti, V.M.; Rota, A.; Citi, S.; Poli, A. B-Mode Ultrasound Examination of Canine Mammary Gland Neoplastic Lesions of Small Size (Diameter < 2 cm). Vet. Res. Commun. 2018, 42, 137–143. [Google Scholar] [CrossRef]
- Lana, S. Tumors of the Mammary Gland. In Withrow & MacEwen’s Small Animal Clinical Oncology; Withrow, S.J., Vail, D.M., Page, R.L., Eds.; Elsevier: St. Louis, MO, USA, 2007; pp. 619–636. [Google Scholar] [CrossRef]
- Ophir, J.; Céspedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A Quantitative Method for Imaging the Elasticity of Biological Tissues. Ultrason. Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; Espadas, L.; Santella, M.; Ezquerra, L.J.; Tarazona, R.; Durán, M.E. Comparison between Histological Features and Strain Elastographic Characteristics in Canine Mammary Carcinomas. Vet. Sci. 2022, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Uscategui, R.A.R.; Maronezi, M.C.; Gasser, B.; Pavan, L.; Gatto, I.R.H.; de Almeida, V.T.; Vicente, W.R.R.; Feliciano, M.A.R. Ultrasonography for Lymph Nodes Metastasis Identification in Bitches with Mammary Neoplasms. Sci. Rep. 2018, 8, 17708. [Google Scholar] [CrossRef]
- Belotta, A.F.; Gomes, M.C.; Rocha, N.S.; Melchert, A.; Giuffrida, R.; Silva, J.P.; Mamprim, M.J. Sonography and Sonoelastography in the Detection of Malignancy in Superficial Lymph Nodes of Dogs. J. Vet. Intern. Med. 2019, 33, 1403–1413. [Google Scholar] [CrossRef]
- Ying, L.; Hou, Y.; Zheng, H.-M.; Lin, X.; Xie, Z.-L.; Hu, Y.-P. Real-Time Elastography for the Differentiation of Benign and Malignant Superficial Lymph Nodes: A Meta-Analysis. Eur. J. Radiol. 2012, 81, 2576–2584. [Google Scholar] [CrossRef]
- Bhatia, K.S.S.; Cho, C.C.M.; Yuen, Y.-H.; Rasalkar, D.D.; King, A.D.; Ahuja, A.T. Real-Time Qualitative Ultrasound Elastography of Cervical Lymph Nodes in Routine Clinical Practice: Interobserver Agreement and Correlation with Malignancy. Ultrasound Med. Biol. 2010, 36, 1990–1997. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Du, J.; Fang, H.; Li, F.; Wang, L. Evaluation of Breast Lesions by Contrast Enhanced Ultrasound: Qualitative and Quantitative Analysis. Eur. J. Radiol. 2012, 81, e444–e450. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, R.; Ouyang, Q.; Chen, L.; Dong, B.; Huihua, Y. Contrast-Enhanced Ultrasound Is Helpful in the Differentiation of Malignant and Benign Breast Lesions. Eur. J. Radiol. 2010, 73, 288–293. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.-X.; Liu, J.-B.; Zhu, Q.-L.; Sun, Q. Evaluation of Breast Lesions with Contrast-Enhanced Ultrasound Using the Microvascular Imaging Technique: Initial Observations. Breast 2008, 17, 532–539. [Google Scholar] [CrossRef]
- Mantziaras, G.; Vasileiou, N.G.; Ioannidi, K.S.; Mavrogianni, V.S.; Gougoulis, D.A.; Fthenakis, G.C.; Petridis, I.G.; Barbagianni, M.S. Use of Contrast-Enhanced Ultrasonographic Examination to Evaluate Health Status of Mammary Glands of Ewes at the End of a Lactation Period. J. Dairy Res. 2018, 85, 39–43. [Google Scholar] [CrossRef]
- Vanderperren, K.; Saunders, J.H.; Van der Vekens, E.; Wydooghe, E.; de Rooster, H.; Duchateau, L.; Stock, E. B-Mode and Contrast-Enhanced Ultrasonography of the Mammary Gland during the Estrous Cycle of Dogs. Anim. Reprod. Sci. 2018, 199, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Stan, F.; Gudea, A.; Damian, A.; Gal, A.F.; Papuc, I.; Pop, A.R.; Martonos, C. Ultrasonographic Algorithm for the Assessment of Sentinel Lymph Nodes That Drain the Mammary Carcinomas in Female Dogs. Animals 2020, 10, 2366. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.R.; Pretorius, D.H. Three-Dimensional Ultrasound Imaging. Ultrasound Med. Biol. 1998, 24, 1243–1270. [Google Scholar] [CrossRef]
- Franz, S.; Hofmann-Parisot, M.M.; Baumgartner, W. Evaluation of Three-Dimensional Ultrasonography of the Bovine Mammary Gland. Am. J. Vet. Res. 2004, 65, 1159–1163. [Google Scholar] [CrossRef]
- Chen, D.-R.; Lai, H.-W. Three-Dimensional Ultrasonography for Breast Malignancy Detection. Expert. Opin. Med. Diagn. 2011, 5, 253–261. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Daniel, G.B. Advanced Imaging for Veterinary Cancer Patients. Vet. Clin. N. Am. Small Anim. Pract. 2007, 37, 1059–1077. [Google Scholar] [CrossRef] [PubMed]
- Farwell, M.D.; Pryma, D.A.; Mankoff, D.A. PET/CT Imaging in Cancer: Current Applications and Future Directions. Cancer 2014, 120, 3433–3445. [Google Scholar] [CrossRef]
- Randall, E.K. PET-Computed Tomography in Veterinary Medicine. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 515–533. [Google Scholar] [CrossRef]
- Black, N.F.; McJames, S.; Rust, T.C.; Kadrmas, D.J. Evaluation of Rapid Dual-Tracer 62 Cu-PTSM + 62 Cu-ATSM PET in Dogs with Spontaneously Occurring Tumors. Phys. Med. Biol. 2008, 53, 217–232. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Peremans, K. PET and SPECT Imaging in Veterinary Medicine. Semin. Nucl. Med. 2014, 44, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yitbarek, D.; Dagnaw, G.G. Application of Advanced Imaging Modalities in Veterinary Medicine: A Review. Vet. Med. Res. Rep. 2022, 13, 117–130. [Google Scholar] [CrossRef]
- Balogh, L.; Andócs, G.; Perge, E.; Vajdovich, P.; Thuróczy, J.; Láng, J.; Jánoki, C.A. Oncological Scintigraphy in Dogs with 99m Technetium MIBI and DMSA(V)—Two Case Reports. Vet. Q. 2001, 23, 52–56. [Google Scholar] [CrossRef]
- Morandi, F. Liver Scintigraphy in Veterinary Medicine. Semin. Nucl. Med. 2014, 44, 15–23. [Google Scholar] [CrossRef]
- Hibbert, A.; Gruffydd-Jones, T.; Barrett, E.L.; Day, M.J.; Harvey, A.M. Feline Thyroid Carcinoma: Diagnosis and Response to High-Dose Radioactive Iodine Treatment. J. Feline Med. Surg. 2009, 11, 116–124. [Google Scholar] [CrossRef]
- Taillefer, R. The Role of 99mTc-Sestamibi and Other Conventional Radiopharmaceuticals in Breast Cancer Diagnosis. Semin. Nucl. Med. 1999, 29, 16–40. [Google Scholar] [CrossRef]
- Branco, P.S.C.; Gutfilen-Schlesinger, G.; Sena, P.; Gutfilen-Schlesinger, G.; Souza, S.A.L.; Gutfilen, B. Detection of Mammary Adenocarcinoma Metastases in a Cat Through99mtc-Thymine Scintigraphy. Vet. Mex. OA 2020, 7, 1–9. [Google Scholar] [CrossRef]
- Daniel, G.B.; Neelis, D.A. Thyroid Scintigraphy in Veterinary Medicine. Semin. Nucl. Med. 2014, 44, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Garamvölgyi, R.; Petrási, Z.; Hevesi, A.; Jakab, C.; Vajda, Z.; Bogner, P.; Repa, I. Magnetic Resonance Imaging Technique for the Examination of Canine Mammary Tumours. Acta Vet. Hung. 2006, 54, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Chaustre, X.L.; Bustamante-Cano, J.J.; Gómez-Parra, F. Linfografía Mediante Imagen Por Resonancia Magnética En Modelo Animal Canino. ITECKNE 2017, 14, 46–61. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, K.; Choi, H.; Lee, Y. Evaluation of Mammary Gland Calcification in Dogs: Radiography and Computed Tomography. J. Anim. Reprod. Biotechnol. 2017, 32, 183–192. [Google Scholar] [CrossRef]
- Soultani, C.; Patsikas, M.N.; Karayannopoulou, M.; Jakovljevic, S.; Chryssogonidis, I.; Papazoglou, L.; Papaioannou, N.; Papadopoulou, P.; Pavlidou, K.; Ilia, G.M.; et al. Assessment of Sentinel Lymph Node Metastasis in Canine Mammary Gland Tumors Using Computed Tomographic Indirect Lymphography. Vet. Radiol. Ultrasound 2017, 58, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, M.A.R.; Vicente, W.R.R.; Silva, M.A.M. Conventional and Doppler Ultrasound for the Differentiation of Benign and Malignant Canine Mammary Tumours. J. Small Anim. Pract. 2012, 53, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Nyman, H.T.; Nielsen, O.L.; McEvoy, F.J.; Lee, M.H.; Martinussen, T.; Hellmén, E.; Kristensen, A.T. Comparison of B-Mode and Doppler Ultrasonographic Findings with Histologic Features of Benign and Malignant Mammary Tumors in Dogs. Am. J. Vet. Res. 2006, 67, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Bulnes, A.; Garcia Fernandez, P.; Mayenco Aguirre, A.M.; Sanchez de la Muela, M. Ultrasonographic Imaging of Canine Mammary Tumours. Vet. Rec. 1998, 143, 687–689. [Google Scholar]
- Feliciano, M.A.R.; Ramirez, R.A.U.; Maronezi, M.C.; Maciel, G.S.; Avante, M.L.; Senhorello, I.L.S.; Mucédola, T.; Gasser, B.; Carvalho, C.F.; Vicente, W.R.R. Accuracy of Four Ultrasonography Techniques in Predicting Histopathological Classification of Canine Mammary Carcinomas. Vet. Radiol. Ultrasound 2018, 59, 444–452. [Google Scholar] [CrossRef]
- Baştan, A.; Özenc, E.; Pir Yağci, İ.; Baki Acar, D. Ultrasonographic Evaluation of Mammary Tumors in Bitches. Kafkas Univ. Vet. Fak. Derg. 2009, 15, 211–216. [Google Scholar] [CrossRef]
- Marquardt, C.; Burkhardt, E.; Failing, K.; Wehrend, A. Sonographic Examination of Mammary Tumors in Bitches. Part I: Individual Criteria Detectable by Sonography and Their Correlation with Tumor Dignity. Tierärztl. Prax. Kleintiere. 2003, 31, 275–283. [Google Scholar] [CrossRef]
- Soler, M.; Dominguez, E.; Lucas, X.; Novellas, R.; Gomes-Coelho, K.V.; Espada, Y.; Agut, A. Comparison between Ultrasonographic Findings of Benign and Malignant Canine Mammary Gland Tumours Using B-Mode, Colour Doppler, Power Doppler and Spectral Doppler. Res. Vet. Sci. 2016, 107, 141–146. [Google Scholar] [CrossRef]
- Guedes, P.E.B.; Daniel, H.B.T.; Rosa Sampaio, K.M.O.; da Silva, E.B.; Ferreira, M.L.; de Lavor, M.S.L.; de Oliveira Clark, R.M.; Wenceslau, A.A.; Said, R.A.; Silva, F.L. Clinical and Ultrasonographic Aspects of Benign and Malignant Mammary Tumors in Female Dogs. Acta Sci. Vet. 2020, 48, 101276. [Google Scholar] [CrossRef]
- Hillaert, A.; Stock, E.; Duchateau, L.; de Rooster, H.; Devriendt, N.; Vanderperren, K. B-Mode and Contrast-Enhanced Ultrasonography Aspects of Benign and Malignant Superficial Neoplasms in Dogs: A Preliminary Study. Animals 2022, 12, 2765. [Google Scholar] [CrossRef]
- Glińska-Suchocka, K.; Jankowski, M.; Kubiak, K.; Spużak, J.; Nicpon, J. Application of Shear Wave Elastography in the Diagnosis of Mammary Gland Neoplasm in Dogs. Pol. J. Vet. Sci. 2013, 16, 477–482. [Google Scholar] [CrossRef]
- Massimini, M.; Gloria, A.; Romanucci, M.; Della Salda, L.; Di Francesco, L.; Contri, A. Strain and Shear-Wave Elastography and Their Relationship to Histopathological Features of Canine Mammary Nodular Lesions. Vet. Sci. 2022, 9, 506. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.; Maronezi, M.C.; Pavan, L.; Castanheira, T.L.; Simões, A.P.R.; Carvalho, C.F.; Canola, J.C.; Vicente, W.R.R. ARFI Elastography as a Complementary Diagnostic Method for Mammary Neoplasia in Female Dogs—Preliminary Results. J. Small Anim. Pract. 2014, 55, 504–508. [Google Scholar] [CrossRef]
- Gasser, B.; Rodriguez, M.G.K.; Uscategui, R.A.R.; Silva, P.A.; Maronezi, M.C.; Pavan, L.; Feliciano, M.A.R.; Vicente, W.R.R. Ultrasonographic Characteristics of Benign Mammary Lesions in Bitches. Vet. Med. 2018, 63, 216–224. [Google Scholar] [CrossRef]
- Feliciano, M.A.R.; Maronezi, M.C.; Brito, M.B.S.; Simões, A.P.R.; Maciel, G.S.; Castanheira, T.L.L.; Garrido, E.; Uscategui, R.R.; Miceli, N.G.; Vicente, W.R.R. Doppler and Elastography as Complementary Diagnostic Methods for Mammary Neoplasms in Female Cats. Arq. Bras. Med. Vet. Zootec. 2015, 67, 935–939. [Google Scholar] [CrossRef]
- Navarro, D.M.; Silva, D.M.F.; Costa, F.S.; Wischral, A. Dopplerfluxometric and Angiogenic Analysis of Canine Mammary Tumors. Arq. Bras. Med. Vet. Zootec. 2018, 70, 1099–1108. [Google Scholar] [CrossRef]
- Dantas Cassali, G.; Cavalheiro Bertagnolli, A.; Ferreira, E.; Araújo Damasceno, K.; de Oliveira Gamba, C.; Bonolo de Campos, C. Canine Mammary Mixed Tumours: A Review. Vet. Med. Int. 2012, 2012, 274608. [Google Scholar] [CrossRef]
- Seiler, S.M.F.; Baumgartner, C.; Hirschberger, J.; Beer, A.J.; Brühschwein, A.; Kreutzmann, N.; Laberke, S.; Wergin, M.C.; Meyer-Lindenberg, A.; Brandl, J.; et al. Comparative Oncology: Evaluation of 2-Deoxy-2-[18F]Fluoro-D-Glucose (FDG) Positron Emission Tomography/Computed Tomography (PET/CT) for the Staging of Dogs with Malignant Tumors. PLoS ONE 2015, 10, e0127800. [Google Scholar] [CrossRef]
- Koo, Y.; Yun, T.; Chae, Y.; Lee, D.; Son, M.; Ku, D.; Kim, H.; Yang, M.; Kang, B. Evaluation of a Dog with Inflammatory Mammary Carcinoma Using 18 F-2-deoxy-2-fluoro-d-glucose Positron Emission Tomography/Computed Tomography. Vet. Med. Sci. 2022, 8, 1361–1365. [Google Scholar] [CrossRef]
- Fidler, I.J.; Brodey, R.S. A Necropsy Study of Canine Malignant Mammary Neoplasms. J. Am. Vet. Med. Assoc. 1967, 151, 710–715. [Google Scholar] [PubMed]
- Otoni, C.C.; Rahal, S.C.; Vulcano, L.C.; Ribeiro, S.M.; Hette, K.; Giordano, T.; Doiche, D.P.; Amorim, R.L. Survey Radiography and Computerized Tomography Imaging of the Thorax in Female Dogs with Mammary Tumors. Acta Vet. Scand. 2010, 52, 20. [Google Scholar] [CrossRef]
- Lekshmi, S.L.; Nair, S.S.; Sajitha, I.S.; Ramankutty, S.; Narayanan, M.K.; John Martin, K.D. Radiographic Assessment of Pulmonary Metastatic Lesions in Superficial Cutaneous and Mammary Neoplasms in Dogs. J. Vet. Anim. Sci. 2021, 52, 393–398. [Google Scholar] [CrossRef]
- Forrest, L.J.; Graybush, C.A. Radiographic Patterns of Pulmonary Metastasis in 25 Cats. Vet. Radiol. Ultrasound 1998, 39, 4–8. [Google Scholar] [CrossRef]
- Fonseca Pinto, A.C.B.C.; Iwasaki, M.; Figueiredo, C.M.; Cortopassi, S.R.G.; Sterman, F.d.A. Tomografia Computadorizada do Tórax de Cadelas Portadoras de Neoplasias Malignas. II—Avaliação dos Campos Pulmonares. Braz. J. Vet. Res. Anim. Sci. 2007, 44, 174–182. [Google Scholar] [CrossRef]
- Fields, E.L.; Robertson, I.D.; Osborne, J.A.; Brown, J.C. Comparison of Abdominal Computed Tomography and Abdominal Ultrasound in Sedated Dogs. Vet. Radiol. Ultrasound 2012, 53, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, C.; Sterman, F.A.; Hagen, S.C.F.; Pinto, A.C.B.C.F.; Oliveira, C.M.; Faustino, M.; Talib, M.S.; Torres, L.N. Avaliação Ultrassonográfica de Linfonodos na Pesquisa de Metástases de Neoplasia Mamária em Cadelas. Pesq. Vet. Bras. 2011, 31, 1006–1013. [Google Scholar] [CrossRef]
- Moraes, N.S.; Borges, N.C. Sonographic Assessment of the Normal and Abnormal Feline Mammary Glands and Axillary and Inguinal Lymph Nodes. Vet. Med. Int. 2021, 2021, 9998025. [Google Scholar] [CrossRef]
- Castelo-Branco, P.S.M.; Sena, P.; Souza, S.A.L.; Lopes, F.P.P.L.; Fonseca, L.M.B.; Scherer, P.O.; Gutfilen, B. Diagnóstico Precoce de Metástase Pulmonar: Comparação entre a Radiografia Convencional e a Cintilografia com Timina-99mTc em Cadela com Tumor de Mama—Relato de Caso. Braz. J. Vet. Med. 2013, 35, 267–271. [Google Scholar]
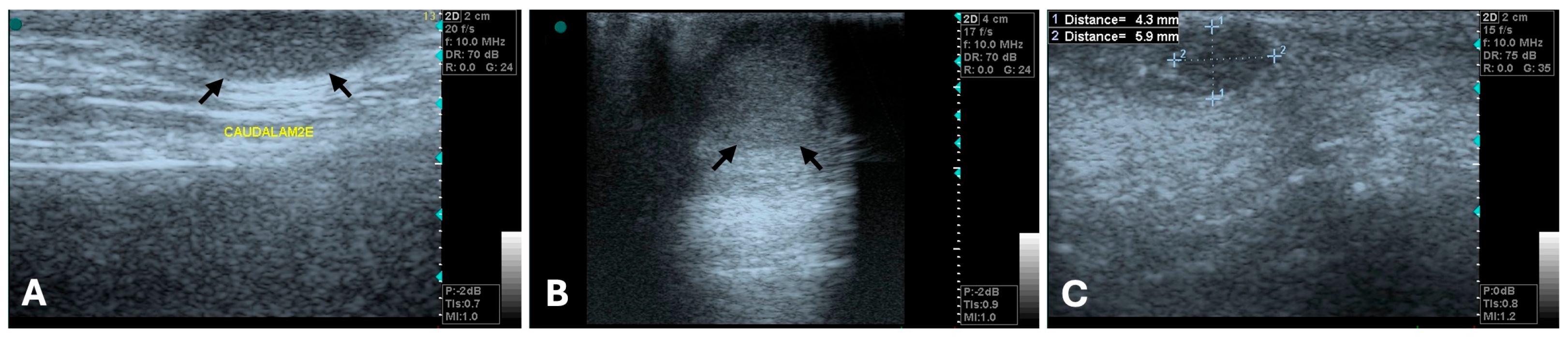
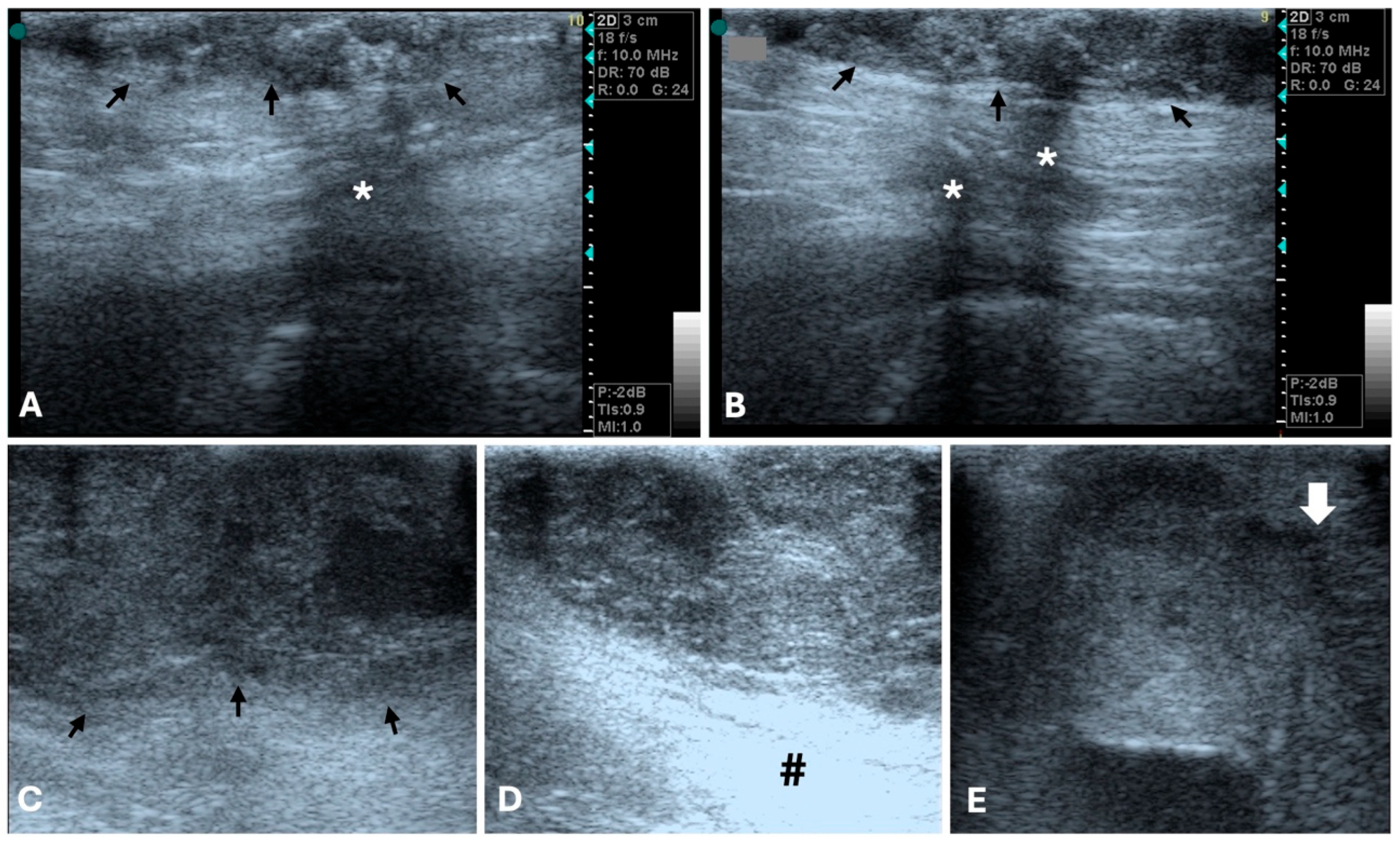
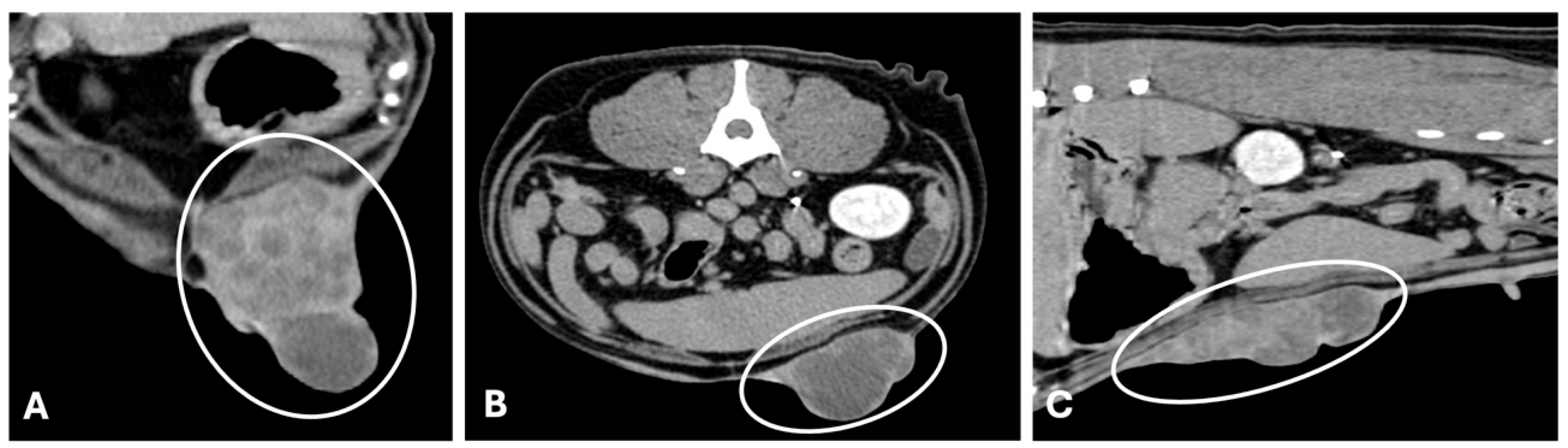
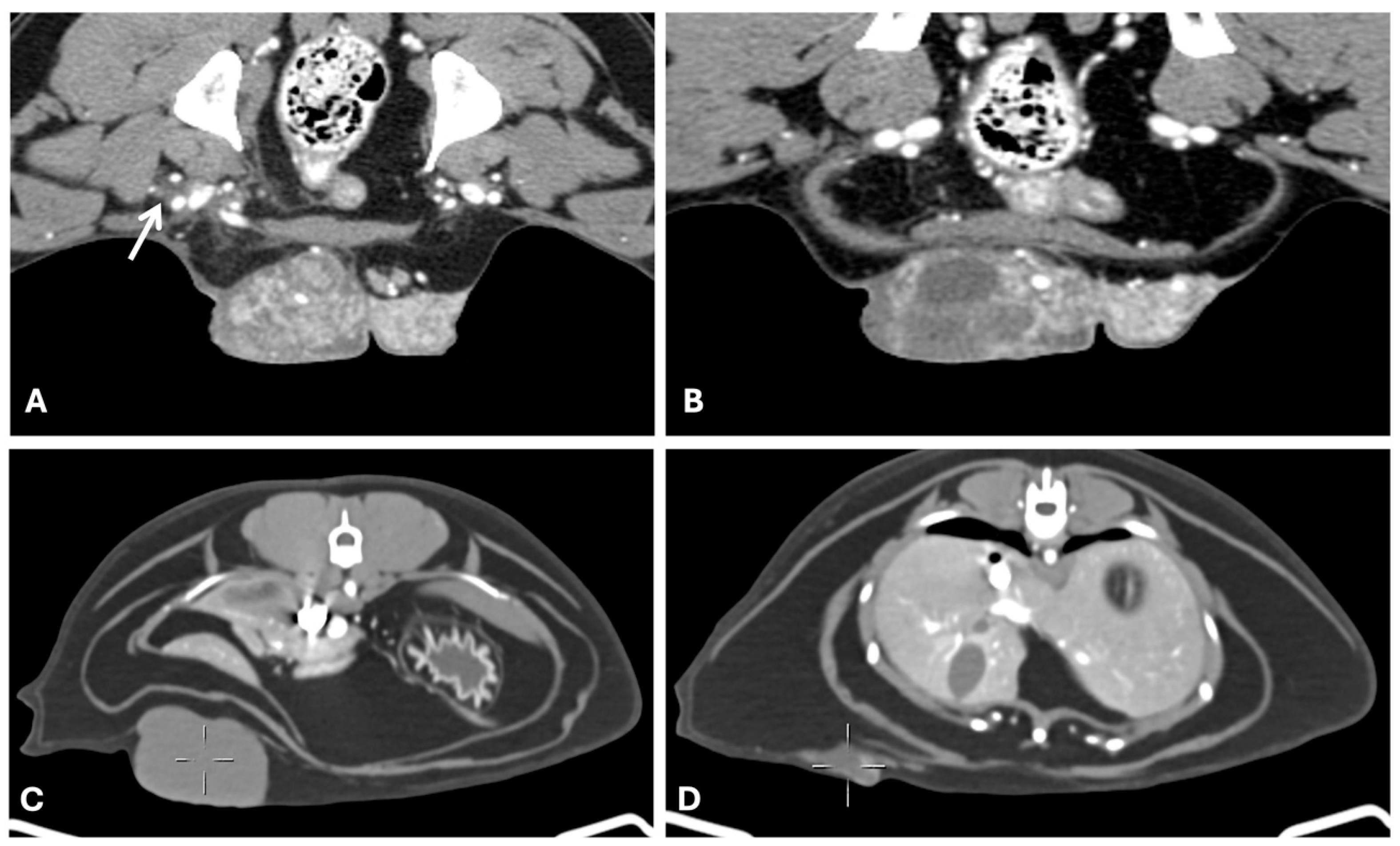
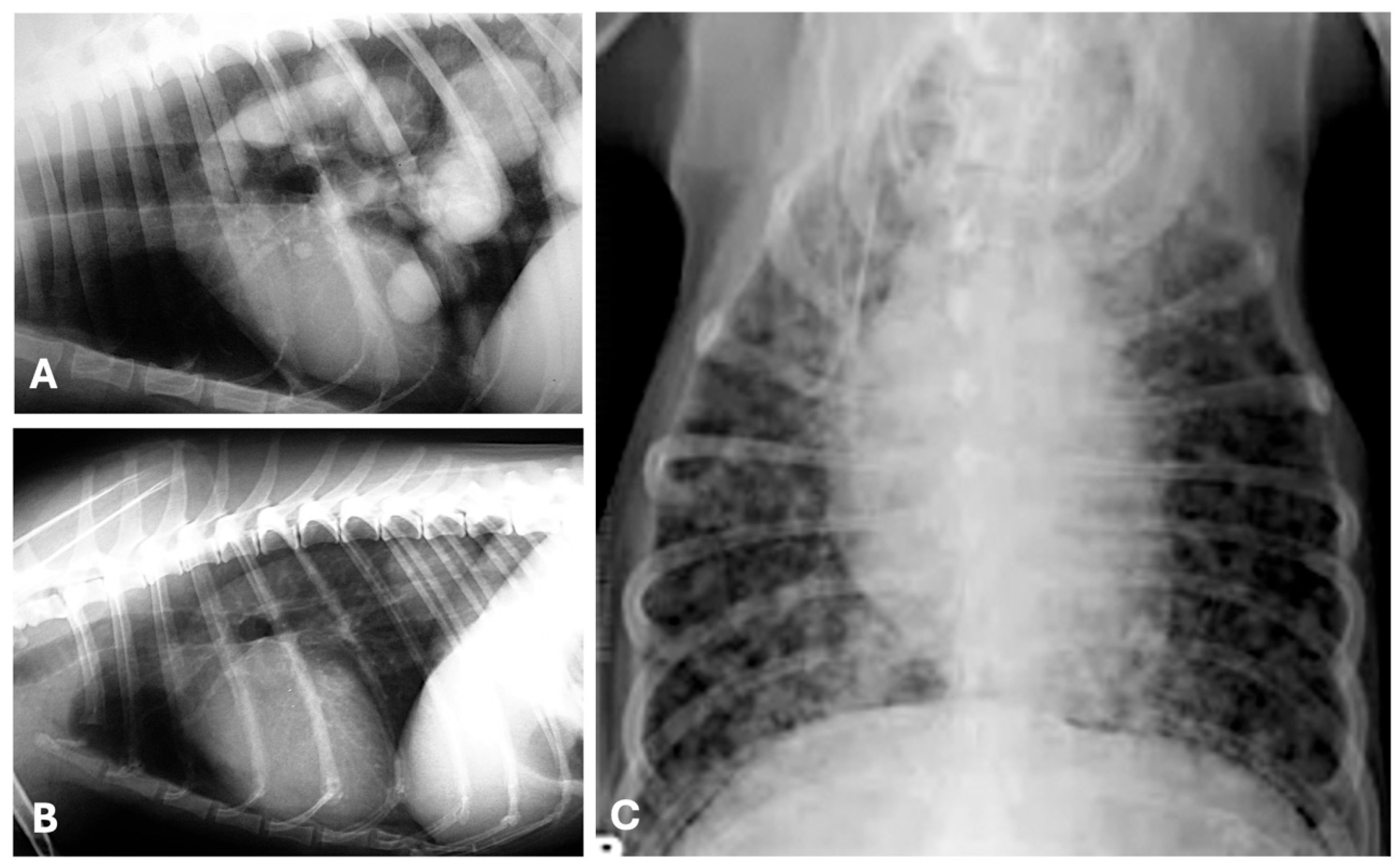
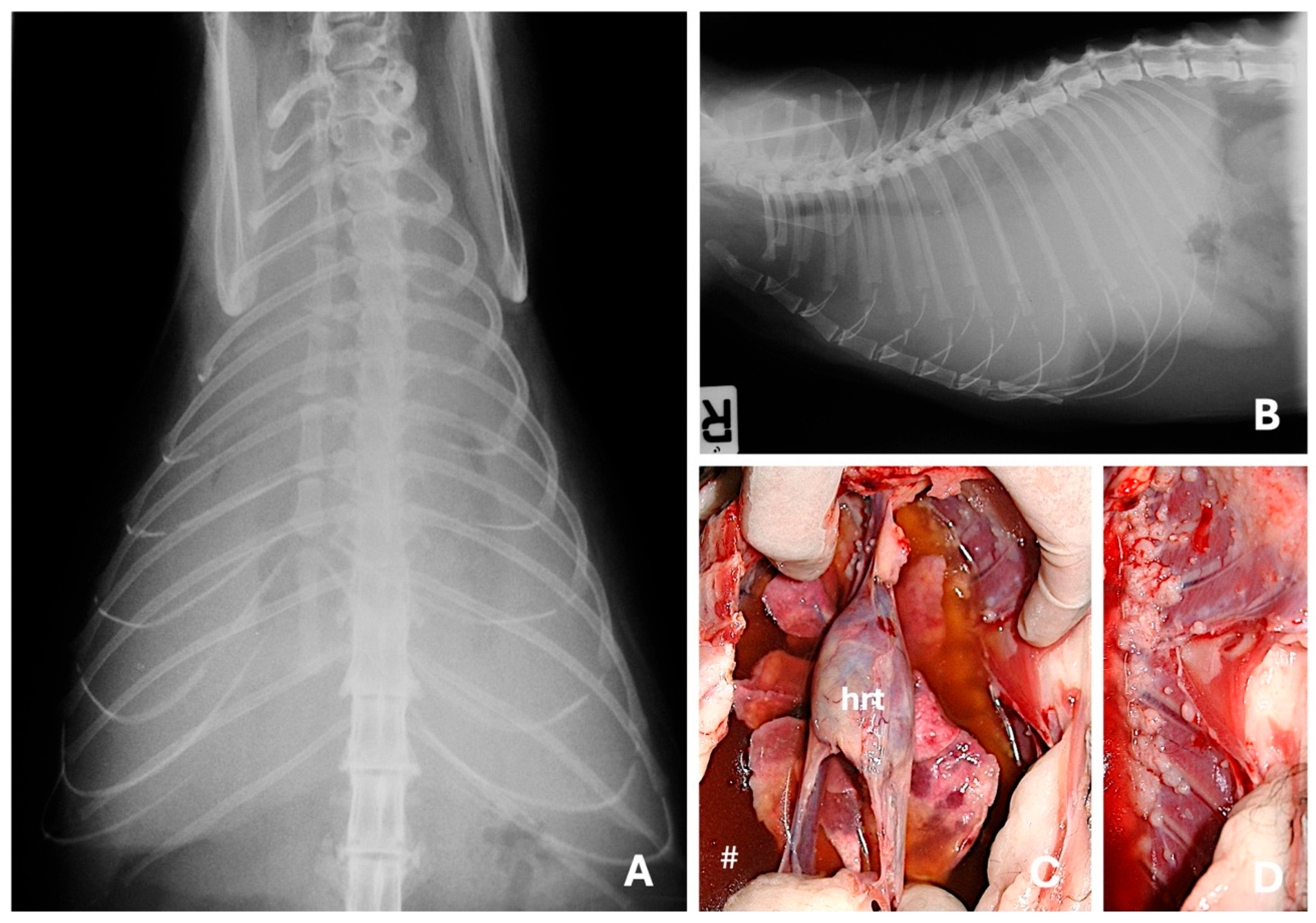
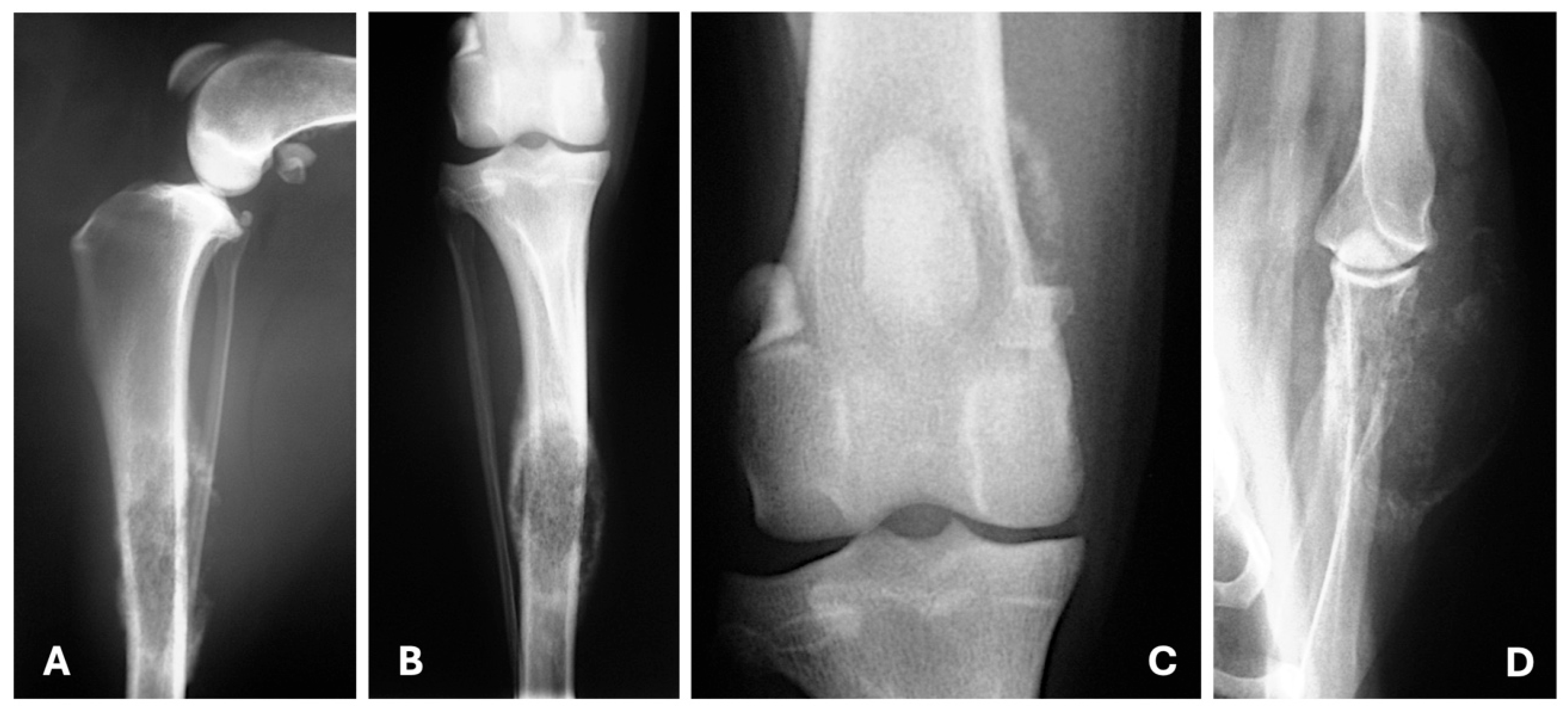
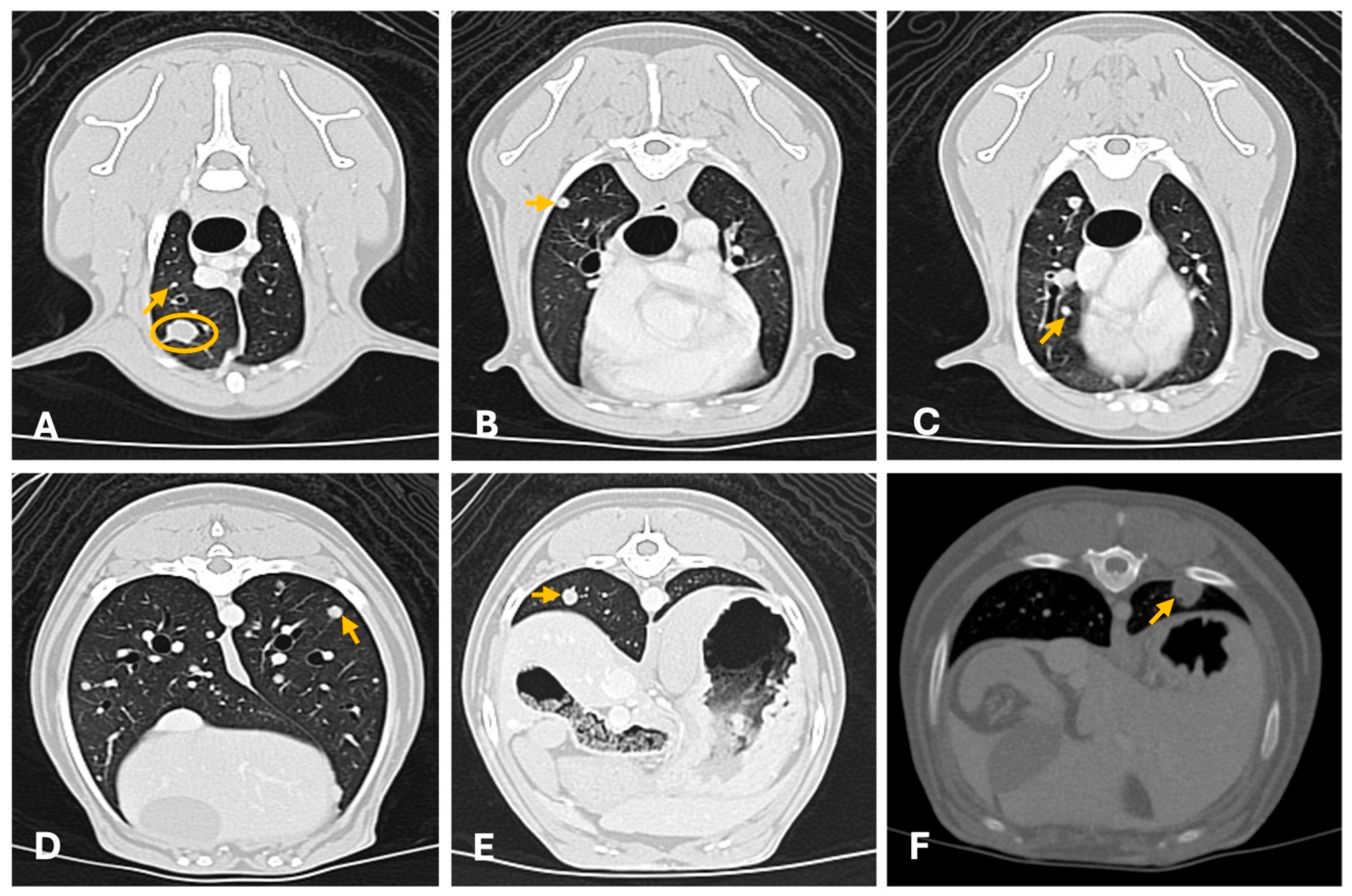
| Imaging Technique | Advantages | Disadvantages | Primary Mass | Metastases |
|---|---|---|---|---|
| RX | Quick, highly available, low cost [37] | Low sensitivity, superimposition of structures, low differentiation of soft tissues [37] | No [72] | 1st line (pulmonary metastases) [30] |
| CT | Removal of superimposition of structures, high sensitivity, contrast resolution and spatial resolution [34] | Low soft tissue contrast, cost, low availability, need for anesthesia [34] | Yes [72] | 2nd line (pulmonary, lymphatic and abdominal metastases) [31,73] |
| B-mode US | Quick, highly available, low cost, safe [37] | Requires proper education of the operator [37] | Yes (size, location, echogenicity) [74] | 1st line (abdominal metastases) [37] |
| Doppler US | Quick, highly available, low cost, safe [37] | Inability to detect small lesions or microcalcifications [75] | Yes (vascularization) [74] | No [37] |
| Elastography | High sensitivity and specificity | Requires specific software and specialized operator | Yes (elasticity) | No [37] |
| CEUS | Progression of vessel formation [20] | Low sensitivity and specificity [20] | Yes [54] | Yes [54] |
| Nuclear medicine | Information through true functional imaging [34] | Little anatomical information, low sensitivity, cost, low availability, need for radioactive tracers [34] | Yes [27,61] | Yes [65] |
| MRI | Differentiation of soft tissues, high resolution [34] | Very high cost, low availability, need for anesthesia [34] | Yes [70] | Yes [71] |
| Benign MGT | Malignant MGT | Comments | |
|---|---|---|---|
| Size | Tend to be smaller [40,75,77] | Tend to be larger [75,77] | There is no consensual cut off |
| Margins/Contour | Usually well-defined, smooth, regular [40,75,76] | Usually ill-defined, irregular, spiculated [76,78,82] | Non-defined margins were not reliable in differentiating benign/malignant lesions [78] |
| Shape | Round to oval [76,78] | Irregular, microlobulated [78] | Malignant tumors often distort surrounding tissue |
| Local invasion | Tend to be isolated [40,76,78] | Tend to invade fascia, muscle, skin [76,82] | Best seen on MRI/CT |
| Echotexture Echogenicity (US) | Homogeneous, hypoechoic or isoechoic [40,75,78] | Heterogeneous, mixed echogenicity [75,76,78] | Heterogenicity due to edema, hemorrhage, calcification, cystic/necrotic areas [75] |
| Posterior acoustic features (US) | Shadowing or enhancement more likely [78,80] | Shadowing [40] or enhancement more likely [75,76,79] | Features not consensual [78,81] |
| Vascularity (Color Doppler US) | Limited angiogenesis [75,81]. Peripheral vascular pattern [74,81] | Increased neovascularization [40,87]. Mixed vascular pattern [74,81] | Several studies report no significant differences in vascular flow [75,81,88], but in the distribution pattern [81] |
| Elastography (strain, shear-wave, ARFI) | Low stiffness and greater deformability [44,83,87] | Lower tissue deformation [40]. Stiffness values exceeding 80–100 kPa [44,83,87] | Malignancy evidence denser stroma matrix and reduced elasticity [85] |
| CEUS | May have low specificity in differentiating benign/malignant tumors [40] | Shorter periods of contrast wash-in and peak enhancement times in complex carcinomas [40,77] | Could be a useful adjunct tool for assessing tumoral perfusion patterns |
| Contrast Enhancement (CT/MRI) | Mild, homogeneous [72] | Strong, heterogeneous enhancement [26] | Dynamic contrast-enhanced MRI may show rapid wash-in/wash-out in malignancy |
| FDG-PET/CT | Glucose uptake < 2 [27] | Glucose uptake > 2 evidenced 100% sensitivity [27] | 100% predictive value if lesion > 1.5 cm + glucose uptake > 2 [27] |
| Cysts | May occur [75] | Cystic degeneration [72] | Proportion of cystic lesions is similar in both [75] |
| Necrosis | Less common [1] | Common, central necrosis [72] | May helps differentiate high-grade malignant tumors [1] |
| Calcifications | Occasional | May be present, coarse or microcalcifications | Accurate detection by CT [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteves-Monteiro, M.; Santos, J.; Fontes-Sousa, A.P.; Baptista, C.S. Diagnostic Imaging Features of Mammary Gland Tumors in Dogs and Cats. Animals 2025, 15, 3506. https://doi.org/10.3390/ani15243506
Esteves-Monteiro M, Santos J, Fontes-Sousa AP, Baptista CS. Diagnostic Imaging Features of Mammary Gland Tumors in Dogs and Cats. Animals. 2025; 15(24):3506. https://doi.org/10.3390/ani15243506
Chicago/Turabian StyleEsteves-Monteiro, Marisa, Joana Santos, Ana Patrícia Fontes-Sousa, and Cláudia S. Baptista. 2025. "Diagnostic Imaging Features of Mammary Gland Tumors in Dogs and Cats" Animals 15, no. 24: 3506. https://doi.org/10.3390/ani15243506
APA StyleEsteves-Monteiro, M., Santos, J., Fontes-Sousa, A. P., & Baptista, C. S. (2025). Diagnostic Imaging Features of Mammary Gland Tumors in Dogs and Cats. Animals, 15(24), 3506. https://doi.org/10.3390/ani15243506








