Definition of Meat Quality Across Different Cattle Breeds
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
- (1)
- On-farm selection (live animal assessment). Before slaughter, distributors or procurement specialists evaluated live cattle based on:
- (i)
- Body Condition Score (BCS), with an Ideal BCS (3–4 on a 5-point scale) to ensure proper fat cover without excess waste;
- (ii)
- Conformation and muscularity, with animals having well-developed muscle mass, particularly in the loin, rump, and shoulder;
- (iii)
- Mobility and health, without signs of lameness, disease, or stress;
- (iv)
- Weight and age, with slaughter targets (500–700 kg live weight, 12–24 months old).
- (2)
- At the slaughterhouse (final selection), additional checks were made:
- (i)
- Fat cover evaluation to reject excessively lean or overly fat animals;
- (ii)
- Conformation grading to assess muscle development using the SEUROP grading system (only S, E, U, and R accepted).
- (3)
- Traceability Tags and RFID (Radio Frequency Identification) chips to ensure that animals meet sourcing standards (organic, antibiotic-free, etc.).
- (4)
- Genetic and feed data for feeding history and genetic markers for tenderness.
- (5)
- Post slaughter (meat selection), carcasses were first classified according to the SEUROP system for conformation and fat cover, and subsequently evaluated for the specific parameters proposed in this study—color, tenderness, and marbling—by trained assessors within the distributor’s quality program.
2.2. Colorimetric Analysis
2.3. Marbling
2.4. Lipid Extraction and Fatty Acid Analysis
2.5. Tenderness
2.6. Statistical Analysis
2.7. Labeling System
| L* | a* | b* | ||||
| Mean | se | Mean | se | Mean | se | |
| Angus | 32.33 b | 0.38 | 19.46 c | 0.17 | 17.54 c | 0.26 |
| Chianina | 34.79 b | 0.67 | 26.64 a | 0.38 | 23.49 b | 0.41 |
| Holstein | 33.78 b | 0.3 | 22.44 b | 0.48 | 21.00 bd | 0.46 |
| German Red Pied | 27.64 a | 0.41 | 19.71 c | 0.29 | 17.57 c | 0.33 |
| Piemontese | 26.39 a | 0.29 | 19.55 c | 0.18 | 15.59 a | 0.23 |
| Polish crossbreed | 31.89 b | 0.37 | 21.37 b | 0.27 | 18.92 cd | 0.29 |
| Tenderness (Compression) (Newton, N) | Tenderness (Shear Force) (Newton, N) | |||||
| Mean | se | Mean | se | |||
| Angus | 48.04 a | 2.94 | 15.42 b | 0.77 | ||
| Chianina | 66.18 b | 2.72 | 15.24 b | 0.82 | ||
| Holstein | 43.44 ac | 4.42 | 15.26 ab | 1.56 | ||
| German Red Pied | 53.35 acd | 2.24 | 12.98 b | 0.65 | ||
| Piemontese | 59.23 bc | 2.05 | 15.22 b | 0.62 | ||
| Polish crossbreed | 63.23 bd | 2.39 | 21.62 a | 1.12 | ||
| Marbling (% on the Total Surface of the Sample) | Total Lipids (% of the Total Sample Weight) | |||||
| Mean | se | Mean | se | |||
| Angus | 27.01 b | 1.52 | 5.35 bc | 0.50 | ||
| Chianina | 14.60 cd | 0.79 | 6.30 b | 0.37 | ||
| Holstein | 17.51 bc | 1.22 | 5.81 b | 0.58 | ||
| German Red Pied | 11.80 ad | 0.62 | 3.09 ac | 0.13 | ||
| Piemontese | 10.23 a | 0.37 | 2.63 a | 0.08 | ||
| Polish crossbreed | 18.83 b | 0.72 | 4.81 b | 0.31 | ||
3. Results
3.1. Colorimetric Analysis
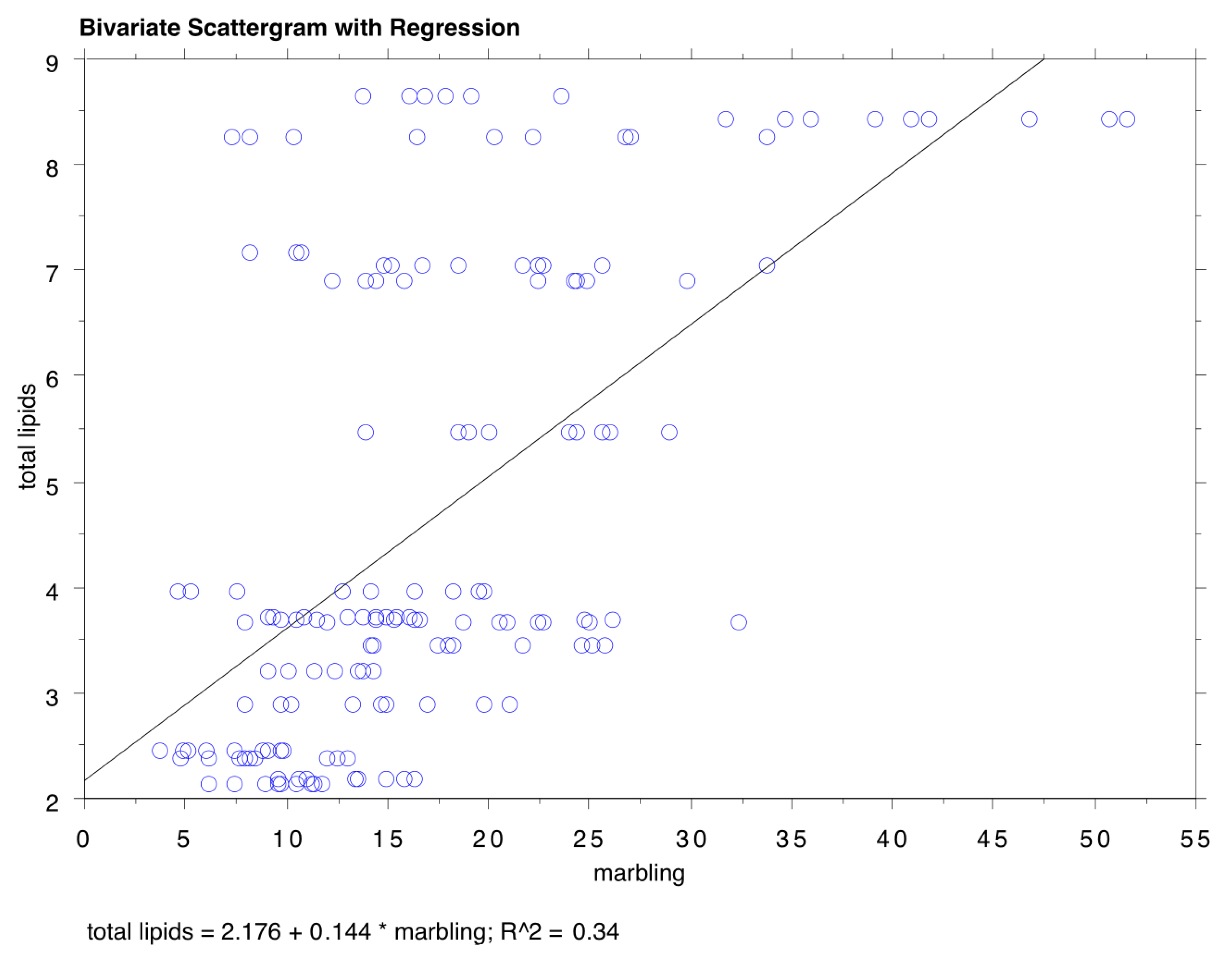
3.2. Marbling, Total Lipids, and Fatty Acid Profile
3.3. Tenderness
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, P. Recent developments in the objective measurement of carcass and meat quality for industrial application. Meat Sci. 2021, 181, 108601. [Google Scholar] [CrossRef]
- Mateescu, R.G.; Oltenacu, P.A.; Garmyn, A.J.; Mafi, G.G.; VanOverbeke, D.L. Strategies to predict and improve eating quality of cooked beef using carcass and meat composition traits in Angus cattle. J. Anim. Sci. 2016, 94, 2160–2171. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Borhan, M.S.; Young, J.; Newman, D.; Berg, E.; Sun, X. A Review on Meat Quality Evaluation Methods Based on Non-Destructive Computer Vision and Artificial Intelligence Technologies. Food Sci. Anim. Resour. 2021, 41, 563–588. [Google Scholar] [CrossRef] [PubMed]
- Hocquette, J.F.; Botreau, R.; Picard, B.; Jacquet, A.; Pethick, D.W.; Scollan, N.D. Opportunities for predicting and manipulating beef quality. Meat Sci. 2012, 92, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Raulet, M.; Clinquart, A.; Prache, S. Construction of beef quality through official quality signs, the example of Label Rouge. Animal 2022, 16 (Suppl. S1), 100357. [Google Scholar] [CrossRef] [PubMed]
- Bernués, A.; Olaizola, A.; Corcoran, K. Labelling information demanded by European consumers and relationships with purchasing motives, quality and safety of meat. Meat Sci. 2003, 65, 1095–1106. [Google Scholar] [CrossRef]
- Gellynck, X.; Verbeke, W.; Vermeire, B. Pathways to increase consumer trust in meat as a safe and wholesome food. Meat Sci. 2006, 74, 161–171. [Google Scholar] [CrossRef]
- Stranieri, S.; Banterle, A. Consumer Interest in Meat Labelled Attributes: Who Cares? Int. Food Agribus. Manag. Rev. 2015, 18, 21–38. [Google Scholar]
- USDA, United States Department of Agricolture. Requirements for Grading Terms on Meat Product Labeling; USDA, United States Department of Agricolture: Washington, DC, USA, 2018. [Google Scholar]
- Gagaoua, M.; Bonnet, M.; Ellies-Oury, M.-P.; De Koning, L.; Picard, B. Reverse phase protein arrays for the identification/validation of biomarkers of beef texture and their use for early classification of carcasses. Food Chem. 2018, 250, 245–252. [Google Scholar] [CrossRef]
- Grunert, K.G.; Bredahl, L.; Brunsø, K. Consumer perception of meat quality and implications for product development in the meat sector—A review. Meat Sci. 2004, 66, 259–272. [Google Scholar] [CrossRef]
- Pethick, D.; Hocquette, J.; Scollan, N.; Dunshea, F. Improving the nutritional, sensory and market value of meat products from sheep and cattle. Animal 2021, 15, 100356. [Google Scholar] [CrossRef]
- Ardeshiri, A.; Rose, J.M. How Australian consumers value intrinsic and extrinsic attributes of beef products. Food Qual. Prefer. 2018, 65, 146–163. [Google Scholar] [CrossRef]
- Emerson, M.R.; Woerner, D.R.; Belk, K.E.; Tatum, J.D. Effectiveness of USDA instrument-based marbling measurements for categorizing beef carcasses according to differences in longissimus muscle sensory attributes. J. Anim. Sci. 2013, 91, 1024–1034. [Google Scholar] [CrossRef]
- Smith, G.C.; Belk, K.E.; Sofos, J.N.; Tatum, J.D.; Williams, S.N. Economic implications of improved color stability in beef. In Antioxidants in Muscle Foods Nutritional Strategies to Improve Quality; John Wiley and Sons: Hoboken, NJ, USA, 2000; pp. 397–426. [Google Scholar]
- Xie, X.; Meng, Q.; Cui, Z.; Ren, L. Effect of cattle breed on meat quality, muscle fiber characteristics, lipid oxidation and Fatty acids in china. Asian-Australas. J. Anim. Sci. 2012, 25, 824–831. [Google Scholar] [CrossRef]
- Corbin, C.H.; O’Quinn, T.G.; Garmyn, A.J.; Legako, J.F.; Hunt, M.R.; Dinh, T.T.N.; Rathmann, R.J.; Brooks, J.C.; Miller, M.F. Sensory evaluation of tender beef strip loin steaks of varying marbling levels and quality treatments. Meat Sci. 2015, 100, 24–31. [Google Scholar] [CrossRef]
- Hunt, M.R.; Garmyn, A.J.; O’Quinn, T.G.; Corbin, C.H.; Legako, J.F.; Rathmann, R.J.; Brooks, J.C.; Miller, M.F. Consumer assessment of beef palatability from four beef muscles from USDA Choice and Select graded carcasses. Meat Sci. 2014, 98, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; DelCurto-Wyffels, H.; Thomson, J.; Boles, J. Fat Deposition and Fat Effects on Meat Quality-A Review. Animals 2022, 12, 1550. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.; Miller, R.; Ha, M.; Wheeler, T.L.; Dunshea, F.; Li, X.; Vaskoska, R.; Purslow, P. Meat Tenderness: Underlying Mechanisms, Instrumental Measurement, and Sensory Assessment. Meat Muscle Biol. 2021, 4, 1–25. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Banks, C.E. Electroanalytical overview: The pungency of chile and chilli products determined via the sensing of capsaicinoids. Analyst 2021, 146, 2769–2783. [Google Scholar] [CrossRef]
- McClure, A.P.; Hopfer, H.; Grün, I.U. Optimizing consumer acceptability of 100% chocolate through roasting treatments and effects on bitterness and other important sensory characteristics. Curr. Res. Food Sci. 2022, 5, 167–174. [Google Scholar] [CrossRef]
- Zheng, Y.-M.; Gao, H.-Y.; Wang, H.-Y.; Zhu, B.-q.; Shi, B.-L.; Zhong, K.; Sun, P.; Zhang, L.-L.; Zhao, L. The relationship between Scoville Units and the suprathreshold intensity of sweeteners and Sichuan pepper oleoresins. J. Sens. Stud. 2021, 36, e12699. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Jiang, S.; Zhang, Y.; Zhang, L.; Liu, Y. Multi-dimensional pungency and sensory profiles of powder and oil of seven chili peppers based on descriptive analysis and Scoville heat units. Food Chem. 2023, 411, 135488. [Google Scholar] [CrossRef]
- Vukasinovic, A.; Fiorani, F.; Costanzi, E.; Cataldi, S.; Pieroni, L.; Sorci, G.; Cenci-Goga, B.T.; Albi, E. Evaluation of quality and biochemical properties of Bovine meat from different rearing systems. Ital. J. Food Sci. 2025, 37, 120–128. [Google Scholar] [CrossRef]
- Pinna, N.; Ianni, F.; Codini, M.; Cenci-Goga, B.T.; Misuraca, M.; Costanzi, E.; Cossignani, L.; Blasi, F. Development of Value-Added Chicken Burgers by Adding Pumpkin Peel Powder as a Sustainable Ingredient. Antioxidants 2025, 14, 648. [Google Scholar] [CrossRef] [PubMed]
- Pinna, N.; Ianni, F.; Selvaggini, R.; Urbani, S.; Codini, M.; Grispoldi, L.; Cenci-Goga, B.T.; Cossignani, L.; Blasi, F. Valorization of Pumpkin Byproducts: Antioxidant Activity and Carotenoid Characterization of Extracts from Peel and Filaments. Foods 2023, 12, 4035. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Cheng, J.-H.; Sun, D.-W.; Pu, H. Marbling Analysis for Evaluating Meat Quality: Methods and Techniques. Compr. Rev. Food Sci. Food Saf. 2015, 14, 523–535. [Google Scholar] [CrossRef]
- Lee, B.; Yoon, S.; Choi, Y.M. Comparison of marbling fleck characteristics between beef marbling grades and its effect on sensory quality characteristics in high-marbled Hanwoo steer. Meat Sci. 2019, 152, 109–115. [Google Scholar] [CrossRef]
- Lee, S.; Lohumi, S.; Lim, H.; Gotoh, T.; Cho, B.-K.; Jung, S. Determination of Intramuscular Fat Content in Beef using Magnetic Resonance Imaging. J. Fac. Agric. Kyushu Univ. 2015, 60, 157–162. [Google Scholar] [CrossRef]
- D’Arco, G.; Blasi, F.; Cossignani, L.; Di Giacomo, F.; Ciavardelli, D.; Ventura, F.; Scipioni, S.; Simonetti, M.S.; Damiani, P. Composition of meat and offal from weaned and fattened rabbits and results of stereospecific analysis of triacylglycerols and phosphatidylcholines. J. Sci. Food Agric. 2012, 92, 952–959. [Google Scholar] [CrossRef]
- Maurelli, S.; Blasi, F.; Cossignani, L.; Bosi, A.; Simonetti, M.S.; Damiani, P. Enzymatic Synthesis of Structured Triacylglycerols Containing CLA Isomers Starting from sn-1,3-Diacylglycerols. J. Am. Oil Chem. Soc. 2009, 86, 127–133. [Google Scholar] [CrossRef]
- Liu, J.; Ellies-Oury, M.P.; Stoyanchev, T.; Hocquette, J.F. Consumer Perception of Beef Quality and How to Control, Improve and Predict It? Focus on Eating Quality. Foods 2022, 11, 1732. [Google Scholar] [CrossRef]
- Piccinetti, C.C.; Ricci, L.A.; Tokle, N.; Radaelli, G.; Pascoli, F.; Cossignani, L.; Palermo, F.; Mosconi, G.; Nozzi, V.; Raccanello, F.; et al. Malnutrition may affect common sole (Solea solea L.) growth, pigmentation and stress response: Molecular, biochemical and histological implications. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2012, 161, 361–371. [Google Scholar] [CrossRef] [PubMed]
- de Huidobro, F.R.; Miguel, E.; Blázquez, B.; Onega, E. A comparison between two methods (Warner–Bratzler and texture profile analysis) for testing either raw meat or cooked meat. Meat Sci. 2005, 69, 527–536. [Google Scholar] [CrossRef]
- Bonny, S.; Polkinghorne, R.; Strydom, P.; Matthews, K.; López-Campos, Ó.; Nishimura, T.; Scollan, N.; Pethick, D.; Hocquette, J.F. Chapter 10—Quality Assurance Schemes in Major Beef-Producing Countries. In New Aspects of Meat Quality; Purslow, P.P., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 223–255. [Google Scholar]
- Motoyama, M.; Sasaki, K.; Watanabe, A. Wagyu and the factors contributing to its beef quality: A Japanese industry overview. Meat Sci. 2016, 120, 10–18. [Google Scholar] [CrossRef]
- Sakowski, T.; Grodkowski, G.; Gołebiewski, M.; Slósarz, J.; Kostusiak, P.; Solarczyk, P.; Puppel, K. Genetic and Environmental Determinants of Beef Quality—A Review. Front. Vet. Sci. 2022, 9, 819605. [Google Scholar] [CrossRef]
- Papanikolopoulou, V.; Tsitsos, A.; Dokou, S.; Priskas, S.; Vouraki, S.; Economou, V.; Stylianaki, I.; Argyriadou, A.; Arsenos, G. Impact of Breed and Slaughter Hygiene on Beef Carcass Quality Traits in Northern Greece. Foods 2025, 14, 1776. [Google Scholar] [CrossRef]
- Tan, Z.; Jiang, H. Molecular and Cellular Mechanisms of Intramuscular Fat Development and Growth in Cattle. Int. J. Mol. Sci. 2024, 25, 2520. [Google Scholar] [CrossRef]
- Kostusiak, P.; Slósarz, J.; Gołębiewski, M.; Sakowski, T.; Puppel, K. Relationship between Beef Quality and Bull Breed. Animals 2023, 13, 2603. [Google Scholar] [CrossRef]
- Park, S.J.; Beak, S.H.; Jung, D.J.S.; Kim, S.Y.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—A review. Asian-Australas. J. Anim. Sci. 2018, 31, 1043–1061. [Google Scholar] [CrossRef] [PubMed]
- Tyra, M.; Ropka-Molik, K.; Terman, A.; Piórkowska, K.; Oczkowicz, M.; Bereta, A. Association between subcutaneous and intramuscular fat content in porcine ham and loin depending on age, breed and FABP3 and LEPR genes transcript abundance. Mol. Biol. Rep. 2013, 40, 2301–2308. [Google Scholar] [CrossRef] [PubMed]
- Ramani, A.; Chamadia, B.; Purohit, P. Comparative analysis of fatty acid profiles in indigenous cattle breeds: A comprehensive evaluation. Int. J. Adv. Biochem. Res. 2024, 8, 378–381. [Google Scholar] [CrossRef]
- Liu, T.; Wu, J.P.; Lei, Z.M.; Zhang, M.; Gong, X.Y.; Cheng, S.R.; Liang, Y.; Wang, J.F. Fatty Acid Profile of Muscles from Crossbred Angus-Simmental, Wagyu-Simmental, and Chinese Simmental Cattles. Food Sci. Anim. Resour. 2020, 40, 563–577. [Google Scholar] [CrossRef]
- Pannier, L.; van de Weijer, T.M.; van der Steen, F.T.H.J.; Kranenbarg, R.; Gardner, G.E. Prediction of chemical intramuscular fat and visual marbling scores with a conveyor vision scanner system on beef portion steaks. Meat Sci. 2023, 199, 109141. [Google Scholar] [CrossRef] [PubMed]
- Benli, H.; Yildiz, D.G. Consumer perception of marbling and beef quality during purchase and consumer preferences for degree of doneness. Anim. Biosci. 2023, 36, 1274–1284. [Google Scholar] [CrossRef]
- Killinger, K.M.; Calkins, C.R.; Umberger, W.J.; Feuz, D.M.; Eskridge, K.M. Consumer sensory acceptance and value for beef steaks of similar tenderness, but differing in marbling level1. J. Anim. Sci. 2004, 82, 3294–3301. [Google Scholar] [CrossRef] [PubMed]
- Konarska, M.; Kuchida, K.; Tarr, G.; Polkinghorne, R.J. Relationships between marbling measures across principal muscles. Meat Sci. 2017, 123, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Warren, H.E.; Scollan, N.D.; Enser, M.; Hughes, S.I.; Richardson, R.I.; Wood, J.D. Effects of breed and a concentrate or grass silage diet on beef quality in cattle of 3 ages. I: Animal performance, carcass quality and muscle fatty acid composition. Meat Sci. 2008, 78, 256–269. [Google Scholar] [CrossRef]
- Brugiapaglia, A.; Lussiana, C.; Destefanis, G. Fatty acid profile and cholesterol content of beef at retail of Piemontese, Limousin and Friesian breeds. Meat Sci. 2014, 96, 568–573. [Google Scholar] [CrossRef]
- Choe, J.H.; Choi, M.H.; Rhee, M.S.; Kim, B.C. Estimation of Sensory Pork Loin Tenderness Using Warner-Bratzler Shear Force and Texture Profile Analysis Measurements. Asian-Australas. J. Anim. Sci. 2016, 29, 1029–1036. [Google Scholar] [CrossRef]
- Herrera, N.J.; Wilkerson, E.K.; Domenech-Perez Perez, K.I.; Ribeiro, F.A.; Chao, M.D. The Relationship between Marbling, Superoxide Dismutase, and Beef TendernessBeef Tenderness. In Nebraska Beef Cattle Reports; University of Nebraska Extension MP105: Lincoln, NE, USA, 2018. [Google Scholar]
- Conter, M. Recent advancements in meat traceability, authenticity verification, and voluntary certification systems. Ital. J. Food Saf. 2024, 14, 12971. [Google Scholar] [CrossRef]
- Špehar, M.; Vincek, D.; Žgur, S. Beef quality: Factors affecting tenderness and marbling. Stočarstvo 2008, 62, 463–478. [Google Scholar]
- Warner, R.D.; Wheeler, T.L.; Ha, M.; Li, X.; Bekhit, A.E.-D.; Morton, J.; Vaskoska, R.; Dunshea, F.R.; Liu, R.; Purslow, P.; et al. Meat tenderness: Advances in biology, biochemistry, molecular mechanisms and new technologies. Meat Sci. 2022, 185, 108657. [Google Scholar] [CrossRef]
- Silva, D.R.G.; de Moura, A.P.R.; Ramos, A.L.S.; Ramos, E.M. Comparison of Warner-Bratzler shear force values between round and square cross-section cores for assessment of beef Longissimus tenderness. Meat Sci. 2017, 125, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Marrone, R.; Salzano, A.; Di Francia, A.; Vollano, L.; Di Matteo, R.; Balestrieri, A.; Anastasio, A.; Barone, C.M.A. Effects of Feeding and Maturation System on Qualitative Characteristics of Buffalo Meat (Bubalus bubalis). Animals 2020, 10, 899. [Google Scholar] [CrossRef]
- Smaldone, G.; Marrone, R.; Vollano, L.; Peruzy, M.F.; Barone, C.M.A.; Ambrosio, R.L.; Anastasio, A. Microbiological, rheological and physical-chemical characteristics of bovine meat subjected to a prolonged ageing period. Ital. J. Food Saf. 2019, 8, 8100. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S.; Zhu, L.G.; Bidner, B.; Meisinger, D.J.; McKeith, F.K. Measuring pork color: Effects of bloom time, muscle, pH and relationship to instrumental parameters. Meat Sci. 2001, 57, 169–176. [Google Scholar] [CrossRef]
- Corlett, M.T.; Pethick, D.W.; Kelman, K.R.; Jacob, R.H.; Gardner, G.E. Consumer Perceptions of Meat Redness Were Strongly Influenced by Storage and Display Times. Foods 2021, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Renerre, M. Biochemical Basis of Fresh Meat Color: Review. In Proceedings of the 45th International Congress of Meat Science and Technology, Yokohama, Japan, 1–6 August 1999; pp. 344–352. [Google Scholar]
- Cenci-Goga, B.T.; Iulietto, M.F.; Sechi, P.; Borgogni, E.; Karama, M.; Grispoldi, L. New Trends in Meat Packaging. Microbiol. Res. 2020, 11, 56–67. [Google Scholar] [CrossRef]
- O’Brien, K.D.; Baker, C.N.; Bush, S.A.; Wolf, K.J. The Meat of the Matter: The Effect of Science-based Information on Consumer Perception of Grass-fed Beef. J. Appl. Commun. 2003, 107, 10. [Google Scholar] [CrossRef]
| From Slaughter to Cutting/Packaging | From Cutting/ Packaging to Arrival at the Lab | From Arrival to Analysis | From Packaging to Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | se | Mean | se | Mean | se | Mean | se | |
| Angus (n = 7 shipments) | 4.29 | 0.64 | 26.71 | 16.64 | 9 | 1 | 35.71 | 16.22 |
| Chianina (n = 7 shipments) | 5 | 0.44 | 0 | 0 | 9 | 0 | 9 | 0.00 |
| German Red Pied (n = 7 shipments) | 3.86 | 0.63 | 3.43 | 0.53 | 7.71 | 0.18 | 11.14 | 0.51 |
| Piemontese (n = 13 shipments) | 3.77 | 0.23 | 3.08 | 0.4 | 6.92 | 0.51 | 10 | 0.32 |
| Polish crossbreed (n = 7 shipments) | 3.71 | 0.47 | 0.57 | 0.2 | 12 | 0 | 12.57 | 0.27 |
| Holstein (n = 2 shipments) | 4 | 0 | 1 | 0 | 7.5 | 3.5 | 8.5 | 3.5 |
| Angus | Chianina | German Red Pied | Piemontese | Polish Crossbreed | Holstein | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | se | Mean | se | Mean | se | Mean | se | Mean | se | Mean | se | |
| C14:0 | 1.81 | 0.08 | 2.11 | 0.09 | 1.27 | 0.03 | 1.91 | 0.10 | 2.17 | 0.03 | 2.64 | 0.07 |
| C14:1 | 0.37 | 0.05 | 0.45 | 0.01 | 0.25 | 0.01 | 0.49 | 0.02 | 0.33 | 0.01 | 0.36 | 0.00 |
| C15:0 | 0.44 | 0.04 | 0.7 | 0.03 | 0.44 | 0.02 | 0.43 | 0.03 | 0.33 | 0.02 | 0.45 | 0.00 |
| C15:1 | 0.17 | 0.01 | 0.26 | 0.02 | 0.16 | 0.00 | 0.17 | 0.01 | 0.15 | 0.01 | 0.16 | 0.00 |
| C16:0 | 24.3 | 0.24 | 24.7 | 0.90 | 22.7 | 0.29 | 25 | 0.54 | 25.4 | 0.43 | 25.9 | 0.10 |
| C16:1n-9 | 0.26 | 0.01 | 0.28 | 0.00 | 0.24 | 0.00 | 0.23 | 0.02 | 0.17 | 0.01 | 0.28 | 0.01 |
| C16:1n-7 | 3.21 | 0.14 | 2.96 | 0.08 | 3.09 | 0.02 | 4.29 | 0.10 | 3.13 | 0.03 | 2.82 | 0.07 |
| C17:0 | 0.97 | 0.04 | 1.26 | 0.05 | 0.93 | 0.03 | 0.75 | 0.03 | 0.69 | 0.06 | 0.79 | 0.01 |
| C17:1 | 1.11 ab | 0.03 | 1.19 ab | 0.05 | 1.62 b | 0.02 | 1.02 ab | 0.01 | 0.79 ab | 0.06 | 0.62 a | 0.02 |
| C18:0 | 15.8 | 0.44 | 18.2 | 0.37 | 14.4 | 0.21 | 12.8 | 0.22 | 12.5 | 0.30 | 16.6 | 0.14 |
| C18:1 trans | 1.19 | 0.14 | 0.96 | 0.10 | 0.83 | 0.04 | 0.73 | 0.03 | 0.71 | 0.04 | 1.15 | 0.07 |
| C18:1n-9 + n7 | 35.9 ab | 0.95 | 33.3 ab | 0.47 | 40 b | 0.56 | 33.5 ab | 0.83 | 30.5 ab | 0.34 | 27.7 a | 0.17 |
| C18:2n6 | 7.87 | 0.23 | 10.1 | 0.89 | 6.02 | 0.30 | 10.2 | 0.62 | 13.8 | 0.67 | 15.7 | 0.27 |
| C18:3n3 | 1.21 | 0.14 | 0.59 | 0.03 | 1.59 | 0.12 | 0.92 | 0.07 | 0.88 | 0.14 | 0.71 | 0.04 |
| 9c, 11t CLA | 0.28 | 0.01 | 0.24 | 0.03 | 0.22 | 0.00 | 0.21 | 0.01 | 0.15 | 0.00 | 0.34 | 0.03 |
| C20:1 | 0.23 | 0.01 | 0.23 | 0.03 | 0.22 | 0.01 | 0.18 | 0.01 | 0.13 | 0.00 | 0.12 | 0.01 |
| 20:3n6 | 0.86 | 0.03 | 0.61 | 0.01 | 0.89 | 0.07 | 1.14 | 0.09 | 1.64 | 0.18 | 0.55 | 0.02 |
| C20:4n6 | 2.62 | 0.20 | 1.57 | 0.13 | 2.43 | 0.25 | 3.73 | 0.46 | 5.56 | 0.56 | 2.56 | 0.05 |
| C20:5 n3 | 0.53 | 0.07 | 0.08 | 0.01 | 0.85 | 0.11 | 0.67 | 0.08 | 0.24 | 0.03 | 0.12 | 0.01 |
| C22:5n3 | 0.86 | 0.11 | 0.2 | 0.03 | 1.51 | 0.18 | 1.44 | 0.23 | 0.7 | 0.06 | 0.4 | 0.03 |
| C22:6n3 | 0.05 | 0.01 | 0.01 | 0.00 | 0.35 | 0.06 | 0.22 | 0.03 | 0.05 | 0.01 | 0.03 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cenci-Goga, B.T.; Costanzi, E.; Blasi, F.; Ianni, F.; Tassinari, M.; Truzzi, C.; Karama, M.; El-Ashram, S.; Saraiva, C.; Martínez-Barbitta, M.; et al. Definition of Meat Quality Across Different Cattle Breeds. Animals 2025, 15, 3467. https://doi.org/10.3390/ani15233467
Cenci-Goga BT, Costanzi E, Blasi F, Ianni F, Tassinari M, Truzzi C, Karama M, El-Ashram S, Saraiva C, Martínez-Barbitta M, et al. Definition of Meat Quality Across Different Cattle Breeds. Animals. 2025; 15(23):3467. https://doi.org/10.3390/ani15233467
Chicago/Turabian StyleCenci-Goga, Beniamino T., Egidia Costanzi, Francesca Blasi, Federica Ianni, Marco Tassinari, Claudio Truzzi, Musafiri Karama, Saeed El-Ashram, Cristina Saraiva, Marcelo Martínez-Barbitta, and et al. 2025. "Definition of Meat Quality Across Different Cattle Breeds" Animals 15, no. 23: 3467. https://doi.org/10.3390/ani15233467
APA StyleCenci-Goga, B. T., Costanzi, E., Blasi, F., Ianni, F., Tassinari, M., Truzzi, C., Karama, M., El-Ashram, S., Saraiva, C., Martínez-Barbitta, M., García-Díez, J., Zerani, M., Guelfi, G., Maranesi, M., Grispoldi, L., & Cossignani, L. (2025). Definition of Meat Quality Across Different Cattle Breeds. Animals, 15(23), 3467. https://doi.org/10.3390/ani15233467














