FABP3 Mediates Lipid Droplet Accumulation and Adhesive Capacity in Bovine Endometrial Epithelial Cells via PGE2/PTGER4/PPAR Axis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. Bulk RNA Sequencing (RNA-Seq)
2.3. Cell Transfection
2.4. Tissue Collection
2.5. Progesterone Determination
2.6. Immunohistochemistry
2.7. RNA Extraction and Real-Time Quantitative PCR
2.8. Western Blot
2.9. Measurement of Cell Viability
2.10. Cell Proliferation Assay
2.11. Immunofluorescent Staining
2.12. Scanning Electron Microscope
2.13. In Vitro Implantation Assay
2.14. BODIPY Staining
2.15. Statistical Analysis
3. Results
3.1. PGE2 Treatment Induces Lipid Droplet Accumulation and Alternation of Cell Morphology in Bovine Endometrial Epithelial Cells
3.2. The Number of Lipid Droplets Is Related to Cell Morphology in Bovine Endometrial Epithelial Cells
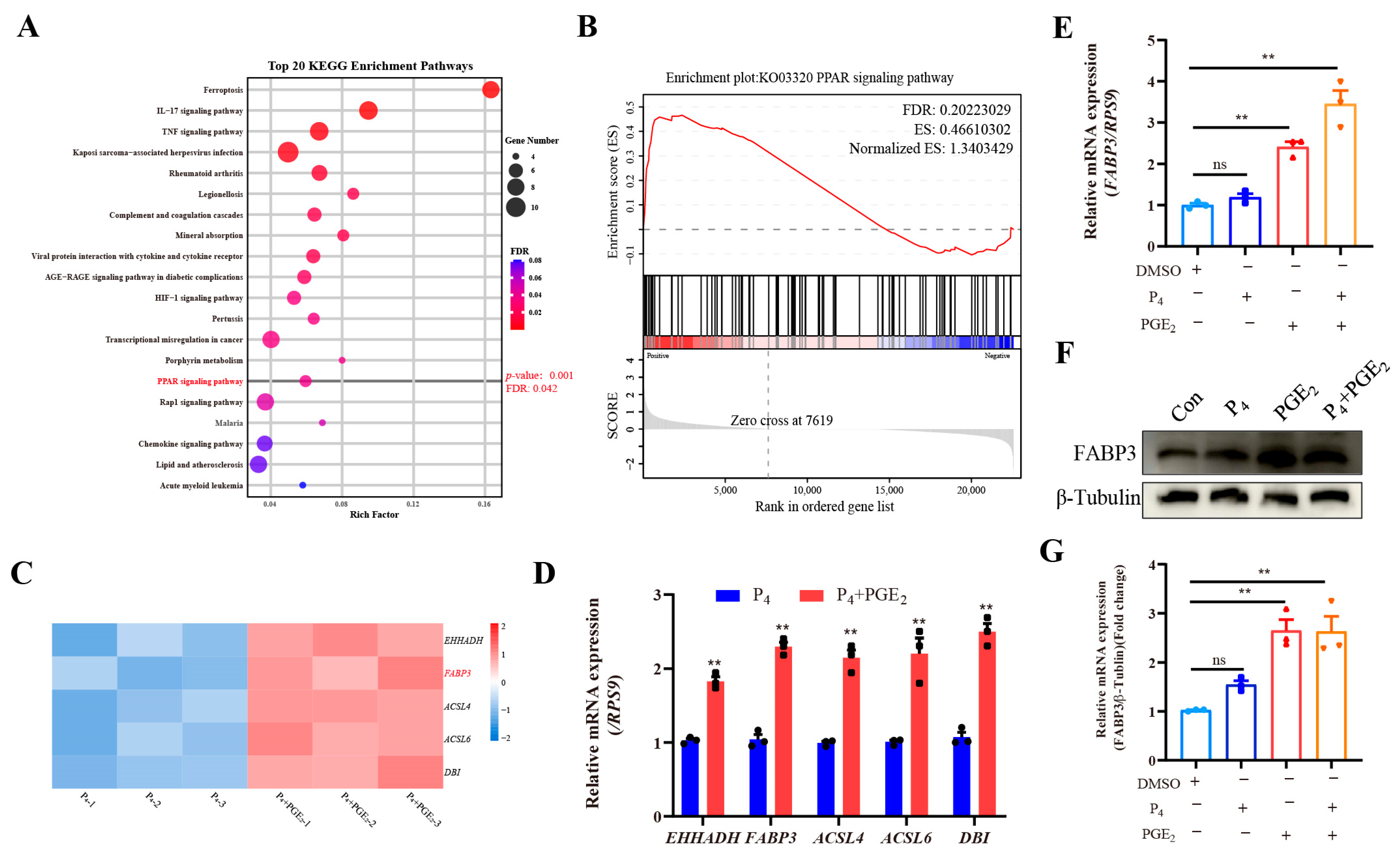
3.3. RNA-Seq Reveals PPAR Signaling Pathway and Its Key Molecules Respond to PGE2 Treatment in Bovine Endometrial Epithelial Cells
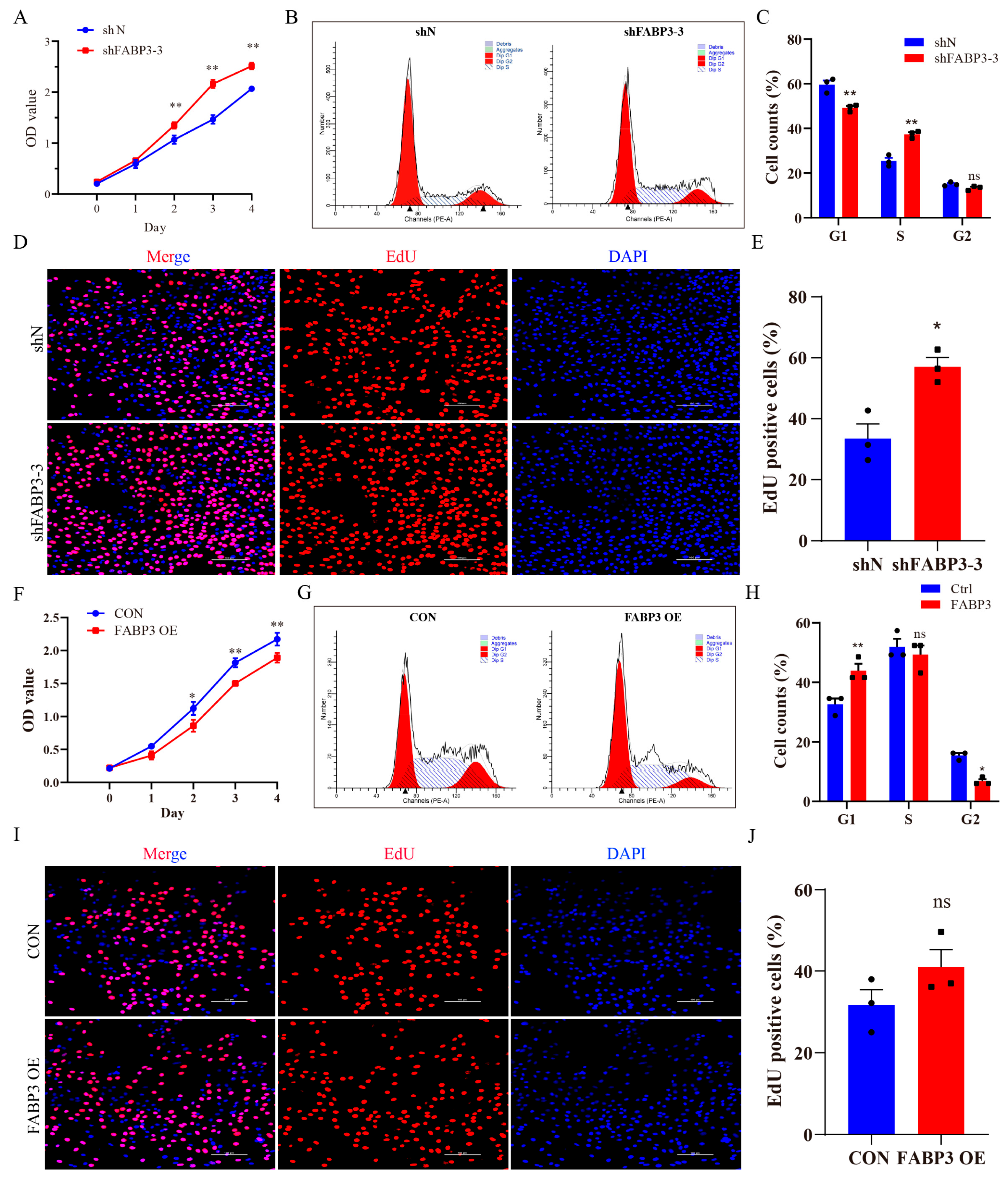
3.4. FABP3 Regulates the Proliferation of Bovine Endometrial Epithelial Cells
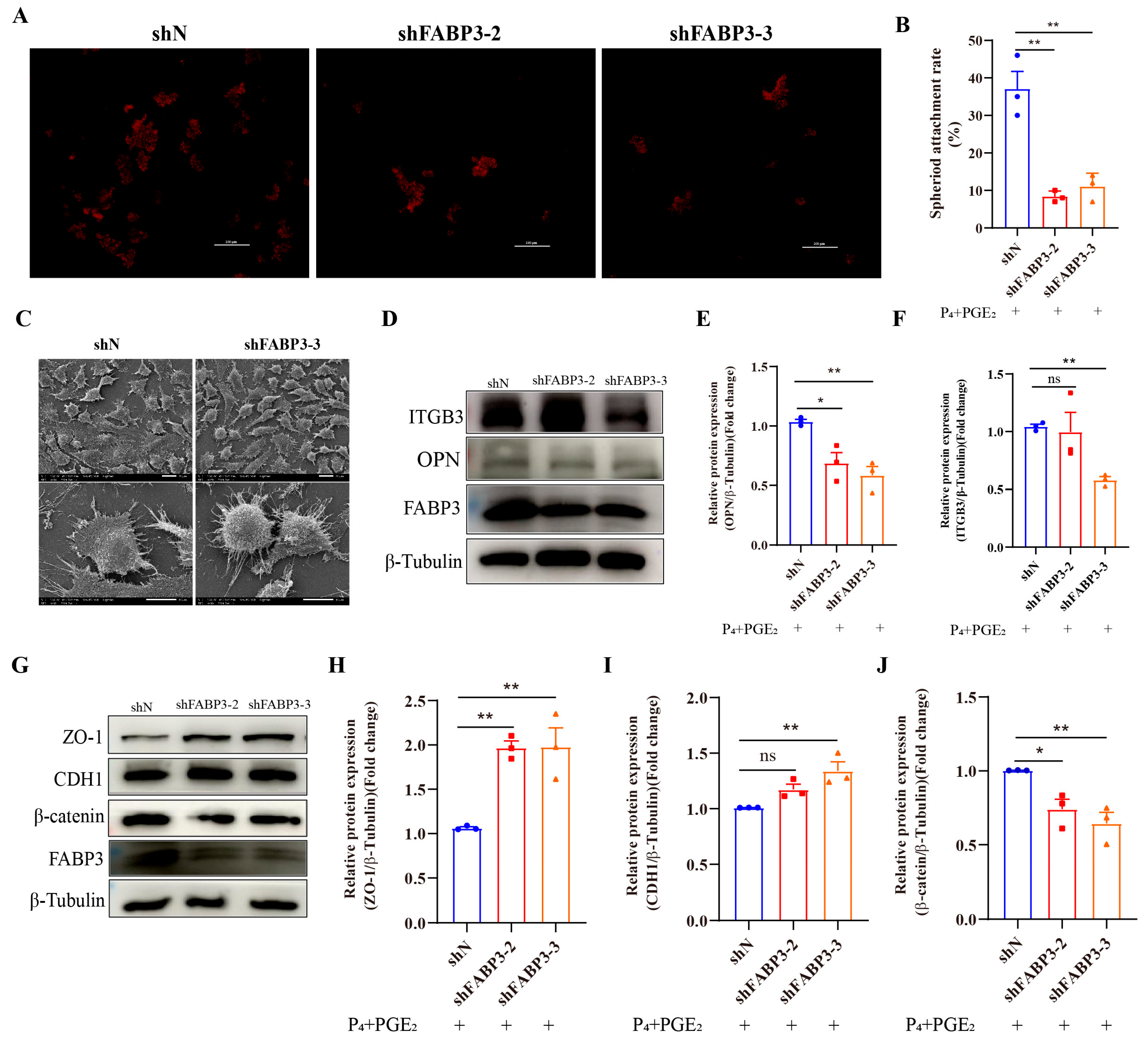
3.5. FABP3 Regulates the Adhesion Ability of Bovine Endometrial Epithelial Cells
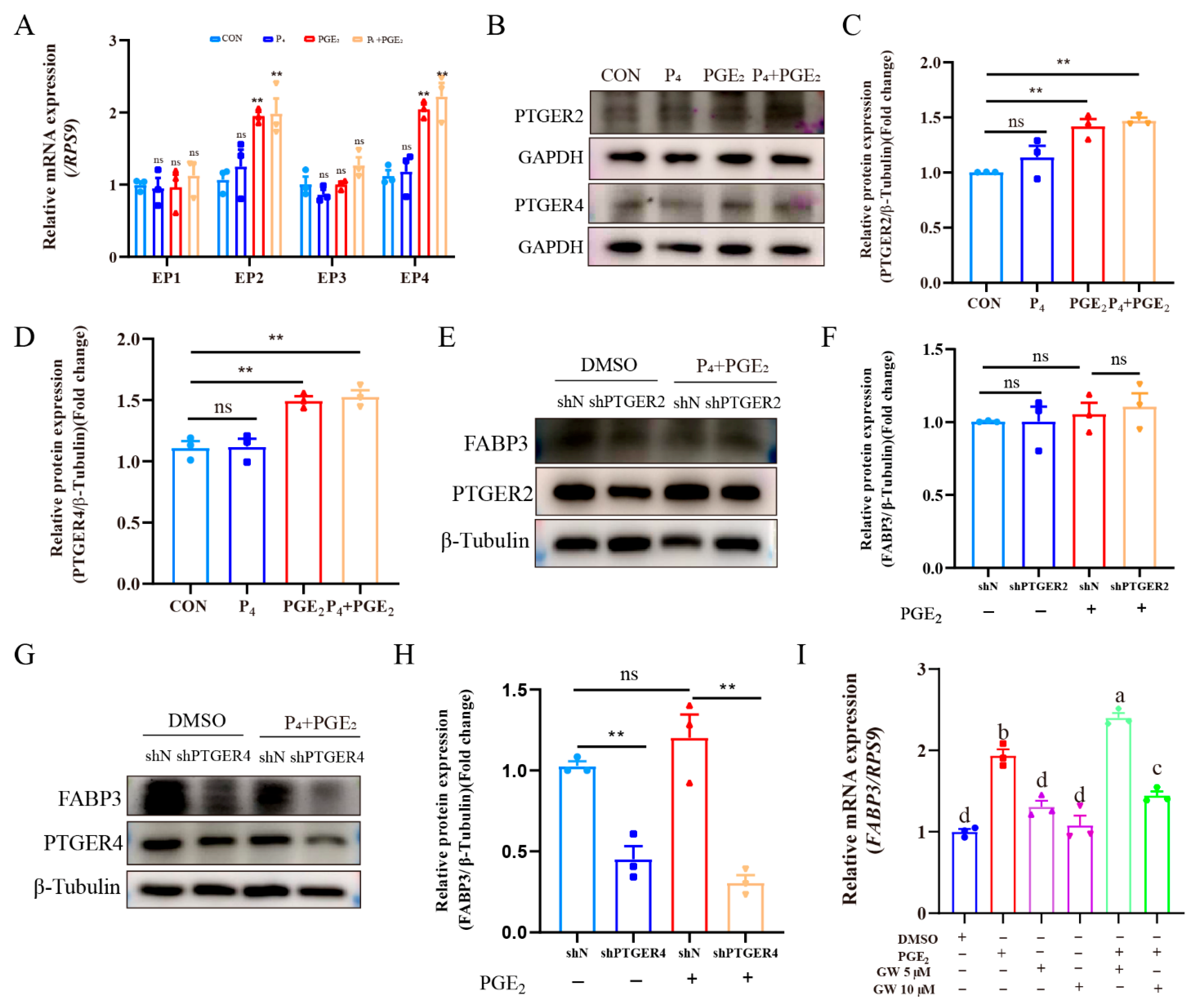
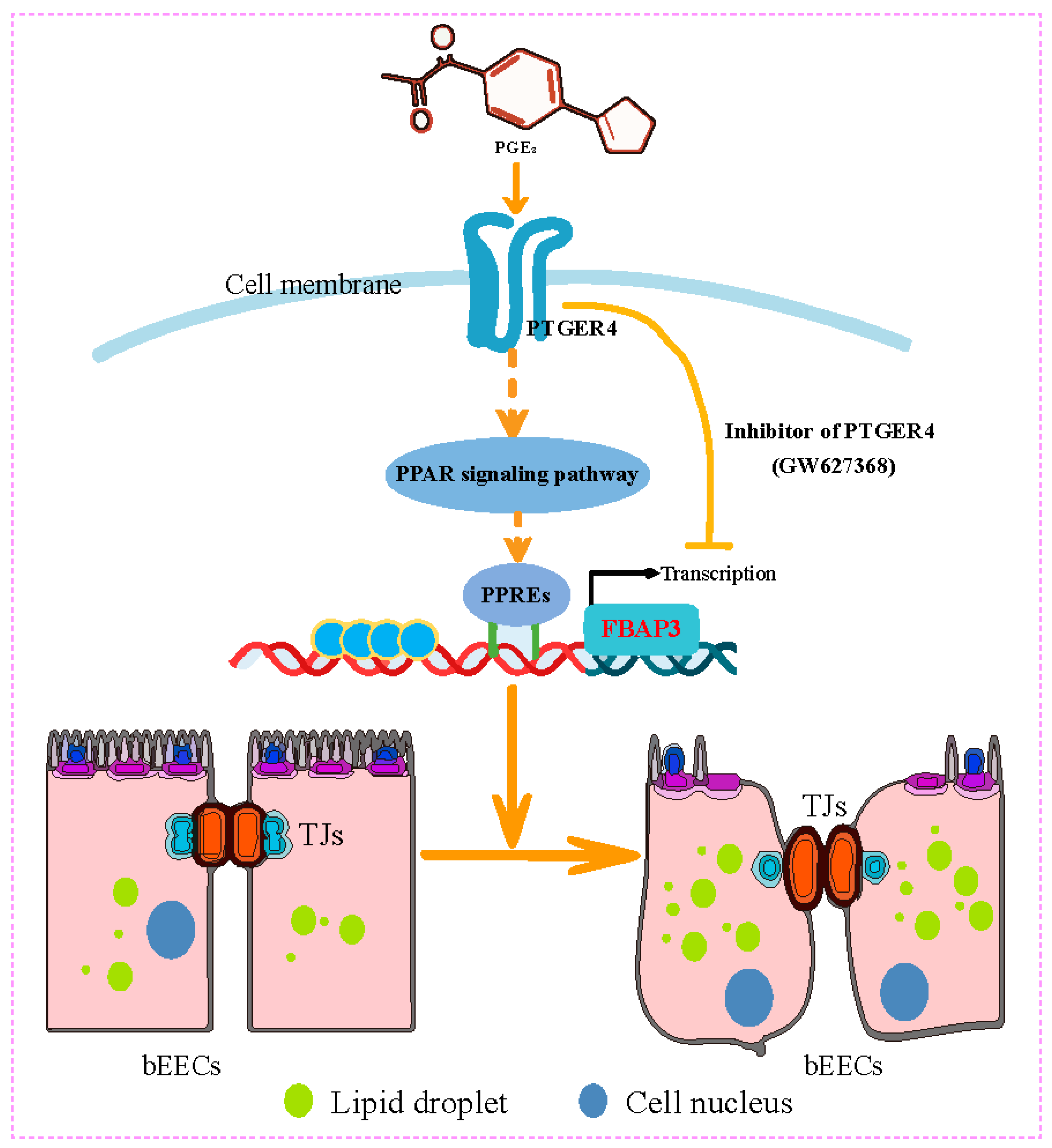
3.6. PGE2 Binds to PTGER4 to Regulate FABP3 Expression of Bovine Endometrial Epithelial Cells
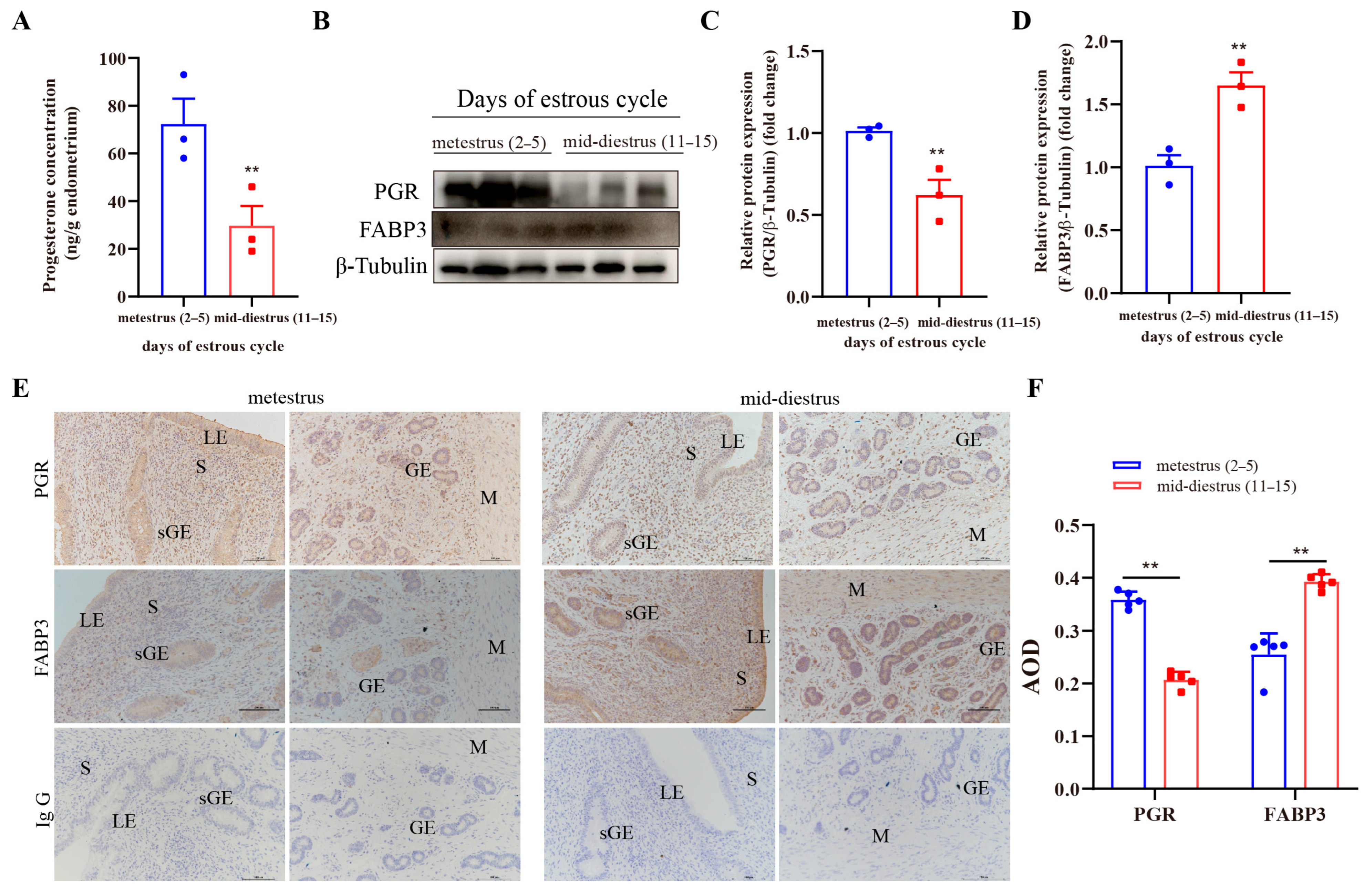
3.7. Immunolocalization and Expression of FABP3 in the Endometrial Tissue of Dairy Cows at Different Stages of Estrus Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moraes, J.G.N.; Behura, S.K.; Geary, T.W.; Hansen, P.J.; Neibergs, H.L.; Spencer, T.E. Uterine influences on conceptus development in fertility-classified animals. Proc. Natl. Acad. Sci. USA 2018, 115, E1749–E1758. [Google Scholar] [CrossRef]
- Ichikawa, R.; Kimura, K.; Nakamura, S.; Ohkura, S.; Matsuyama, S. Effects of intrauterine extracellular vesicle microRNAs on embryonic gene expression in low-fertility cows. FASEB J. 2024, 38, e70116. [Google Scholar] [CrossRef]
- Moraes, J.G.N.; Behura, S.K.; Geary, T.W.; Spencer, T.E. Analysis of the uterine lumen in fertility-classified heifers: I. Glucose, prostaglandins, and lipids. Biol. Reprod. 2020, 102, 456–474. [Google Scholar] [CrossRef]
- Juengel, J.L.; Mosaad, E.M.O.; Mitchell, M.D.; Phyn, C.V.C.; French, M.C.; Meenken, E.D.; Burke, C.R.; Meier, S. Relationships between prostaglandin concentrations, a single nucleotide polymorphism in HSD17B12, and reproductive performance in dairy cows. J. Dairy Sci. 2022, 105, 4643–4652. [Google Scholar] [CrossRef] [PubMed]
- Arosh, J.A.; Banu, S.K.; McCracken, J.A. Novel concepts on the role of prostaglandins on luteal maintenance and maternal recognition and establishment of pregnancy in ruminants. J. Dairy Sci. 2016, 99, 5926–5940. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, S.E.; Schulke, K.; Groebner, A.E.; Reichenbach, H.D.; Angioni, C.; Geisslinger, G.; Meyer, H.H. Quantitative characterization of prostaglandins in the uterus of early pregnant cattle. Reproduction 2009, 138, 371–382. [Google Scholar] [CrossRef]
- Arosh, J.A.; Banu, S.K.; Chapdelaine, P.; Emond, V.; Kim, J.J.; MacLaren, L.A.; Fortier, M.A. Molecular cloning and characterization of bovine prostaglandin E2 receptors EP2 and EP4: Expression and regulation in endometrium and myometrium during the estrous cycle and early pregnancy. Endocrinology 2003, 144, 3076–3091. [Google Scholar] [CrossRef] [PubMed]
- Dorniak, P.; Bazer, F.W.; Spencer, T.E. Prostaglandins regulate conceptus elongation and mediate effects of interferon tau on the ovine uterine endometrium. Biol. Reprod. 2011, 84, 1119–1127. [Google Scholar] [CrossRef]
- Dorniak, P.; Bazer, F.W.; Wu, G.; Spencer, T.E. Conceptus-derived prostaglandins regulate endometrial function in sheep. Biol. Reprod. 2012, 87, 1–7. [Google Scholar] [CrossRef]
- Brooks, K.; Burns, G.; Spencer, T.E. Conceptus elongation in ruminants: Roles of progesterone, prostaglandin, interferon tau and cortisol. J. Anim. Sci. Biotechnol. 2014, 5, 53. [Google Scholar] [CrossRef]
- Wu, W.X.; Coksaygan, T.; Chakrabarty, K.; Collins, V.; Rose, J.C.; Nathanielsz, P.W. Sufficient progesterone-priming prior to estradiol stimulation is required for optimal induction of the cervical prostaglandin system in pregnant sheep at 0.7 gestations. Biol. Reprod. 2005, 73, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Han, Y.; Cheng, M.; Yan, L.; Gao, K.; Zhou, D.; Wang, A.; Lin, P.; Jin, Y. Metabolomic effects of intrauterine meloxicam perfusion on histotroph in dairy heifers during diestrus. Front. Vet. Sci. 2025, 12, 1528530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Han, Y.; Wang, S.; Cheng, M.; Yan, L.; Zhou, D.; Wang, A.; Lin, P.; Jin, Y. The Impact of Uterus-Derived Prostaglandins on the Composition of Uterine Fluid During the Period of Conceptus Elongation in Dairy Heifers. Int. J. Mol. Sci. 2025, 26, 1792. [Google Scholar] [CrossRef] [PubMed]
- Simintiras, C.A.; Sánchez, J.M.; McDonald, M.; Lonergan, P. The biochemistry surrounding bovine conceptus elongation†. Biol. Reprod. 2019, 101, 328–337. [Google Scholar] [CrossRef]
- Simintiras, C.A.; Sánchez, J.M.; McDonald, M.; Lonergan, P. Progesterone alters the bovine uterine fluid lipidome during the period of elongation. Reproduction 2019, 157, 399–411. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Santos, J.E.; Thatcher, W.W. Role of lipids on elongation of the preimplantation conceptus in ruminants. Reproduction 2016, 152, R115–R126. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Wordinger, R.J.; Dickey, J.F.; Ellicott, A.R. Histochemical evaluation of the lipid droplet content of bovine oviductal and endometrial epithelial cells. J. Reprod. Fertil. 1977, 49, 113–114. [Google Scholar] [CrossRef]
- Brinsfield, T.H.; Hawk, H.W. Control by progesterone of the concentration of lipid droplets in epithelial cells of the sheep endometrium. J. Anim. Sci. 1973, 36, 919–922. [Google Scholar] [CrossRef]
- King, K.; Ticiani, E.; Sprícigo, J.F.W.; Carvalho, M.R.; Mion, B.; Bertolini, M.; Contreras, G.A.; Ribeiro, E.S. Dynamics of lipid droplets in the endometrium and fatty acids and oxylipins in the uterine lumen, blood, and milk of lactating cows during diestrus. J. Dairy Sci. 2021, 104, 3676–3692. [Google Scholar] [CrossRef]
- Michler, S.; Schöffmann, F.A.; Robaa, D.; Volmer, J.; Hinderberger, D. Fatty acid binding to the human transport proteins FABP3, FABP4, and FABP5 from a Ligand’s perspective. J. Biol. Chem. 2024, 300, 107396. [Google Scholar] [CrossRef]
- Bauersachs, S.; Ulbrich, S.E.; Reichenbach, H.D.; Reichenbach, M.; Büttner, M.; Meyer, H.H.; Spencer, T.E.; Minten, M.; Sax, G.; Winter, G.; et al. Comparison of the effects of early pregnancy with human interferon, alpha 2 (IFNA2), on gene expression in bovine endometrium. Biol. Reprod. 2012, 86, 46. [Google Scholar] [CrossRef]
- O’Keeffe, G.; Hammel, S.; Owens, R.A.; Keane, T.M.; Fitzpatrick, D.A.; Jones, G.W.; Doyle, S. RNA-seq reveals the pan-transcriptomic impact of attenuating the gliotoxin self-protection mechanism in Aspergillus fumigatus. BMC Genom. 2014, 15, 894. [Google Scholar] [CrossRef]
- Yi, C.; Liu, J.; Deng, W.; Luo, C.; Qi, J.; Chen, M.; Xu, H. Macrophage elastase (MMP12) critically contributes to the development of subretinal fibrosis. J. Neuroinflamm. 2022, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Lin, P.F.; Li, X.; Sun, J.; Zhang, Z.; Du, E.; Wang, A.; Jin, Y.P. Construction and expression of lentiviral vectors encoding recombinant mouse CREBZF in NIH 3T3 cells. Plasmid 2014, 76, 24–31. [Google Scholar] [CrossRef]
- Yang, D.; Jiang, T.; Lin, P.; Chen, H.; Wang, L.; Wang, N.; Zhao, F.; Wang, A.; Jin, Y. Knock-down of apoptosis inducing factor gene protects endoplasmic reticulum stress-mediated goat granulosa cell apoptosis. Theriogenology 2017, 88, 89–97. [Google Scholar] [CrossRef]
- Ireland, J.J.; Murphee, R.L.; Coulson, P.B. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J. Dairy Sci. 1980, 63, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Fields, M.J.; Fields, P.A. Morphological characteristics of the bovine corpus luteum during the estrous cycle and pregnancy. Theriogenology 1996, 45, 1295–1325. [Google Scholar] [CrossRef]
- Kowalik, M.K.; Dobrzyn, K.; Rekawiecki, R.; Kotwica, J. Expression of membrane progestin receptors (mPRs) α, β and γ in the bovine uterus during the oestrous cycle and pregnancy. Theriogenology 2019, 140, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Z.; Gao, K.; Fu, R.; Chen, H.; Lin, P.; Wang, A.; Jin, Y. MSX1 Regulates Goat Endometrial Function by Altering the Plasma Membrane Transformation of Endometrial Epithelium Cells during Early Pregnancy. Int. J. Mol. Sci. 2023, 24, 4121. [Google Scholar] [CrossRef]
- Gao, Z.; Shao, D.; Zhao, C.; Liu, H.; Zhao, X.; Wei, Q.; Ma, B. The High Level of RANKL Improves IκB/p65/Cyclin D1 Expression and Decreases p-Stat5 Expression in Firm Udder of Dairy Goats. Int. J. Mol. Sci. 2023, 24, 8841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, S.; Yang, L.; Gao, L.; Ning, C.; Xu, M.; Deng, S.; Gan, S. Profile of miRNAs induced during sheep fat tail development and roles of four key miRNAs in proliferation and differentiation of sheep preadipocytes. Front. Vet. Sci. 2024, 11, 1491160. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Mei, C.; Raza, S.H.A.; Cheng, G.; Ning, Y.; Zhang, L.; Zan, L. Arginine (315) is required for the PLIN2-CGI-58 interface and plays a functional role in regulating nascent LDs formation in bovine adipocytes. Genomics 2024, 116, 110817. [Google Scholar] [CrossRef]
- Sun, M.; Song, P.; Zhao, Y.; Li, B.; Wang, P.; Cong, Z.; Hua, S. Mechanisms of LPS-induced epithelial mesenchymal transition in bEECs. Theriogenology 2024, 216, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, R.; Liu, R.; Song, P.; Lin, P.; Chen, H.; Zhou, D.; Wang, A.; Jin, Y. YPEL3 Negatively Regulates Endometrial Function via the Wnt/β-Catenin Pathways during Early Pregnancy in Goats. Animals 2022, 12, 2973. [Google Scholar] [CrossRef]
- Maity, S.; Ambatipudi, K. Quantitative proteomics of milk whey reveals breed and season specific variation in protein abundance in Holstein Friesian cow and Murrah buffalo. Res. Vet. Sci. 2019, 125, 244–252. [Google Scholar] [CrossRef]
- Zhou, M.; Barkema, H.W.; Gao, J.; Yang, J.; Wang, Y.; Kastelic, J.P.; Khan, S.; Liu, G.; Han, B. MicroRNA miR-223 modulates NLRP3 and Keap1, mitigating lipopolysaccharide-induced inflammation and oxidative stress in bovine mammary epithelial cells and murine mammary glands. Vet. Res. 2023, 54, 78. [Google Scholar] [CrossRef]
- Yang, W.; Jin, M.; Wang, Y.; Zhao, H.; Wang, X.; Guo, Y.; Li, C.; Xiao, B.; Zhang, H.; Kiran, F.; et al. NR1D1 activation alleviates inflammatory response through inhibition of IL-6 expression in bovine endometrial epithelial cells. Int. J. Biol. Macromol. 2024, 283, 137642. [Google Scholar] [CrossRef]
- Yuan, N.; Xiao, L.; Chen, J.; Liu, B.; Ren, S.; Sheng, X.; Qi, X.; Wang, Y.; Chen, C.; Guo, K.; et al. CREG1 promotes bovine placental trophoblast cells exosome release by targeting IGF2R and participates in regulating organoid differentiation via exosomes transport. Int. J. Biol. Macromol. 2024, 274, 133298. [Google Scholar] [CrossRef]
- Giedt, M.S.; Thomalla, J.M.; White, R.P.; Johnson, M.R.; Lai, Z.W.; Tootle, T.L.; Welte, M.A. Adipose triglyceride lipase promotes prostaglandin-dependent actin remodeling by regulating substrate release from lipid droplets. Development 2023, 150, dev201516. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, Z.; Lu, Y.; Ma, C.; Li, J.; Yu, K.; Wu, J.; Che, X.; Liu, X.; Huang, X.; et al. Cytoskeleton regulates lipid droplet fusion and lipid storage by controlling lipid droplet movement. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2025, 1870, 159610. [Google Scholar] [CrossRef] [PubMed]
- Skjerven, O. Phosphatase, fat, and carbohydrate content of normal bovine endometrium; biopsy studies of cyclic variations. Fertil. Steril. 1956, 7, 31–43. [Google Scholar] [CrossRef]
- Boshier, D.P.; Fairclough, R.J.; Holloway, H. Assessment of sheep blastocyst effects on neutral lipids in the uterine caruncular epithelium. J. Reprod. Fertil. 1987, 79, 569–573. [Google Scholar] [CrossRef]
- Liang, J.; Li, K.; Chen, K.; Liang, J.; Qin, T.; He, J.; Shi, S.; Tan, Q.; Wang, Z. Regulation of ARHGAP19 in the endometrial epithelium: A possible role in the establishment of uterine receptivity. Reprod. Biol. Endocrinol. 2021, 19, 2. [Google Scholar] [CrossRef]
- Owusu-Akyaw, A.; Krishnamoorthy, K.; Goldsmith, L.T.; Morelli, S.S. The role of mesenchymal-epithelial transition in endometrial function. Hum. Reprod. Update 2019, 25, 114–133. [Google Scholar] [CrossRef]
- Fanning, A.S.; Jameson, B.J.; Jesaitis, L.A.; Anderson, J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998, 273, 29745–29753. [Google Scholar] [CrossRef]
- Belardi, B.; Hamkins-Indik, T.; Harris, A.R.; Kim, J.; Xu, K.; Fletcher, D.A. A Weak Link with Actin Organizes Tight Junctions to Control Epithelial Permeability. Dev. Cell 2020, 54, 792–804.e7. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.; Weis, W.I. Structure and biochemistry of cadherins and catenins. Cold Spring Harb. Perspect. Biol. 2009, 1, a003053. [Google Scholar] [CrossRef] [PubMed]
- Jalali, B.M.; Lukasik, K.; Witek, K.; Baclawska, A.; Skarzynski, D.J. Changes in the expression and distribution of junction and polarity proteins in the porcine endometrium during early pregnancy period. Theriogenology 2020, 142, 196–206. [Google Scholar] [CrossRef]
- Zhang, B.; Song, C.; Zhou, B.; Zhang, J.; Dong, W.; Zhang, Y.; Zhao, X.; Zhang, Q. CTNNB1 and CDH1 Regulate Trophoblast Cell Adhesion and Junction Formation in Yak Placental Tissue at Different Gestational Stages. Animals 2025, 15, 876. [Google Scholar] [CrossRef]
- Zhao, L.; Zheng, X.; Liu, J.; Zheng, R.; Yang, R.; Wang, Y.; Sun, L. PPAR signaling pathway in the first trimester placenta from in vitro fertilization and embryo transfer. Biomed. Pharmacother. 2019, 118, 109251. [Google Scholar] [CrossRef]
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Zhao, X.; Wu, S.; Li, F.; Wang, Y.; Liu, B.; Zhang, Y.; Gao, X.; Wang, Y.; et al. Peroxisome proliferator-activated receptors: A key link between lipid metabolism and cancer progression. Clin. Nutr. 2024, 43, 332–345. [Google Scholar] [CrossRef]
- Zhang, W.; Raza, S.H.A.; Li, B.; Yang, W.; Khan, R.; Aloufi, B.H.; Zhang, G.; Zuo, F.; Zan, L. LncBNIP3 Inhibits Bovine Intramuscular Preadipocyte Differentiation via the PI3K-Akt and PPAR Signaling Pathways. J. Agric. Food Chem. 2024, 72, 24260–24271. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, B.; Liang, H.; Ji, H.; Wang, Z.; Song, X.; Zhu, H.; Song, S.; Yuan, W.; Wu, Q.; et al. Gestational organophosphate esters (OPEs) exposure in association with placental DNA methylation levels of peroxisome proliferator-activated receptors (PPARs) signaling pathway-related genes. Sci. Total Environ. 2024, 947, 174569. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Sun, X.; Tranguch, S.; Zhang, H.; Jia, X.; Wang, D.; Das, S.K.; Desvergne, B.; Wahli, W.; et al. Stage-specific integration of maternal and embryonic peroxisome proliferator-activated receptor delta signaling is critical to pregnancy success. J. Biol. Chem. 2007, 282, 37770–37782. [Google Scholar] [CrossRef]
- Shi, H.B.; Zhang, C.H.; Xu, Z.A.; Lou, G.G.; Liu, J.X.; Luo, J.; Loor, J.J. Peroxisome proliferator-activated receptor delta regulates lipid droplet formation and transport in goat mammary epithelial cells. J. Dairy Sci. 2018, 101, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.R.; Sinedino, L.D.P.; Spencer, T.E. Paracrine and endocrine actions of interferon tau (IFNT). Reproduction 2017, 154, F45–F59. [Google Scholar] [CrossRef] [PubMed]
- Warren, W.; Osborn, M.; Yates, A.; O’Sullivan, S. The emerging role of fatty acid-binding protein 3 (FABP3) in cancer. Drug Discov. Today 2025, 30, 104504. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.; Brabletz, T.; Ceppi, P. Targeting EMT in Cancer with Repurposed Metabolic Inhibitors. Trends Cancer 2020, 6, 942–950. [Google Scholar] [CrossRef]
- Wei, D.; Su, Y.; Leung, P.C.K.; Li, Y.; Chen, Z.J. Roles of bone morphogenetic proteins in endometrial remodeling during the human menstrual cycle and pregnancy. Hum. Reprod. Update 2024, 30, 215–237. [Google Scholar] [CrossRef]
- Bauersachs, S.; Mitko, K.; Ulbrich, S.E.; Blum, H.; Wolf, E. Transcriptome studies of bovine endometrium reveal molecular profiles characteristic for specific stages of estrous cycle and early pregnancy. Exp. Clin. Endocrinol. Diabetes 2008, 116, 371–384. [Google Scholar] [CrossRef]
- Quinn, K.E.; Matson, B.C.; Wetendorf, M.; Caron, K.M. Pinopodes: Recent advancements, current perspectives, and future directions. Mol. Cell. Endocrinol. 2020, 501, 110644. [Google Scholar] [CrossRef]
- Kumro, F.G.; O’Neil, E.V.; Ciernia, L.A.; Moraes, J.G.N.; Spencer, T.E.; Lucy, M.C. Scanning electron microscopy of the surface epithelium of the bovine endometrium. J. Dairy Sci. 2020, 103, 12083–12090. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Frank, J.W.; Burghardt, R.C.; Bazer, F.W.; Johnson, G.A. Integrins and OPN localize to adhesion complexes during placentation in sheep. Reproduction 2020, 160, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Bai, R.; Kusama, K. Integration of molecules to construct the processes of conceptus implantation to the maternal endometrium. J. Anim. Sci. 2018, 96, 3009–3021. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; FitzGerald, G.A. Genetic and pharmacological analysis of prostanoid receptor function. J. Clin. Investig. 2001, 108, 25–30. [Google Scholar] [CrossRef]
- Arosh, J.A.; Banu, S.K.; Chapdelaine, P.; Fortier, M.A. Temporal and tissue-specific expression of prostaglandin receptors EP2, EP3, EP4, FP, and cyclooxygenases 1 and 2 in uterus and fetal membranes during bovine pregnancy. Endocrinology 2004, 145, 407–417. [Google Scholar] [CrossRef]
- Nishimura, T.; Zhao, X.; Gan, H.; Koyasu, S.; Remold, H.G. The prostaglandin E2 receptor EP4 is integral to a positive feedback loop for prostaglandin E2 production in human macrophages infected with Mycobacterium tuberculosis. FASEB J. 2013, 27, 3827–3836. [Google Scholar] [CrossRef]
- Ohmura, T.; Tian, Y.; Sarich, N.; Ke, Y.; Meliton, A.; Shah, A.S.; Andreasson, K.; Birukov, K.G.; Birukova, A.A. Regulation of lung endothelial permeability and inflammatory responses by prostaglandin A2: Role of EP4 receptor. Mol. Biol. Cell 2017, 28, 1622–1635. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Mao, W.; Fu, C.; Shen, Y.; Wang, Y.; Liu, B.; Cao, J. 17β-estradiol regulates prostaglandin E2 and F2α synthesis and function in endometrial explants of cattle. Anim. Reprod. Sci. 2020, 216, 106466. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Yan, Y.; Cheng, M.; Guo, T.; Gao, K.; Wang, A.; Lin, P.; Zhou, D.; Jin, Y. FABP3 Mediates Lipid Droplet Accumulation and Adhesive Capacity in Bovine Endometrial Epithelial Cells via PGE2/PTGER4/PPAR Axis. Animals 2025, 15, 3417. https://doi.org/10.3390/ani15233417
Zhang B, Yan Y, Cheng M, Guo T, Gao K, Wang A, Lin P, Zhou D, Jin Y. FABP3 Mediates Lipid Droplet Accumulation and Adhesive Capacity in Bovine Endometrial Epithelial Cells via PGE2/PTGER4/PPAR Axis. Animals. 2025; 15(23):3417. https://doi.org/10.3390/ani15233417
Chicago/Turabian StyleZhang, Beibei, Yutong Yan, Ming Cheng, Tengfei Guo, Kangkang Gao, Aihua Wang, Pengfei Lin, Dong Zhou, and Yaping Jin. 2025. "FABP3 Mediates Lipid Droplet Accumulation and Adhesive Capacity in Bovine Endometrial Epithelial Cells via PGE2/PTGER4/PPAR Axis" Animals 15, no. 23: 3417. https://doi.org/10.3390/ani15233417
APA StyleZhang, B., Yan, Y., Cheng, M., Guo, T., Gao, K., Wang, A., Lin, P., Zhou, D., & Jin, Y. (2025). FABP3 Mediates Lipid Droplet Accumulation and Adhesive Capacity in Bovine Endometrial Epithelial Cells via PGE2/PTGER4/PPAR Axis. Animals, 15(23), 3417. https://doi.org/10.3390/ani15233417




