Nicotinamide Mononucleotide Enhances Boar Sperm Quality via Maintaining Mitochondrial Function During Liquid Storage
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Media Preparation
2.2. Animals, Semen Collection and Processing
2.3. Sperm Motility
2.4. Evaluation of Sperm Viability and Acrosome Integrity
2.5. Evaluation of Mitochondrial Membrane Potential
2.6. Measure of Sperm ATP Level
2.7. Evaluation of NAD+ and NADH Content
2.8. Western Blotting
2.9. Thermo-Resistance Test
2.10. Capacitation and Acrosome Reaction Assessment
2.11. Assessment of Sperm-Tissue Fragment Binding Capacity
2.12. Statistical Analysis
3. Results
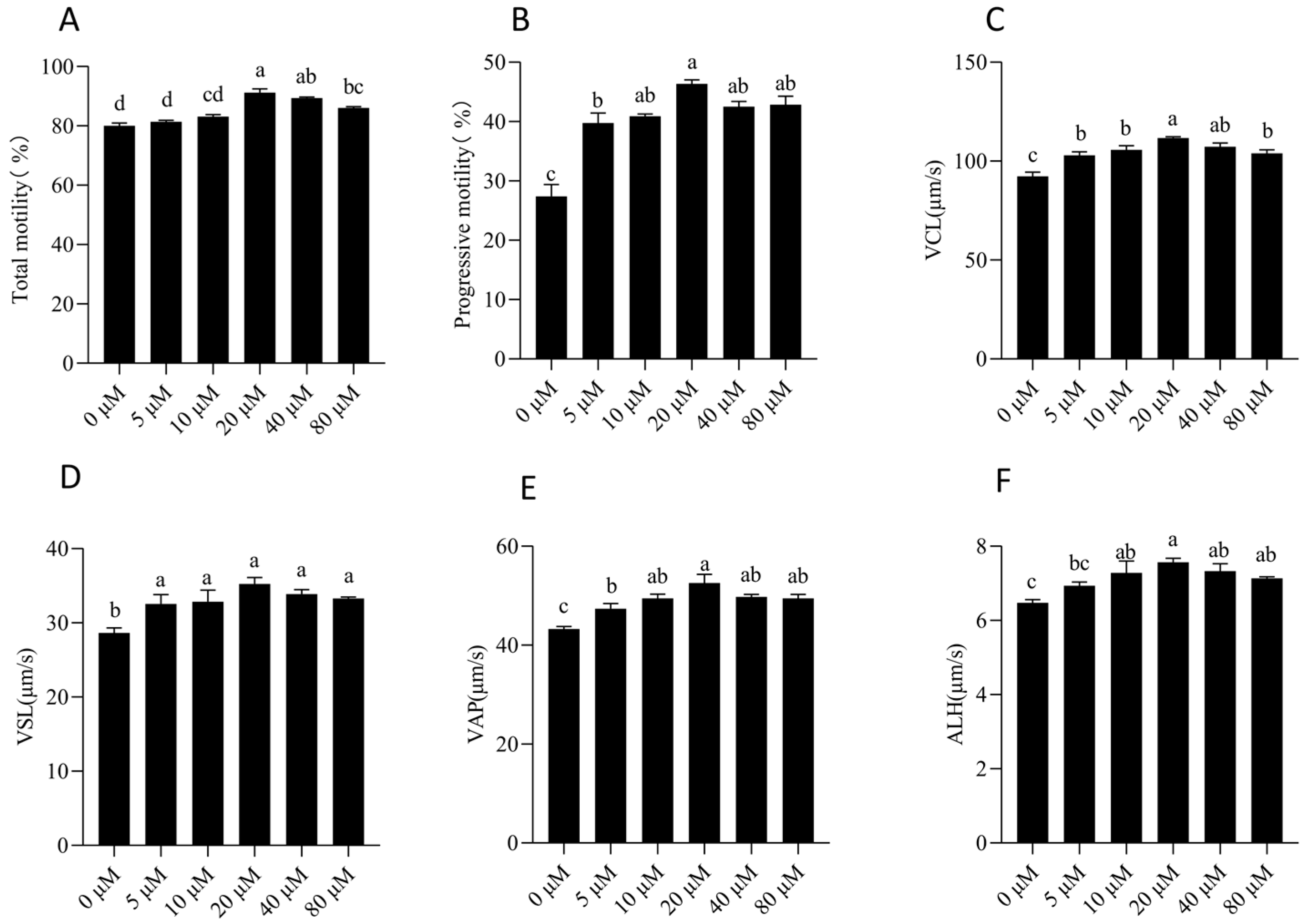
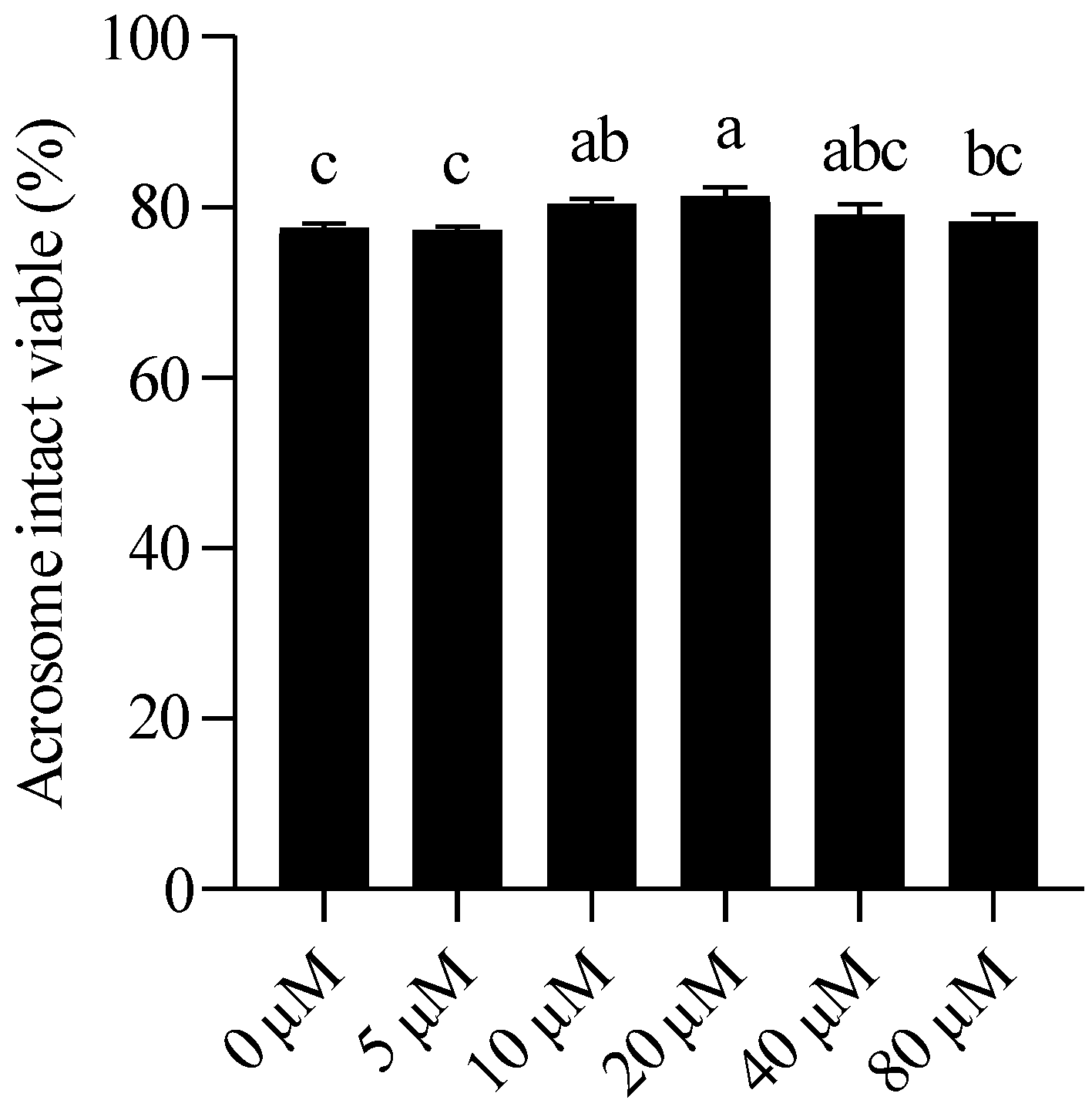
3.1. NMN Enhanced Sperm Motility and Viability and Acrosome Integrity During Liquid Storage
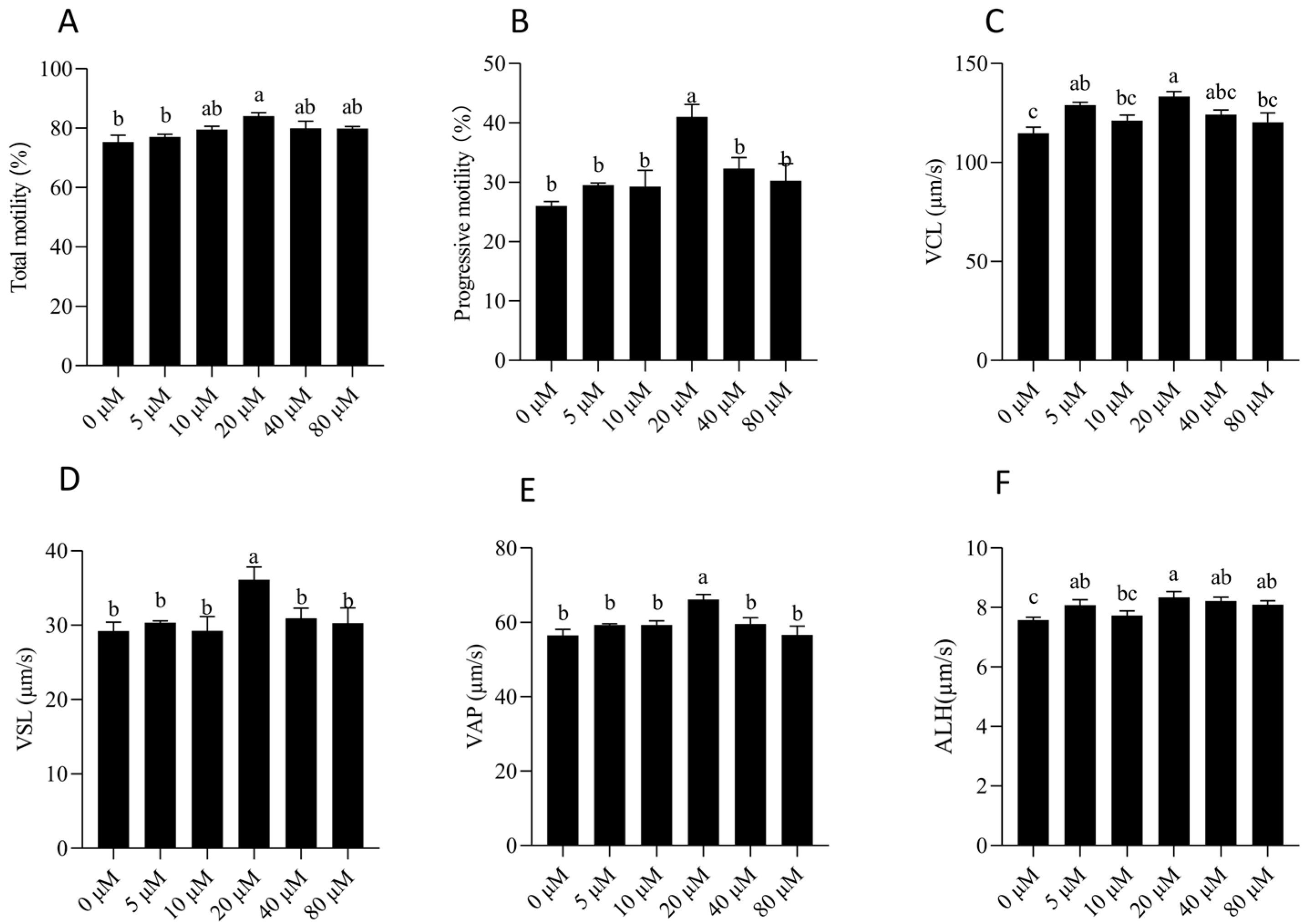
3.2. NMN Improved Sperm Thermo-Resistance After Storage
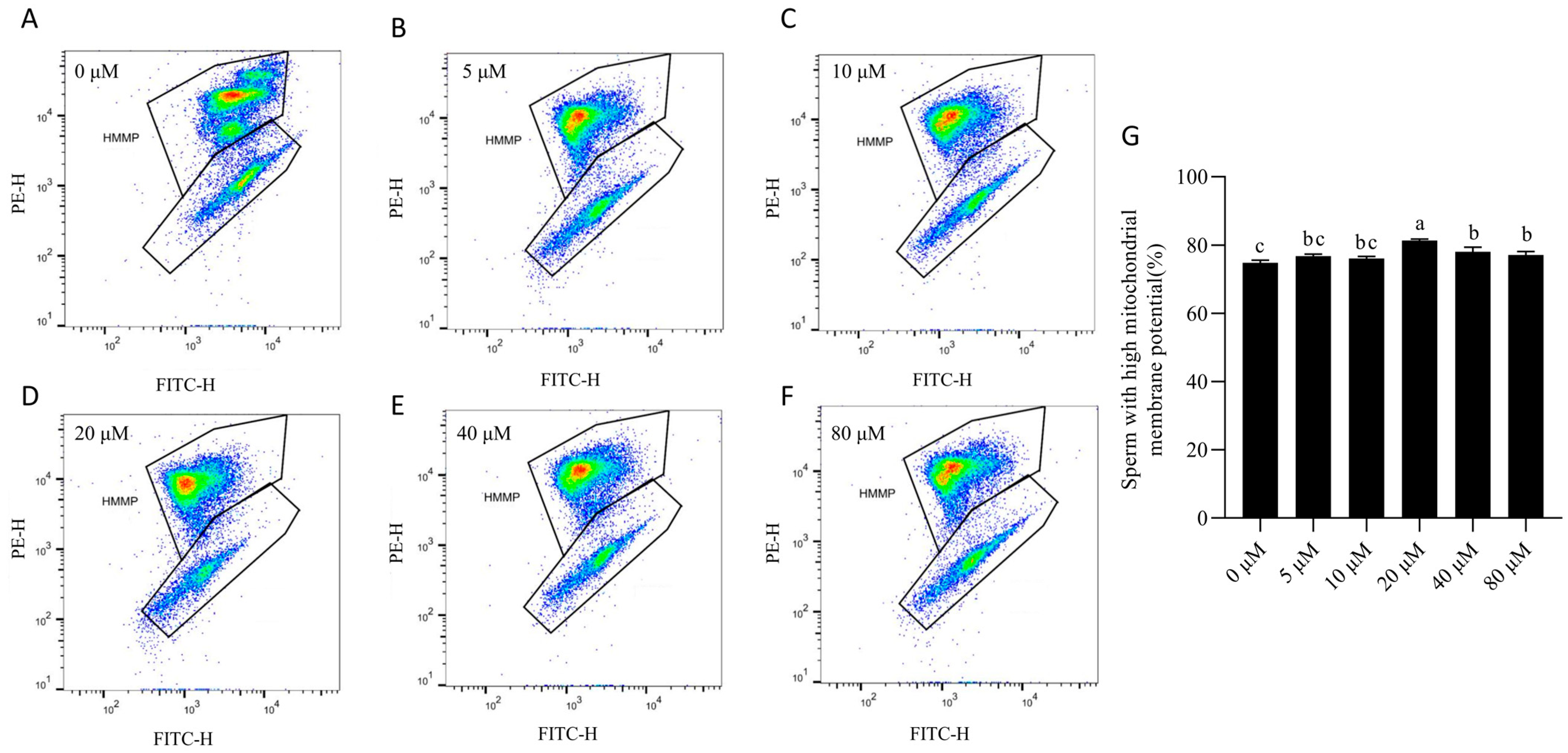
3.3. NMN Preserved Boar Sperm Mitochondrial Membrane Potential
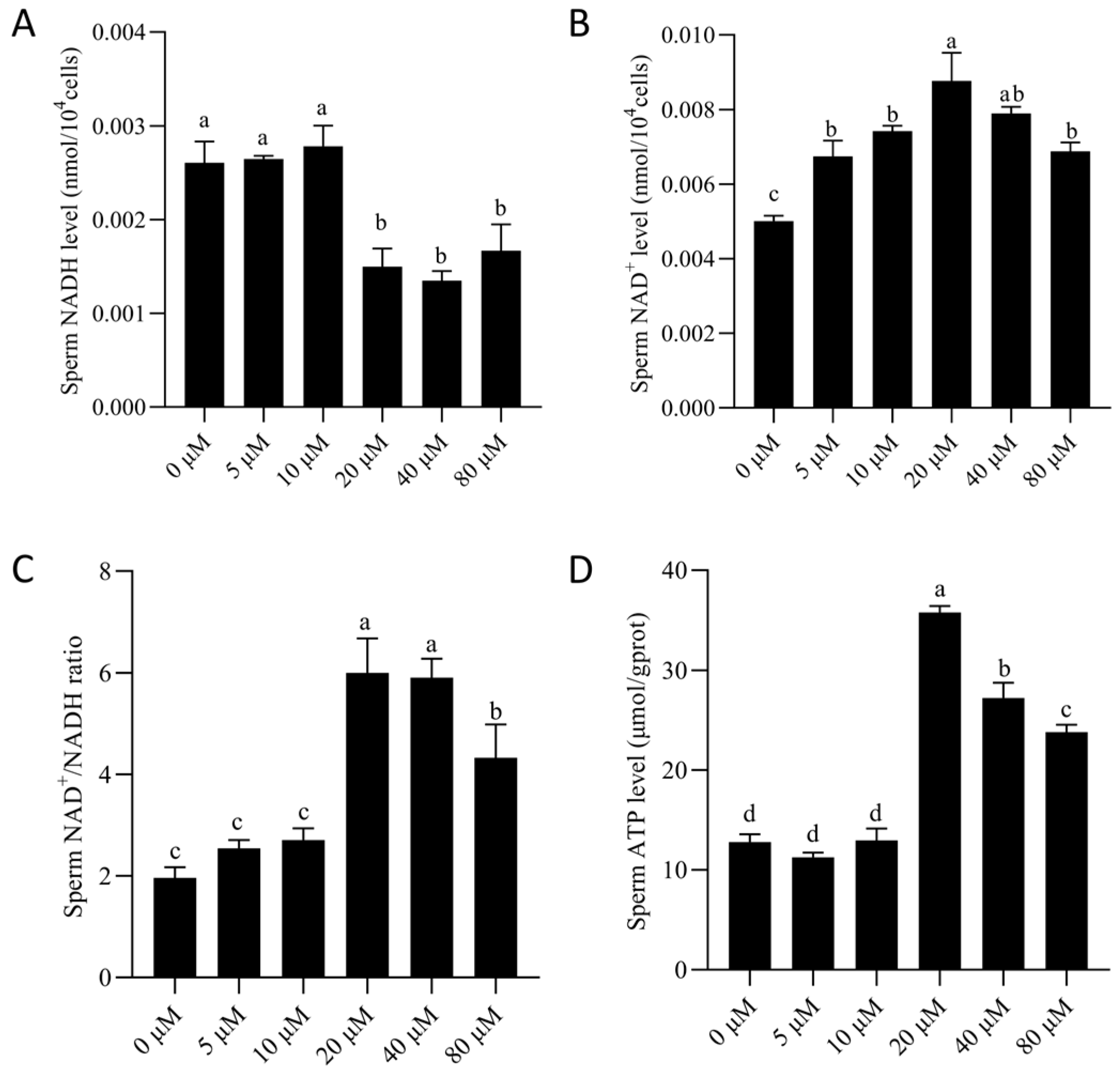
3.4. NMN Supplementation Improved Mitochondrial Metabolic Activity of Boar Sperm by Maintaining NAD+ Homeostasis
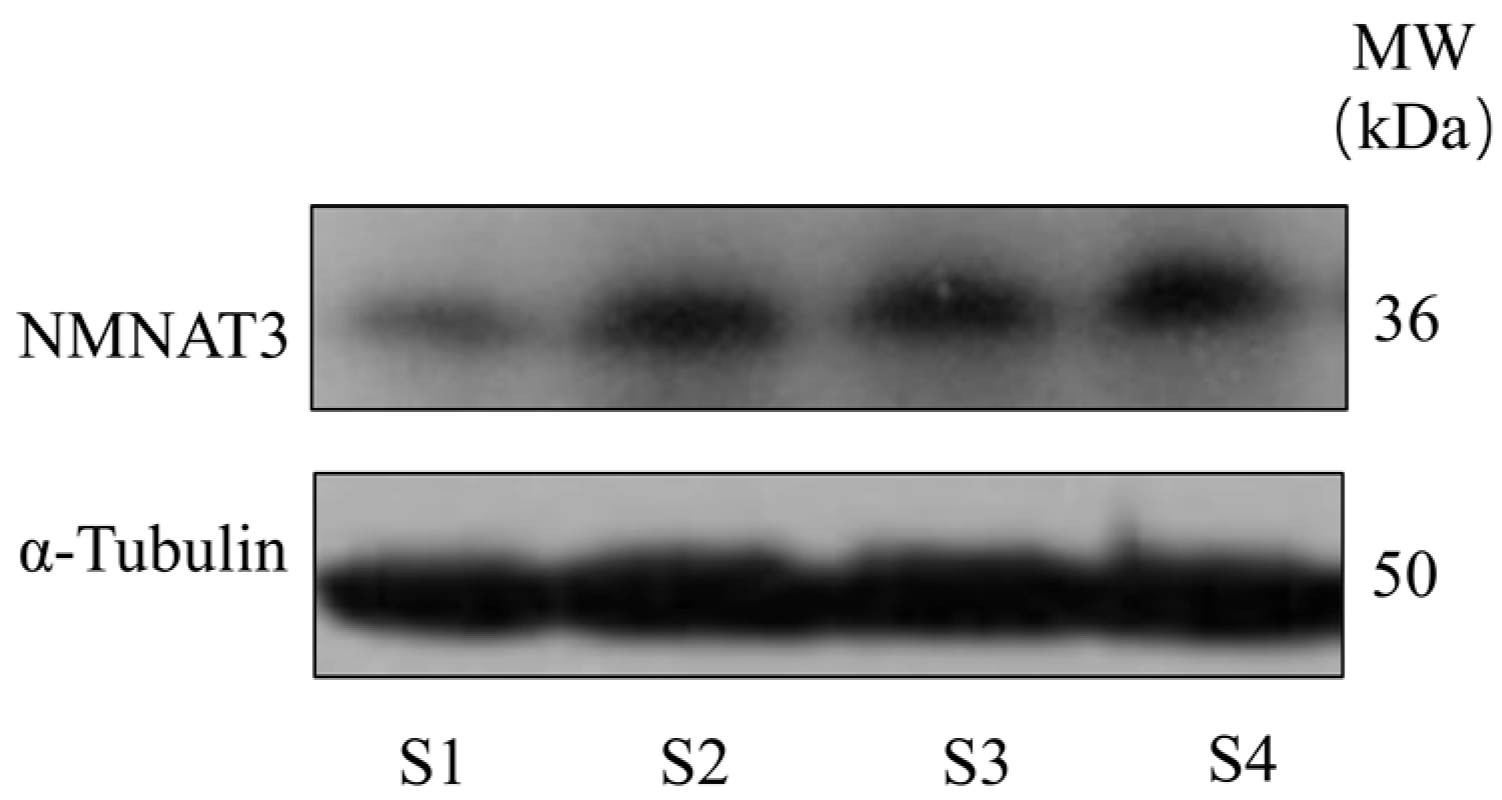
3.5. Expression of NMNAT3 in Boar Sperm
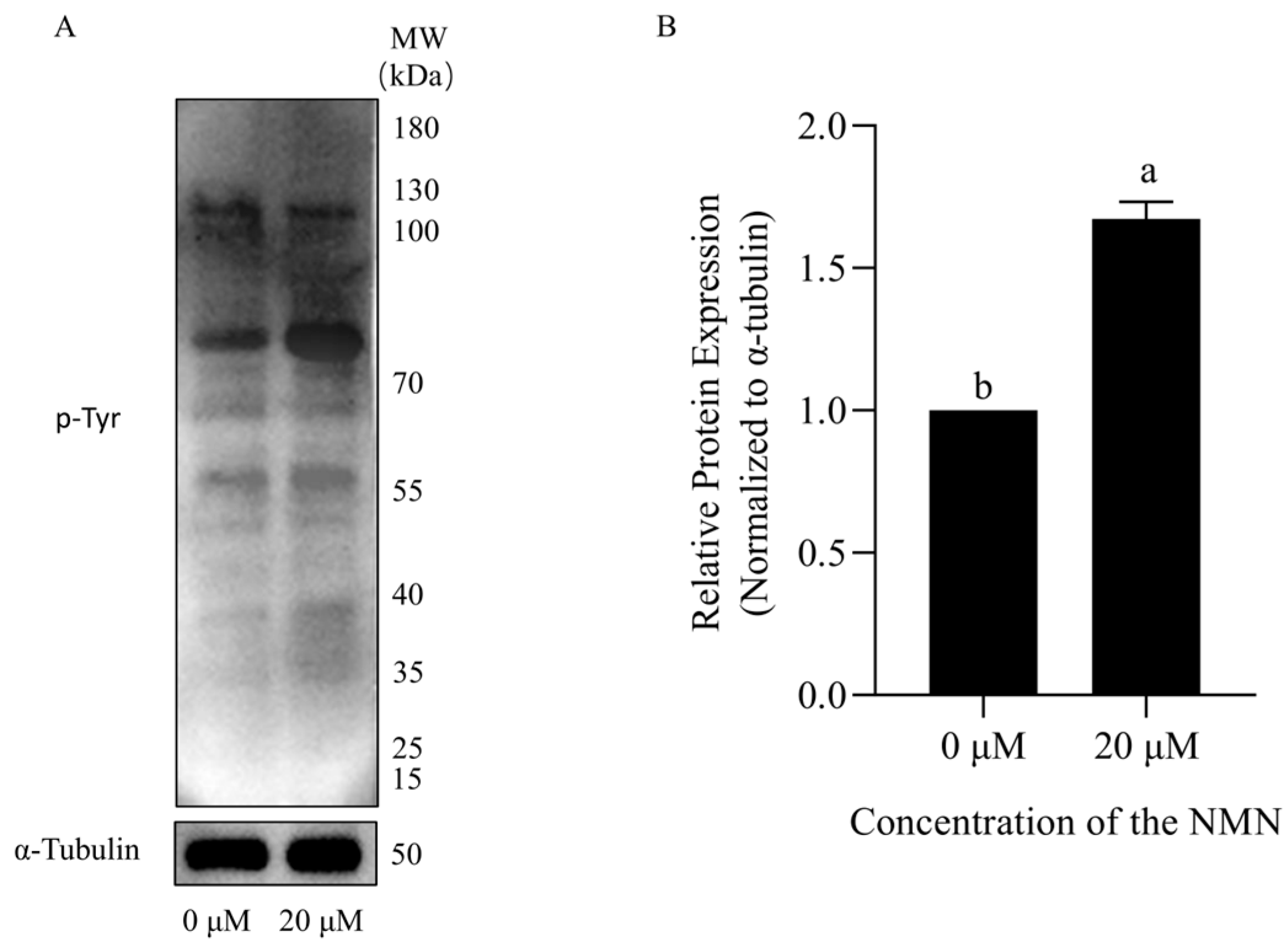
3.6. NMN Maintained Boar Sperm Fertility Ability During Liquid Storage
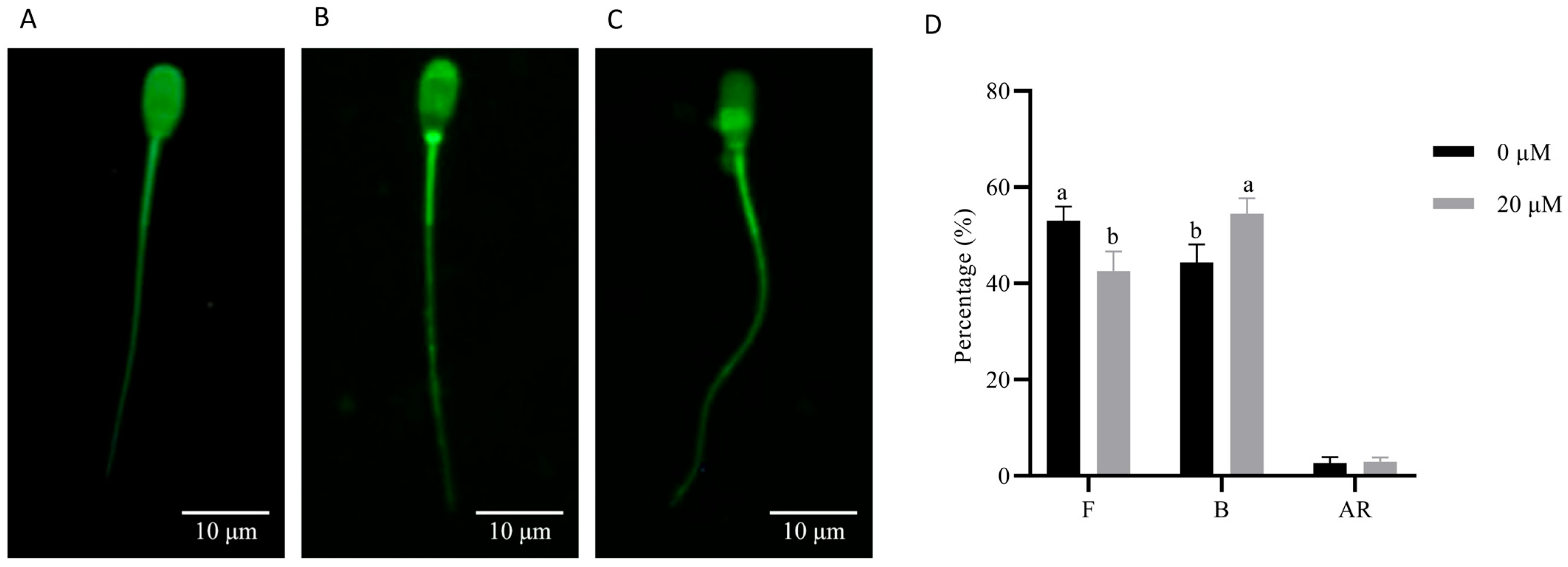
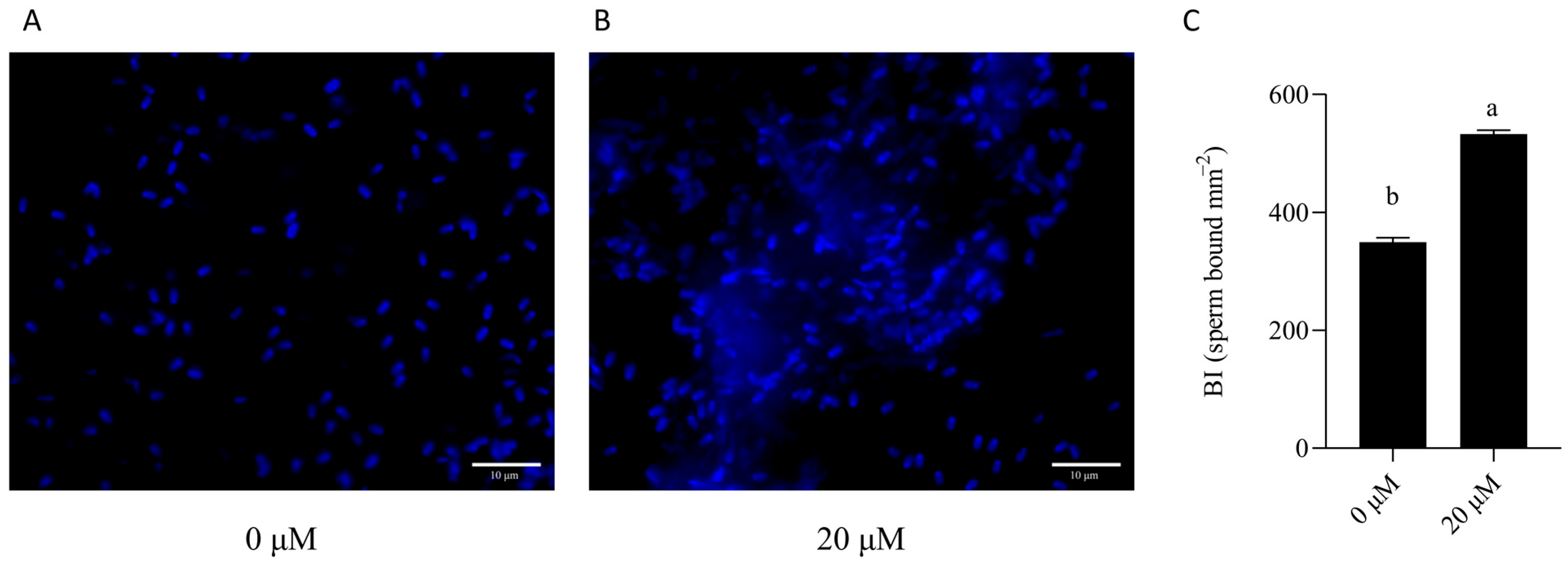
3.7. NMN Improved the Binding Capacity of Sperm Stored for 7 Days to Oviduct Explants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwon, W.S.; Rahman, M.S.; Lee, J.S.; Yoon, S.J.; Park, Y.J.; Pang, M.G. Discovery of Predictive Biomarkers for Litter Size in Boar Spermatozoa. Mol. Cell. Proteom. 2015, 14, 1230–1240. [Google Scholar] [CrossRef]
- Tourmente, M.; Villar-Moya, P.; Rial, E.; Roldan, E.R. Differences in Atp Generation Via Glycolysis and Oxidative Phosphorylation and Relationships with Sperm Motility in Mouse Species. J. Biol. Chem. 2015, 290, 20613–20626. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gong, Y.; He, B.; Zhao, R. Relationships between Mitochondrial DNA Content, Mitochondrial Activity, and Boar Sperm Motility. Theriogenology 2017, 87, 276–283. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Singh, D.; Agarwal, A.; Henkel, R. Causes and Consequences of Sperm Mitochondrial Dysfunction. Andrologia 2021, 53, e13666. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, A.; Zou, Y.; Su, N.; Loscalzo, J.; Yang, Y. In Vivo Monitoring of Cellular Energy Metabolism Using Sonar, a Highly Responsive Sensor for Nad(+)/Nadh Redox State. Nat. Protoc. 2016, 11, 1345–1359. [Google Scholar] [CrossRef]
- Ye, B.; Pei, Y.; Wang, L.; Meng, D.; Zhang, Y.; Zou, S.; Li, H.; Liu, J.; Xie, Z.; Tian, C.; et al. Nad(+) Supplementation Prevents Sting-Induced Senescence in Cd8(+) T Cells by Improving Mitochondrial Homeostasis. J. Cell. Biochem. 2024, 125, e30522. [Google Scholar] [CrossRef]
- Vahedi Raad, M.; Firouzabadi, A.M.; Niaki, M.T.; Henkel, R.; Fesahat, F. The Impact of Mitochondrial Impairments on Sperm Function and Male Fertility: A Systematic Review. Reprod. Biol. Endocrinol. 2024, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Anwar, K.; Thaller, G.; Saeed-Zidane, M. Sperm-Borne Mitochondrial Activity Influenced by Season and Age of Holstein Bulls. Int. J. Mol. Sci. 2024, 25, 13064. [Google Scholar] [CrossRef]
- Ruiz-Pesini, E.; Diez, C.; Lapeña, A.C.; Pérez-Martos, A.; Montoya, J.; Alvarez, E.; Arenas, J.; López-Pérez, M.J. Correlation of Sperm Motility with Mitochondrial Enzymatic Activities. Clin. Chem. 1998, 44, 1616–1620. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, W.; Zou, P.; Jiang, F.; Zeng, Y.; Chen, Q.; Sun, L.; Yang, H.; Zhou, N.; Wang, X.; et al. Mitochondrial Functionality Modifies Human Sperm Acrosin Activity, Acrosome Reaction Capability and Chromatin Integrity. Hum. Reprod. 2019, 34, 3–11. [Google Scholar] [CrossRef]
- Boguenet, M.; Bouet, P.E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their Role in Spermatozoa and in Male Infertility. Hum. Reprod. Update 2021, 7, 97–719. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, P.; Pintos-Polasky, P.; Rosa-Villagran, L.; Skowronek, M.F.; Cassina, A.; Sapiro, R. Mitochondrial Metabolism Determines the Functional Status of Human Sperm and Correlates with Semen Parameters. Front. Cell. Dev. Biol. 2022, 10, 926684. [Google Scholar] [CrossRef]
- Sanocka, D.; Kurpisz, M. Reactive Oxygen Species and Sperm Cells. Reprod. Biol. Endocrinol. 2004, 2, 12. [Google Scholar] [CrossRef]
- Rogers Lynette, K. Cellular Targets of Oxidative Stress. J. Curr. Opin. Toxicol. 2020, 20, 48–54. [Google Scholar] [CrossRef]
- Akbarinejad, V.; Fathi, R.; Shahverdi, A.; Esmaeili, V.; Rezagholizadeh, A.; Ghaleno, L.R. The Relationship of Mitochondrial Membrane Potential, Reactive Oxygen Species, Adenosine Triphosphate Content, Sperm Plasma Membrane Integrity, and Kinematic Properties in Warmblood Stallions. J. Equine Vet. Sci. 2020, 94, 03267. [Google Scholar] [CrossRef]
- Hashimoto, S.; Gamage, U.; Inoue, Y.; Iwata, H.; Morimoto, Y. Nicotinamide Mononucleotide Boosts the Development of Bovine Oocyte by Enhancing Mitochondrial Function and Reducing Chromosome Lagging. Sci. Rep. 2025, 15, 310. [Google Scholar] [CrossRef]
- Ramírez-Martín, N.; Buigues, A.; Rodríguez-Varela, C.; Martínez, J.; Blázquez-Simón, P.; Rodríguez-Hernández, C.; Pellicer, N.; Pellicer, A.; Escriba, M.A.; Herraiz, S. Nicotinamide Mononucleotide Supplementation Improves Oocyte Developmental Competence in Different Ovarian Damage Conditions. J. Am. J. Obstet. Herraiz Gynecol. 2025, 233, 112.e1–112.e20. [Google Scholar] [CrossRef]
- Miao, Y.; Cui, Z.; Zhu, X.; Gao, Q.; Xiong, B. Supplementation of Nicotinamide Mononucleotide Improves the Quality of Postovulatory Aged Porcine Oocytes. J. Mol. Cell. Biol. 2022, 14, mjac025. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell. Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chai, J.; Cao, C.; Wang, X.; Pang, W. Supplementing Boar Diet with Nicotinamide Mononucleotide Improves Sperm Quality Probably through the Activation of the Sirt3 Signaling Pathway. Antioxidants 2024, 13, 507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qin, X.; Bojan, N.; Cao, C.; Chai, J.; Pang, W. Nicotinamide Mononucleotide Enhances Porcine Sperm Quality by Activating the Sirt3-Sod2/Ros Pathway and Promoting Oxidative Phosphorylation. Anim. Reprod. Sci. 2025, 275, 107797. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, W.; Li, R.; Zeng, W. Reducing the Glucose Level in Pre-Treatment Solution Improves Post-Thaw Boar Sperm Quality. Front. Vet. Sci. 2022, 9, 856536. [Google Scholar] [CrossRef]
- Ded, L.; Dostalova, P.; Zatecka, E.; Dorosh, A.; Komrskova, K.; Peknicova, J. Fluorescent Analysis of Boar Sperm Capacitation Process in Vitro. Reprod. Biol. Endocrinol. 2019, 17, 109. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Zeng, W.; Adetunji, A.O.; Min, L.; Shimada, M.; Zhu, Z. Succinylation Regulates Boar Sperm Linear Motility Via Reprogramming Glucose Metabolism. Commun. Biol. 2025, 8, 1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Q.; Min, L.; Cao, H.; Adetunji, A.O.; Zhou, K.; Zhu, Z. Pyrroloquinoline Quinone Improved Boar Sperm Quality Via Maintaining Mitochondrial Function During Cryopreservation. Antioxidants 2025, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.; Jakop, U.; Jung, M.; Cabezón, F. Influences on Thermo-Resistance of Boar Spermatozoa. Theriogenology 2019, 127, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Fraser, L.R.; Abeydeera, L.R.; Niwa, K. Ca(2+)-Regulating Mechanisms That Modulate Bull Sperm Capacitation and Acrosomal Exocytosis as Determined by Chlortetracycline Analysis. Mol. Reprod. Dev. 1995, 40, 233–241. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, X.; Liu, S.; Hoque, S.A.M.; Min, L.; Ding, N.; Zhu, Z. Vibration Emissions Reduce Boar Sperm Quality Via Disrupting Its Metabolism. Biology 2024, 13, 370. [Google Scholar] [CrossRef]
- Youngson, N.A.; Uddin, G.M.; Das, A.; Martinez, C.; Connaughton, H.S.; Whiting, S.; Yu, J.; Sinclair, D.A.; Aitken, R.J.; Morris, M.J. Impacts of Obesity, Maternal Obesity and Nicotinamide Mononucleotide Supplementation on Sperm Quality in Mice. Reproduction 2019, 158, 169–179. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, H.; Yang, Q.; Li, Y.; Wang, R.; Adetunji, A.O.; Min, L. Β-Nicotinamide Mononucleotide Improves Chilled Ram Sperm Quality in Vitro by Reducing Oxidative Stress Damage. Anim. Biosci. 2024, 37, 852–861. [Google Scholar] [CrossRef]
- Schulze, M.; Ammon, C.; Schaefer, J.; Luther, A.M.; Jung, M.; Waberski, D. Impact of Different Dilution Techniques on Boar Sperm Quality and Sperm Distribution of the Extended Ejaculate. Anim. Reprod. Sci. 2017, 182, 138–145. [Google Scholar] [CrossRef]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef]
- Titov, D.V.; Cracan, V.; Goodman, R.P.; Peng, J.; Grabarek, Z.; Mootha, V.K. Complementation of Mitochondrial Electron Transport Chain by Manipulation of the Nad+/Nadh Ratio. Science 2016, 352, 231–235. [Google Scholar] [CrossRef]
- Høyland, L.E.; VanLinden, M.R.; Niere, M.; Strømland, Ø.; Sharma, S.; Dietze, J.; Tolås, I.; Lucena, E.; Bifulco, E.; Sverkeli, L.J.; et al. Subcellular Nad(+) Pools Are Interconnected and Buffered by Mitochondrial Nad(). Nat. Metab. 2024, 6, 2319–2337. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.Y.; Ma, R.X.; Zou, B.; Zhang, Y.; Wu, M.M.; Yao, Y.; Li, J. Nicotinamide Mononucleotide Combined with Pj-34 Protects Microglial Cells from Lipopolysaccharide-Induced Mitochondrial Impairment through Nmnat3-Parp1 Axis. J. Transl. Med. 2025, 23, 279. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. Cd38 Dictates Age-Related Nad Decline and Mitochondrial Dysfunction through an Sirt3-Dependent Mechanism. Cell. Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Yang, X.; Gong, J.; Lv, J.; Yuan, X.; Shi, M.; Fu, C.; Tan, B.; Fan, Z.; Chen, L.; et al. Patterns of Alteration in Boar Semen Quality from 9 to 37 months Old and Improvement by Protocatechuic Acid. J. Anim. Sci. Biotechnol. 2024, 15, 78. [Google Scholar] [CrossRef]
- Hu, R.; Yang, X.; Wang, L.; Su, D.; He, Z.; Li, J.; Gong, J.; Zhang, W.; Ma, S.; Shi, M. Gut Microbiota Dysbiosis and Oxidative Damage in High–Fat Diet–Induced Impairment of Spermatogenesis: Role of Protocatechuic Acid Intervention. J. Food Front. 2024, 5, 2566–2578. [Google Scholar] [CrossRef]
- Prolla, T.A.; Denu, J.M. Nad+ Deficiency in Age-Related Mitochondrial Dysfunction. Cell Metab. 2014, 19, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Weide, T.; Mills, K.; Shofner, I.; Breitzman, M.W.; Kerns, K. Metabolic Shift in Porcine Spermatozoa During Sperm Capacitation-Induced Zinc Flux. Int. J. Mol. Sci. 2024, 25, 7919. [Google Scholar] [CrossRef]
- Sansegundo, E.; Tourmente, M.; Roldan, E.R.S. Energy Metabolism and Hyperactivation of Spermatozoa from Three Mouse Species under Capacitating Conditions. Cells 2022, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Kerns, K. Sperm Capacitation as a Predictor of Boar Fertility. Mol. Reprod. Dev. 2023, 90, 594–600. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, H.; Meng, Q.; Min, L.; Zhang, M.; Adetunji, A.O.; Li, W.; Zhu, Z. Nicotinamide Mononucleotide Enhances Boar Sperm Quality via Maintaining Mitochondrial Function During Liquid Storage. Animals 2025, 15, 3383. https://doi.org/10.3390/ani15233383
Liu Y, Zhang H, Meng Q, Min L, Zhang M, Adetunji AO, Li W, Zhu Z. Nicotinamide Mononucleotide Enhances Boar Sperm Quality via Maintaining Mitochondrial Function During Liquid Storage. Animals. 2025; 15(23):3383. https://doi.org/10.3390/ani15233383
Chicago/Turabian StyleLiu, Yongjin, Hongyan Zhang, Qingzhe Meng, Lingjiang Min, Min Zhang, Adedeji O. Adetunji, Wenjing Li, and Zhendong Zhu. 2025. "Nicotinamide Mononucleotide Enhances Boar Sperm Quality via Maintaining Mitochondrial Function During Liquid Storage" Animals 15, no. 23: 3383. https://doi.org/10.3390/ani15233383
APA StyleLiu, Y., Zhang, H., Meng, Q., Min, L., Zhang, M., Adetunji, A. O., Li, W., & Zhu, Z. (2025). Nicotinamide Mononucleotide Enhances Boar Sperm Quality via Maintaining Mitochondrial Function During Liquid Storage. Animals, 15(23), 3383. https://doi.org/10.3390/ani15233383




