Red LED Light Irradiation Increases the Resistance Against Environmental Stress of Frozen Bovine Sperm Thawed in Suboptimal Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Samples
2.2. Evaluation of Post-Thaw Sperm Integrity and Functionality
2.2.1. Sperm Viability
2.2.2. Acrosome Integrity
2.2.3. Sperm Motility
2.2.4. Chromatin Condensation
2.2.5. Sperm DNA Fragmentation
2.2.6. Antioxidant Capacity
2.2.7. Nitro Blue Tetrazolium Chloride Test
2.2.8. Mitochondrial Membrane Potential
2.3. Flow Cytometry Analyses
2.3.1. Evaluation of Intracellular ROS Levels (DHE/YO-PRO-1 and CM-H2DCFDA/PI)
2.3.2. Mitochondria-Associated ROS Production (MitoSOX/Mitotracker Deep Red/YO-PRO-1)
2.4. Artificial Insemination Trials
2.5. Statistical Analyses
3. Results
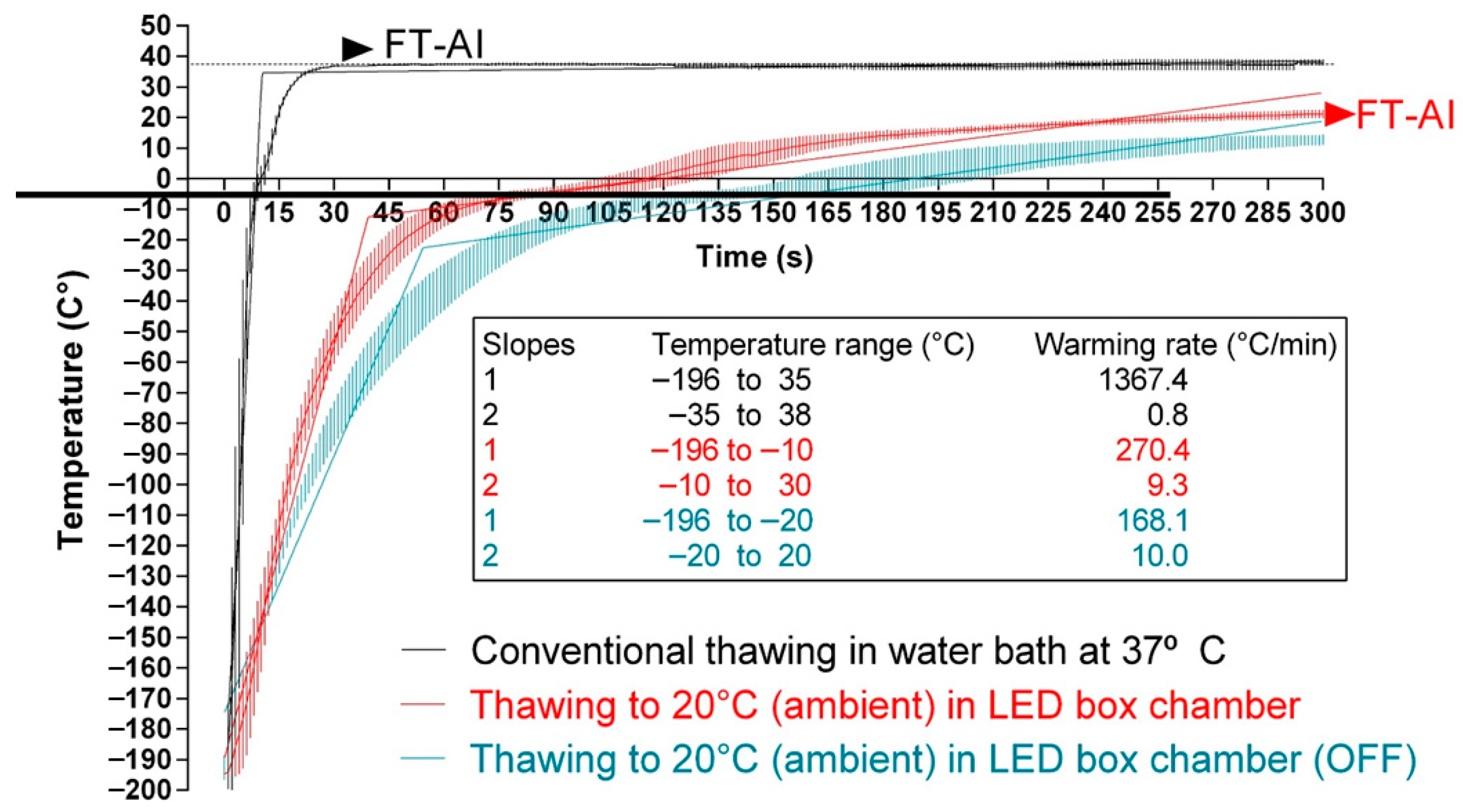
3.1. Thawing Dynamics of Sperm Straws
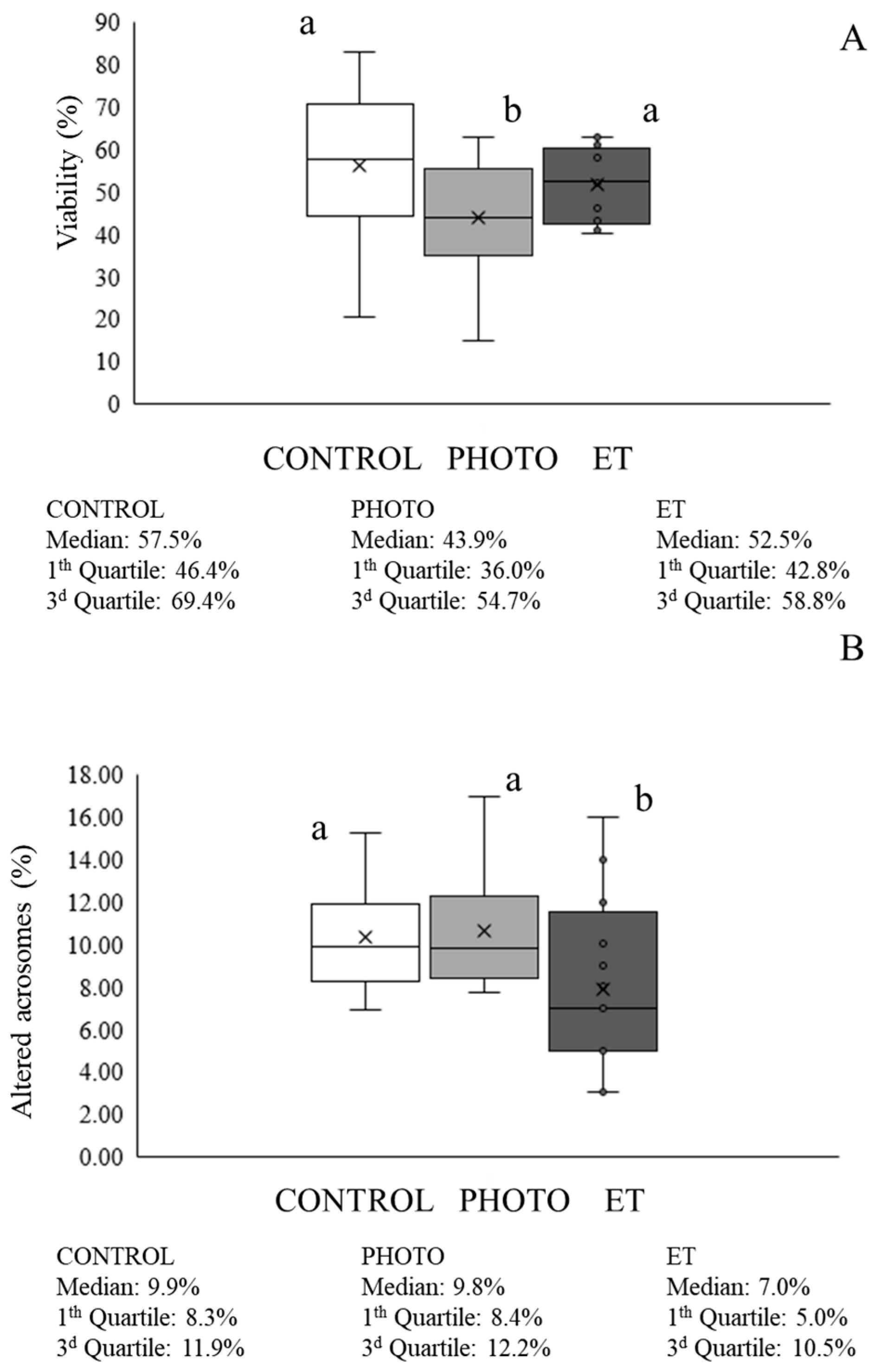
3.2. Effects of Red-Light Irradiation on Sperm Viability and Acrosome Integrity
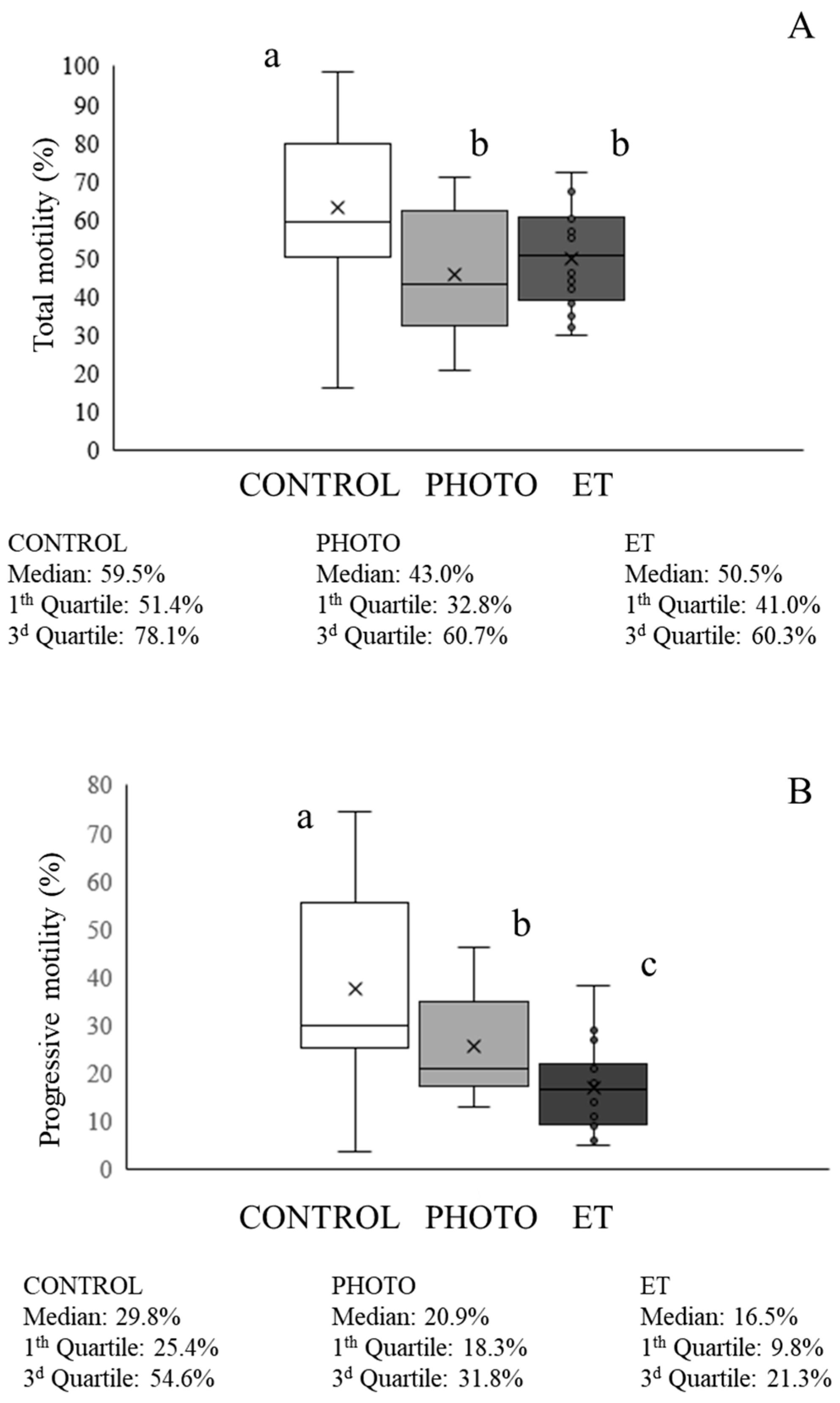
3.3. Effects of Red-Light Irradiation on Sperm Motility
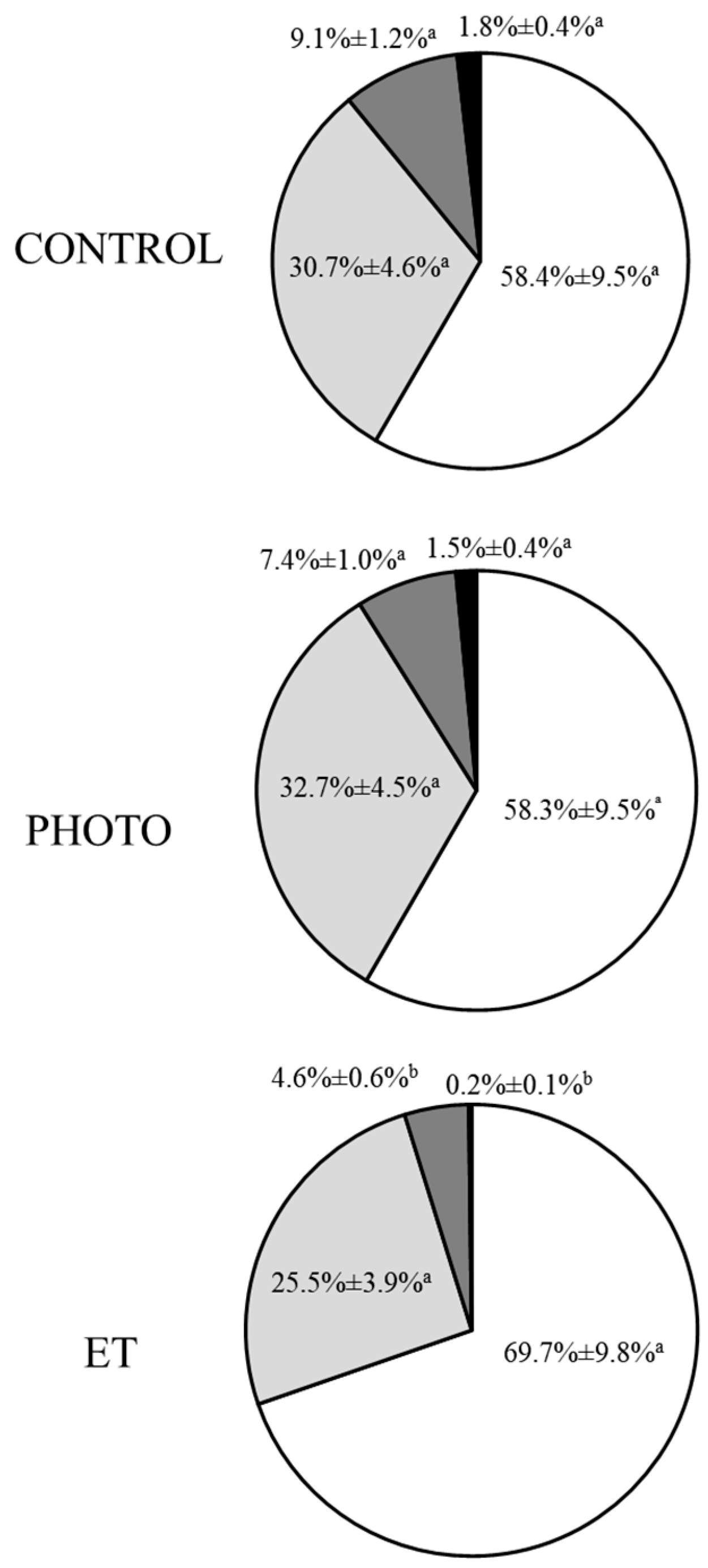
3.4. Effects of Red-Light Irradiation on Motile Sperm Subpopulations
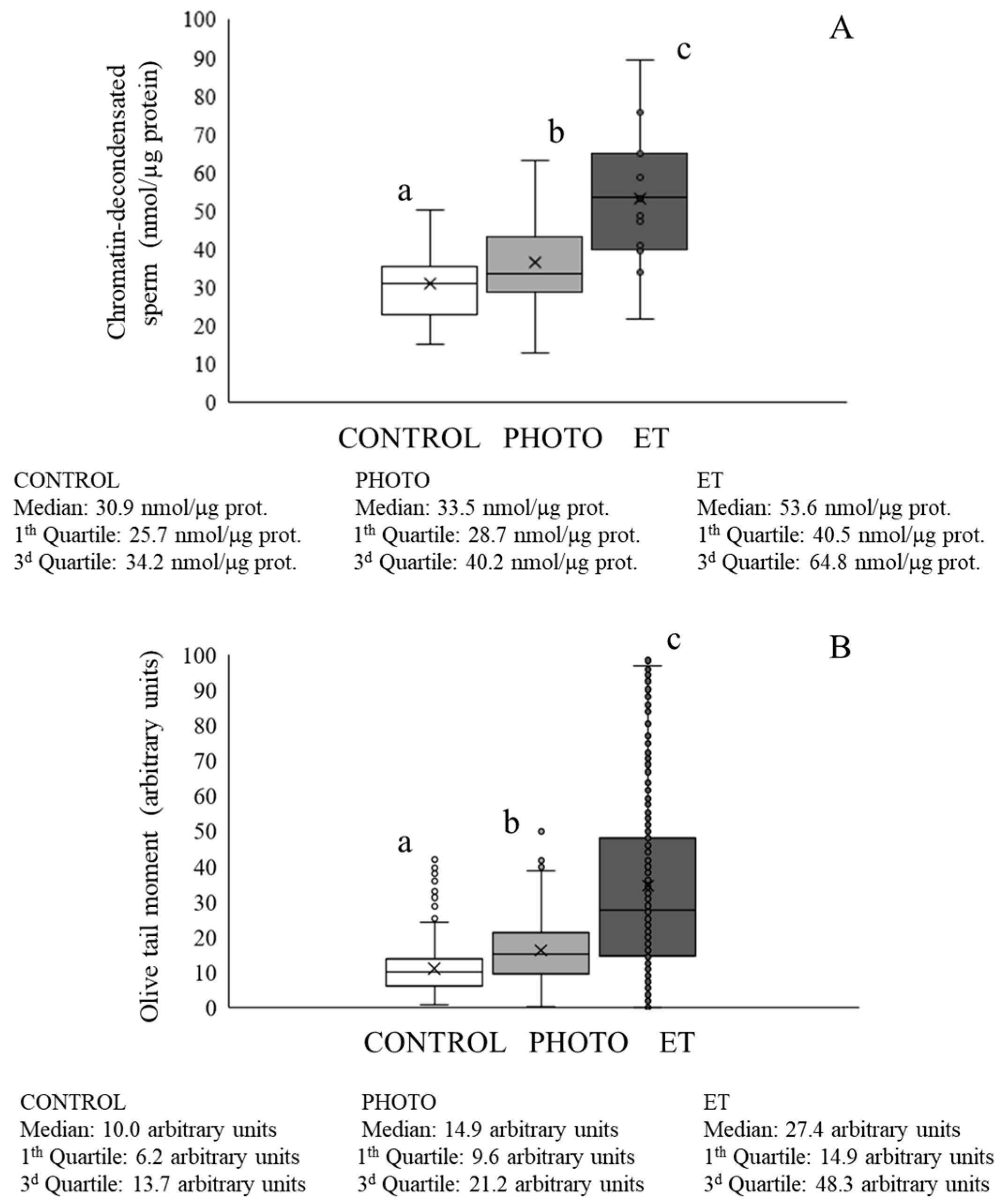
3.5. Effects of Red-Light Irradiation on Chromatin Condensation and DNA Fragmentation
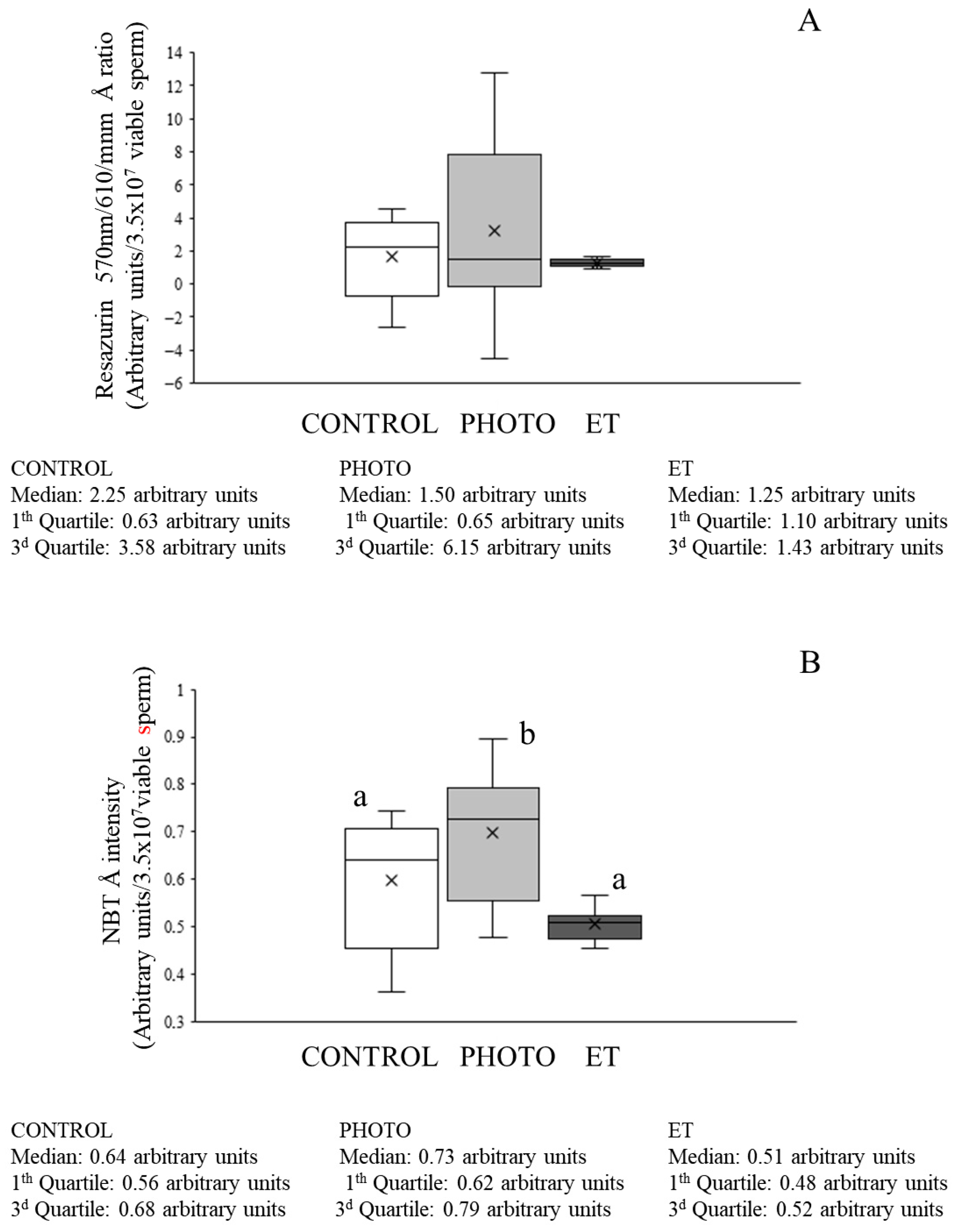
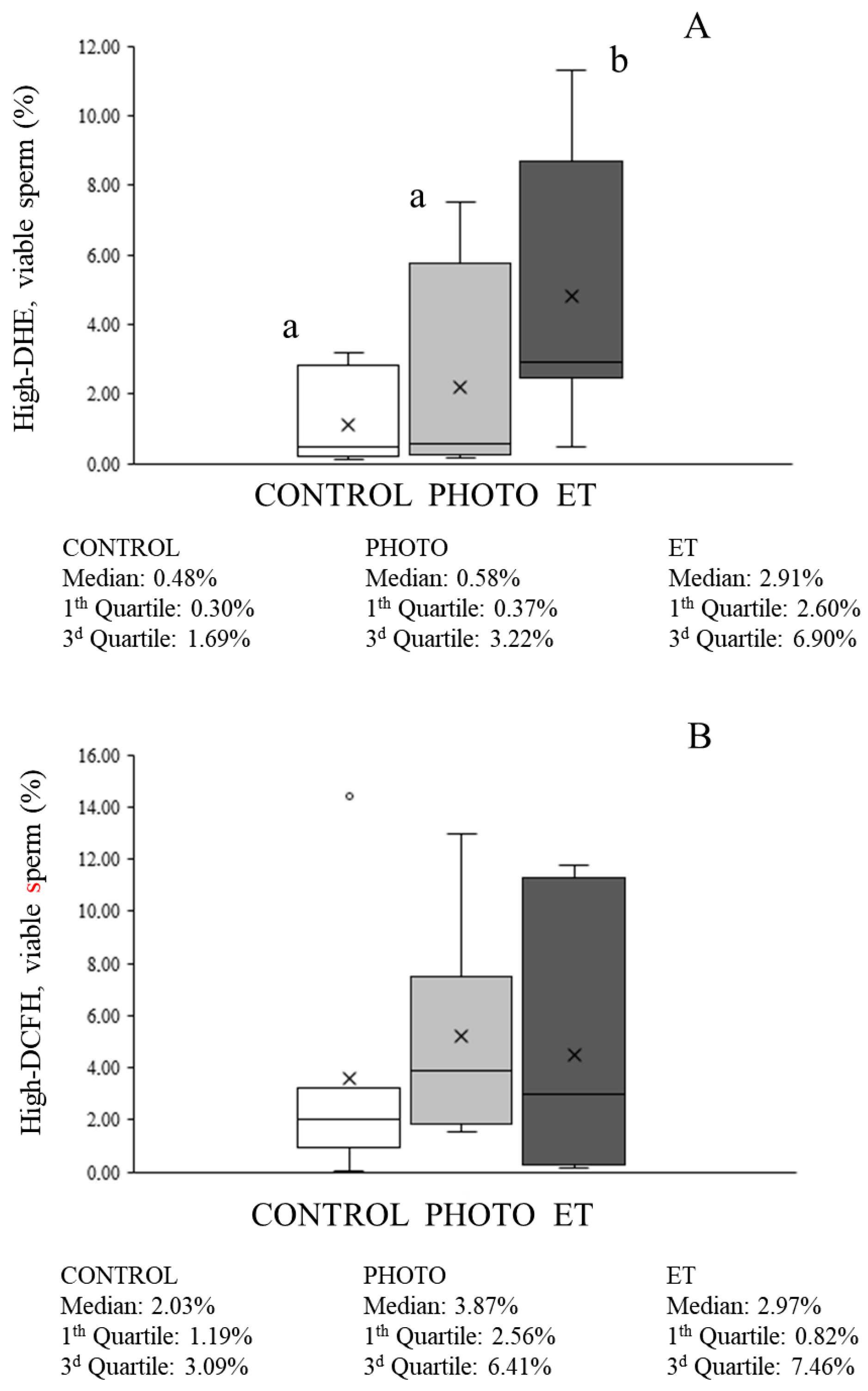
3.6. Effects of Red-Light Irradiation on ROS Levels and Overall Antioxidant Capacity
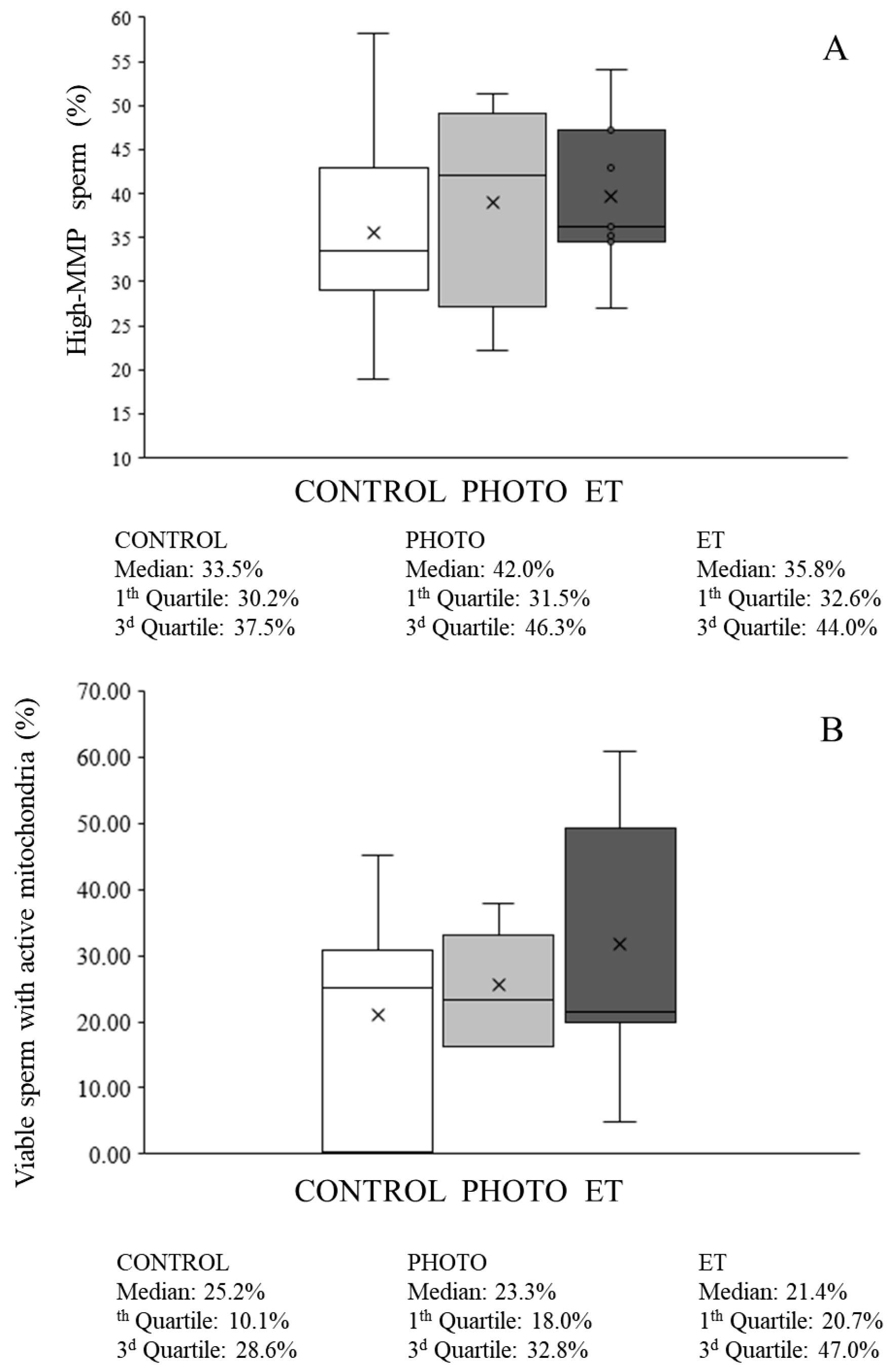
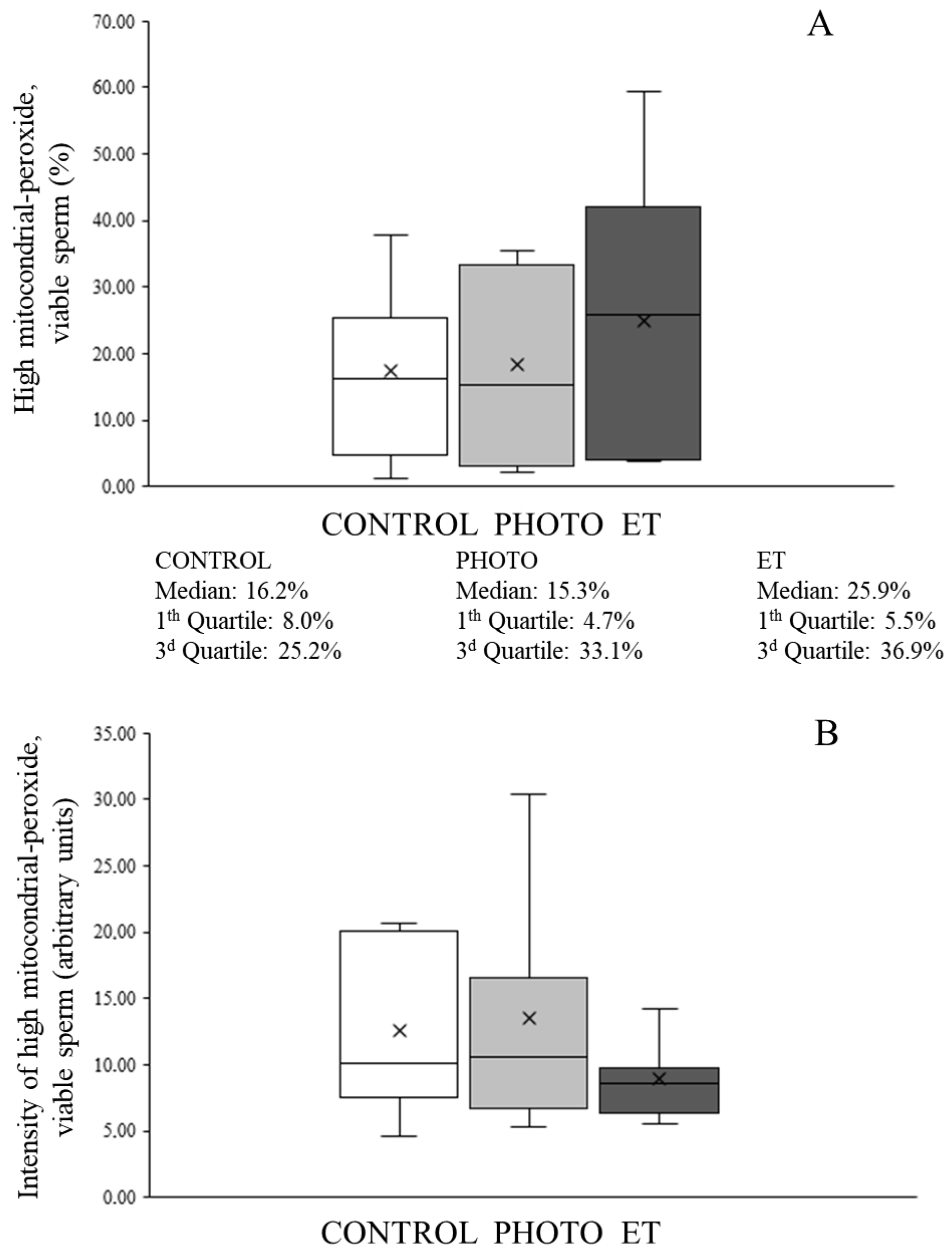
3.7. Effects of Red-Light Irradiation on Mitochondrial Function
3.8. Effects of Red-Light Irradiation on In Vivo Reproductive Performance
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foote, R.H.; Parks, J.E. Factors affecting preservation and fertility of bull sperm: A brief review. Reprod. Fertil. Dev. 1993, 5, 665–673. [Google Scholar] [CrossRef]
- Yánez-Ortiz, I.; Catalán, J.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 2022, 246, 106904. [Google Scholar] [CrossRef] [PubMed]
- Ugur, M.R.; Saber Abdelrahman, A.; Evans, H.C.; Gilmore, A.A.; Hitit, M.; Arifiantini, R.I.; Purwantara, B.; Kaya, A.; Memili, E. Advances in cryopreservation of bull sperm. Front. Vet. Sci. 2019, 6, 268. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Maxwell, W.M. A review on reproductive biotechnologies for conservation of endangered mammalian species. Anim. Reprod. Sci. 2007, 99, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Foote, R.H. Review: Dairy cattle reproductive physiology research and management—Past progress and future prospects. J. Dairy Sci. 1996, 79, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Holden, S.A.; Fernandez-Fuertes, B.; Murphy, C.; Whelan, H.; O’Gorman, A.; Brennan, L.; Butler, S.T.; Lonergan, P.; Fair, S. Relationship between in vitro sperm functional assessments, seminal plasma composition, and field fertility after AI with either non-sorted or sex-sorted bull semen. Theriogenology 2017, 87, 221–228. [Google Scholar] [CrossRef]
- Barth, A.D. Factors affecting fertility with artificial insemination. Vet. Clin. N. Am. Food Anim. Pract. 1993, 9, 275–289. [Google Scholar] [CrossRef]
- Pezo, F.; Zambrano, F.; Uribe, P.; Ramírez-Reveco, A.; Romero, F.; Sánchez, R. LED-based red light photostimulation improves short-term response of cooled boar semen exposed to thermal stress at 37 °C. Andrologia 2019, 51, e13237. [Google Scholar] [CrossRef]
- Catalán, J.; Llavanera, M.; Bonilla-Correal, S.; Papas, M.; Gacem, S.; Rodríguez-Gil, J.E.; Yeste, M.; Miró, J. Irradiating frozen-thawed stallion sperm with red-light increases their resilience to withstand post-thaw incubation at 38 °C. Theriogenology 2020, 157, 85–95. [Google Scholar] [CrossRef]
- Blanco Prieto, O.; Catalán, J.; Lleonart, M.; Bonet, S.; Yeste, M.; Rodríguez-Gil, J.E. Red-light stimulation of boar semen prior to artificial insemination improves field fertility in farms: A worldwide survey. Reprod. Domest. Anim. 2019, 54, 1145–1148. [Google Scholar] [CrossRef]
- Crespo, S.; Martínez, M.; Gadea, J. Photo stimulation of seminal doses with red LED light from Duroc boars and resultant fertility in Iberian sows. Animals 2021, 11, 1656. [Google Scholar] [CrossRef]
- Deng, J.; Bezold, D.; Jessen, H.J.; Walther, A. Multiple light control mechanisms in ATP-fueled non-equilibrium DNA systems. Angew. Chem. Int. Ed. Engl. 2020, 59, 12084–12092. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cerezales, S.; Boryshpolets, S.; Afanzar, O.; Eisenbach, M. Involvement of opsins in mammalian sperm thermotaxis. Sci. Rep. 2015, 5, 16146. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Prieto, O.; Maside, C.; Peña, A.; Ibáñez-Príncep, J.; Bonet, S.; Yeste, M.; Rodríguez-Gil, J.E. The effects of red LED light on pig sperm function rely upon mitochondrial electron chain activity rather than on a PKC-mediated mechanism. Front. Cell Dev. Biol. 2022, 10, 930855. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.; Papas, M.; Gacem, S.; Mateo-Otero, Y.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Red-light irradiation of horse spermatozoa increases mitochondrial activity and motility through changes in the motile sperm subpopulation structure. Biology 2020, 9, 254. [Google Scholar] [CrossRef]
- Catalán, J.; Papas, M.; Trujillo-Rojas, L.; Blanco-Prieto, O.; Bonilla-Correal, S.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Red LED light acts on the mitochondrial electron chain of donkey sperm and its effects depend on the time of exposure to light. Front. Cell Dev. Biol. 2020, 8, 8588261. [Google Scholar] [CrossRef]
- Córdova, A.; Strobel, P.; Vallejo, A.; Valenzuela, P.; Ulloa, O.; Burgos, R.A.; Menarim, B.; Rodríguez-Gil, J.E.; Ratto, M.; Ramírez-Reveco, A. Use of hypometabolic TRIS extenders and high cooling rate refrigeration for cryopreservation of stallion sperm: Presence and sensitivity of 5′ AMP-activated protein kinase (AMPK). Cryobiology 2014, 69, 473–481. [Google Scholar] [CrossRef]
- Larson, J.L.; Miller, D.J. Simple histochemical stain for acrosomes on sperm from several species. Mol. Reprod. Dev. 1999, 52, 445–449. [Google Scholar] [CrossRef]
- Ramírez-Reveco, A.; Hernández, J.L.; Aros, P. Long-term storing of frozen semen at −196 °C does not affect the post-thaw sperm quality of bull semen. In Cryopreservation in Eukaryots; Marco-Jiménez, F., Akdemir, H., Eds.; IntechOpen: London, UK, 2016; Chapter 6; pp. 91–102. [Google Scholar] [CrossRef]
- Miguel-Jimenez, S.; Rivera Del Alamo, M.M.; Álvarez-Rodríguez, M.; Hidalgo, C.O.; Peña, A.I.; Muiño, R.; Rodríguez-Gil, J.E.; Mogas, T. In vitro assessment of egg yolk-, soya bean lecithin- and liposome-based extenders for cryopreservation of dairy bull semen. Anim. Reprod. Sci. 2020, 215, 106315. [Google Scholar] [CrossRef]
- Ijiri, T.W.; Vadnais, M.L.; Huang, A.P.; Lin, A.M.; Levin, L.R.; Buck, J.; Gerton, G.L. Thiol changes during epididymal maturation: A link to flagellar angulation in mouse spermatozoa? Andrology 2014, 2, 65–75. [Google Scholar] [CrossRef]
- Flores, E.; Ramió-Lluch, L.; Bucci, D.; Fernández-Novell, J.M.; Peña, A.; Rodríguez-Gil, J.E. Freezing-thawing induces alterations in histone H1-DNA binding and the breaking of protein-DNA disulfide bonds in boar sperm. Theriogenology 2011, 76, 1450–1464. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Garcia-Bonavila, E.; Hidalgo, C.O.; Catalán, J.; Miró, J.; Yeste, M. Species-specific differences in sperm chromatin decondensation between eutherian mammals underlie distinct lysis requirements. Front. Cell Dev. Biol. 2012, 9, 669182. [Google Scholar] [CrossRef]
- Zrimšek, P.; Kunc, J.; Kosec, M.; Mrkun, J. Spectrophotometric application of resazurin reduction assay to evaluate boar semen quality. Int. J. Androl. 2004, 27, 57–62. [Google Scholar] [CrossRef]
- Tunc, O.; Thompson, J.; Tremellen, K. Development of the NBT assay as a marker of sperm oxidative stress. Int. J. Androl. 2010, 33, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Spidlen, J.; Boyce, K.; Cai, J.; Crosbie, N.; Dalphin, M.; Furlong, J.; Gasparetto, M.; Goldberg, M.; Goralczyk, E.M.; et al. MIFlowCyt: The minimum information about a flow cytometry experiment. Cytometry 2008, 73A, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Petrunkina, A.M.; Waberski, D.; Bollwein, H.; Sieme, H. Identifying non-sperm particles during flow cytometric physiological assessment: A simple approach. Theriogenology 2010, 73, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Yeste, M.; Rivera Del Alamo, M.M.; Domingo, J.; Casao, A.; Rodriguez-Gil, J.E.; Pérez-Pé, R.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Effect of seminal plasma proteins on the motile sperm subpopulations in ram ejaculates. Reprod. Fertil. Dev. 2017, 29, 394–405. [Google Scholar] [CrossRef]
- Riva, N.S.; Ruhlmann, C.; Iaizzo, R.S.; Marcial López, C.A.; Martínez, A.G. Comparative analysis between slow freezing and ultra-rapid freezing for human sperm cryopreservation. JBRA Assist. Reprod. 2018, 22, 331–337. [Google Scholar] [CrossRef]
- Ruddle, A.C.; George, S.J.; Armitage, W.J.; Alexander, E.L.; Mitchell, D.C. A simplified technique for the cryopreservation of vein allografts. Eur. J. Vasc. Endovasc. Surg. 2000, 19, 233–237. [Google Scholar] [CrossRef][Green Version]
- Fang, Y.; Zhao, C.; Xiang, H.; Jia, G.; Zhong, R. Melatonin improves cryopreservation of ram sperm by inhibiting mitochondrial permeability transition pore opening. Reprod. Domest. Anim. 2020, 55, 1240–1249. [Google Scholar] [CrossRef]
- Pegg, D.E. The history and principles of cryopreservation. Semin. Reprod. Med. 2002, 20, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Prieto, O.; Catalán, J.; Rojas, L.T.; Delgado-Bermúdez, A.; Llavanera, M.; Rigau, T.; Bonet, S.; Yeste, M.; Rivera Del Álamo, M.M.; Rodríguez-Gil, J.E. Medium-term effects of the diluted pig semen irradiation with red LED light on the integrity of nucleoprotein structure and resilience to withstand thermal stress. Theriogenology 2020, 157, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.; Papas, M.; Gacem, S.; Noto, F.; Delgado-Bermúdez, A.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Effects of red-light irradiation on the function and survival of fresh and liquid-stored donkey semen. Theriogenology 2020, 149, 88–97. [Google Scholar] [CrossRef]
- Blanco-Prieto, O.; Catalán, J.; Trujillo-Rojas, L.; Peña, A.; Rivera Del Álamo, M.M.; Llavanera, M.; Bonet, S.; Fernández-Novell, J.M.; Yeste, M.; Rodríguez-Gil, J.E. Red LED light acts on the mitochondrial electron chain of mammalian sperm via light-time exposure-dependent mechanisms. Cells 2020, 9, 2546. [Google Scholar] [CrossRef]
- Hallap, T.; Nagy, S.; Jaakma, U.; Johannisson, A.; Rodriguez-Martinez, H. Mitochondrial activity of frozen-thawed spermatozoa assessed by MitoTracker Deep Red 633. Theriogenology 2005, 63, 2311–2322. [Google Scholar] [CrossRef] [PubMed]
- Varela, E.; Rojas, M.; Restrepo, G. Membrane stability and mitochondrial activity of bovine sperm frozen with low-density lipoproteins and trehalose. Reprod. Domest. Anim. 2020, 55, 146–153. [Google Scholar] [CrossRef]
- Algieri, C.; Blanco-Prieto, O.; Llavanera, M.; Yeste, M.; Spinaci, M.; Mari, G.; Bucci, D.; Nesci, S. Effects of cryopreservation on the mitochondrial bioenergetics of bovine sperm. Reprod. Domest. Anim. 2003, 58, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Bulkeley, E.A.; Foutouhi, A.; Wigney, K.; Santistevan, A.C.; Collins, C.; McNabb, B.; Meyers, S. Effects from disruption of mitochondrial electron transport chain function on bull sperm motility. Theriogenology 2021, 176, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Prieto, O.; Mislei, B.; Martínez-Pastor, F.; Spinaci, M.G.; Mari, G.; Bucci, D. Study of mitochondrial function in thawed bull spermatozoa using selective electron transfer chain inhibidors. Theriogenology 2023, 208, 8–14. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pastor, F. What is the importance of sperm subpopulations? Anim. Reprod. Sci. 2022, 46, 106844. [Google Scholar] [CrossRef]
- Carvajal, M.A.; Alaniz, A.J.; Gutiérrez-Gómez, C.; Vergara, P.M.; Sejian, V.; Bozinovic, F. Increasing importance of heat stress for cattle farming under future global climate scenarios. Sci. Total Environ. 2021, 801, 149661. [Google Scholar] [CrossRef]
- Ritter, C.; Beaver, A.; von Keyserlingk, M.A.G. The complex relationship between welfare and reproduction in cattle. Reprod. Domest. Anim. 2019, 54 (Suppl. S3), 29–37. [Google Scholar] [CrossRef] [PubMed]
- Beever, D.E.; Wathes, D.C.; Taylor, V.J. Nutritional implications on the fertility of high yielding dairy cows. Cattle Pract. 2004, 12, 31–39. [Google Scholar]
- Januskauskas, A.; Johannisson, A.; Rodriguez-Martinez, H. Subtle membrane changes in cryopreserved bull semen in relation with sperm viability, chromatin structure, and field fertility. Theriogenology 2003, 60, 743–758. [Google Scholar] [CrossRef]
- Gliozzi, T.M.; Turri, F.; Manes, S.; Cassinelli, C.; Pizzi, F. The combination of kinetic and flow cytometric semen parameters as a tool to predict fertility in cryopreserved bull semen. Animal 2017, 11, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Hawk, H.W. Transport and fate of spermatozoa after insemination of cattle. J. Dairy Sci. 1987, 70, 1487–1503. [Google Scholar] [CrossRef]
- Yeste, M.; Codony, F.; Estrada, E.; Lleonart, M.; Balasch, S.; Peña, A.; Bonet, S.; Rodríguez-Gil, J.E. Specific LED-based red light photo-stimulation procedures improve overall sperm function and reproductive performance of boar ejaculates. Sci. Rep. 2016, 6, 22569. [Google Scholar] [CrossRef]
| CONTROL | PHOTO | ET | |
|---|---|---|---|
| VCL (µm/s) | 104.2 ± 0.6 a | 98.6 ± 0.7 c | 51.1 ± 1.9 b |
| VSL (µm/s) | 50.2 ± 0.7 a | 41.8 ± 0.8 c | 25.9 ± 1.0 b |
| VAP (µm/s) | 63.7 ± 0.6 a | 54.0 ± 0.7 c | 32.4 ± 1.1 b |
| LIN (%) | 50.6 ± 0.8 a | 45.4 ± 0.9 b | 50.9 ± 1.0 a |
| STR (%) | 77.1 ± 0.7 | 75.3 ± 0.8 | 79.8 ± 0.5 |
| WOB (%) | 63.2 ± 0.6 a | 57.8 ± 0.7 b | 63.4 ± 0.9 a |
| mALH (µm) | 4.16 ± 0.02 a | 4.45 ± 0.02 c | 2.38 ± 0.07 b |
| BCF (Hz) | 10.4 ± 0.2 a | 9.2 ± 0.2 b | 9.4 ± 0.2 ab |
| DNC (µm2/s) | 182.9 ± 22.0 a | 165.5 ± 10.9 a | 123.2 ± 8.3 b |
| mDNC (%xµm) | 143.6 ± 11.0 a | 141.2 ± 4.7 a | 121.0 ± 2.51 b |
| Subpopulation 1 | Subpopulation 2 | Subpopulation 3 | Subpopulation 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CONTROL | PHOTO | ET | CONTROL | PHOTO | ET | CONTROL | PHOTO | ET | CONTROL | PHOTO | ET | |
| VCL (µm/s) | 39.9 ± 0.3 a | 38.8 ± 0.4 a | 39.9 ± 0.9 ª | 86.4 ± 0.5 a | 82.0 ± 0.5 b | 73.4 ± 0.3 c | 125.6 ± 0.8 a | 116.5 ± 1.1 b | 114.2 ± 1.0 b | 161.1 ± 1.9 a | 157.3 ± 2.4 a | 217.2 ± 3.0 b |
| VSL (µm/s) | 23.3 ± 0.4 a | 21.5 ± 0.5 a | 20.3 ± 0.4 b | 53.2 ± 0.5 a | 44.9 ± 0.6 b | 39.8 ± 0.4 c | 66.0 ± 1.0 a | 51.4 ± 1.3 b | 48.2 ± 1.4 b | 54.3 ± 2.3 a | 49.2 ± 2.8 a | 24.5 ± 2.0 b |
| VAP (µm/s) | 28.2 ± 0.4 ª | 26.4 ± 0.4 ab | 25.9 ± 0.4 b | 60.0 ± 0.5 a | 51.7 ± 0.6 b | 47.6 ± 0.4 c | 77.7 ± 0.9 a | 63.1 ± 1.2 b | 63.5 ± 1.2 b | 82.9 ± 2.0 a | 74.9 ± 2.5 a | 49.5 ± 1.5 b |
| LIN (%) | 55.7 ± 0.5 a | 54.1 ± 0.5 a | 48.0 ± 0.6 b | 60.1 ± 0.6 a | 53.9 ± 0.7 b | 53.8 ± 0.7 b | 51.0 ± 1.2 a | 43.0 ± 1.5 b | 41.4 ± 1.4 b | 33.4 ± 2.6 a | 30.7 ± 3.3 a | 11.3 ± 1.2 b |
| STR (%) | 76.2 ± 0.4 a | 75.0 ± 0.5 a | 70.5 ± 0.4 b | 86.1 ± 0.6 a | 84.2 ± 0.6 a | 81.3 ± 0.5 b | 82.4 ± 1.0 a | 78.9 ± 1.3 a | 73.5 ± 1.12 b | 64.1 ± 2.4 a | 63.1 ± 3.0 a | 49.5 ± 1.4 b |
| WOB (%) | 70.1 ± 0.3 a | 68.5 ± 0.4 a | 63.2 ± 0.4 b | 68.3 ± 0.5 a | 62.4 ± 0.5 b | 64.6 ± 0.5 b | 60.6 ± 0.8 a | 53.2 ± 1.1 b | 55.2 ± 1.2 b | 50.8 ± 1.9 a | 46.9 ± 2.4 a | 22.8 ± 1.6 b |
| mALH (µm) | 1.86 o ± 0.01 a | 1.91 ± 0.02 a | 1.97 ± 0.03 a | 3.22 ± 0.02 a | 3.42 ± 0.02 b | 3.20 ± 0.02 ª | 4.83 ± 0.03 a | 5.19 ± 0.04 b | 5.01 ± 0.05 b | 6.97 ± 0.08 a | 7.27 ± 0.10 a | 13.85 ± 0.15 b |
| BCF (Hz) | 6.9 ± 0.1 a | 6.2 ± 0.1 b | 6.6 ± 0.2 ab | 11.3 ± 0.1 a | 10.6 ± 0.1 b | 9.9 ± 0.1 b | 12.2 ± 0.2 a | 10.3 ± 0.3 b | 10.1 ± 0.4 b | 10.3 ± 0.5 a | 9.7 ± 0.6 a | 1.0 ± 0.1 b |
| DNC (µm2/s) | 78.6 ± 1.5 a | 79.3 ± 1.7 a | 81.1 ± 2.0 a | 277.3 ± 2.0 a | 279.8 ± 2.3 a | 236.8 ± 2.0 b | 600.6 ± 3.7 a | 599.5 ± 4.8 ab | 576.3 ± 4.5 b | 1120.9 ± 8.4 a | 1140.1 ± 10.6 a | 3007.7 ± 22.1 b |
| mDNC (%xµm) | 97.8 ± 1.0 a | 94.4 ± 1.1 a | 88.7 ± 1.3 b | 183.7 ± 1.3 a | 175.3 ± 1.5 b | 164.1 ± 1.5 b | 235.0 ± 2.5 a | 214.3 ± 3.2 b | 201.8 ± 3.4 c | 220.9 ± 5.6 a | 215.5 ± 7.1 a | 156.1 ± 6.7 b |
| Trial | Geographical Coordinates of Farm | Altitude Above the Sea (m) | Temperature (°C) | Relative Humidity (%) | Animal Type | n Control | n Photo | PR Control (%) | PR Photo (%) | Variation in NRRs (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (Farm 1) | 40°54′36″ S/73°36′58″ W | 214 | 19 | 80 | Angus cows | 81 | 35 | 45.7 (37/81) | 60.0 (21/35) | +31.3 |
| 2 (Farm 2) | 39°46′42″ S/73°14′30″ O | 15 | 17 | 85 | Dairy Holstein cows | 113 | 65 | 52.2 (59/113) | 66.1 (43/65) | +26.6 |
| Overall | - | - | - | - | - | 194 | 100 | 49.4 (96/194) | 64.0 (64/100) | +29.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Prieto, O.; Maside, C.; Gruzmacher, A.; Ortiz, M.; Ratto, M.; Urra, F.J.; Vera, T.; Strobel, P.; Catalán, J.; Mislei, B.; et al. Red LED Light Irradiation Increases the Resistance Against Environmental Stress of Frozen Bovine Sperm Thawed in Suboptimal Conditions. Animals 2025, 15, 3353. https://doi.org/10.3390/ani15223353
Blanco-Prieto O, Maside C, Gruzmacher A, Ortiz M, Ratto M, Urra FJ, Vera T, Strobel P, Catalán J, Mislei B, et al. Red LED Light Irradiation Increases the Resistance Against Environmental Stress of Frozen Bovine Sperm Thawed in Suboptimal Conditions. Animals. 2025; 15(22):3353. https://doi.org/10.3390/ani15223353
Chicago/Turabian StyleBlanco-Prieto, Olga, Carolina Maside, Andrea Gruzmacher, Manuel Ortiz, Marcelo Ratto, Francisco Javier Urra, Tomás Vera, Pablo Strobel, Jaime Catalán, Beatrice Mislei, and et al. 2025. "Red LED Light Irradiation Increases the Resistance Against Environmental Stress of Frozen Bovine Sperm Thawed in Suboptimal Conditions" Animals 15, no. 22: 3353. https://doi.org/10.3390/ani15223353
APA StyleBlanco-Prieto, O., Maside, C., Gruzmacher, A., Ortiz, M., Ratto, M., Urra, F. J., Vera, T., Strobel, P., Catalán, J., Mislei, B., Bucci, D., Yeste, M., Rodríguez-Gil, J. E., & Ramírez-Reveco, A. (2025). Red LED Light Irradiation Increases the Resistance Against Environmental Stress of Frozen Bovine Sperm Thawed in Suboptimal Conditions. Animals, 15(22), 3353. https://doi.org/10.3390/ani15223353







