Transcriptome Profiling Reveals Stage-Specific Regulation of Lipid Metabolism in Orbital Fat of Bighead Carp (Hypophthalmichthys nobilis)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. RNA Extraction and Quality Evaluation
2.3. RNA Sequencing and Data Analysis
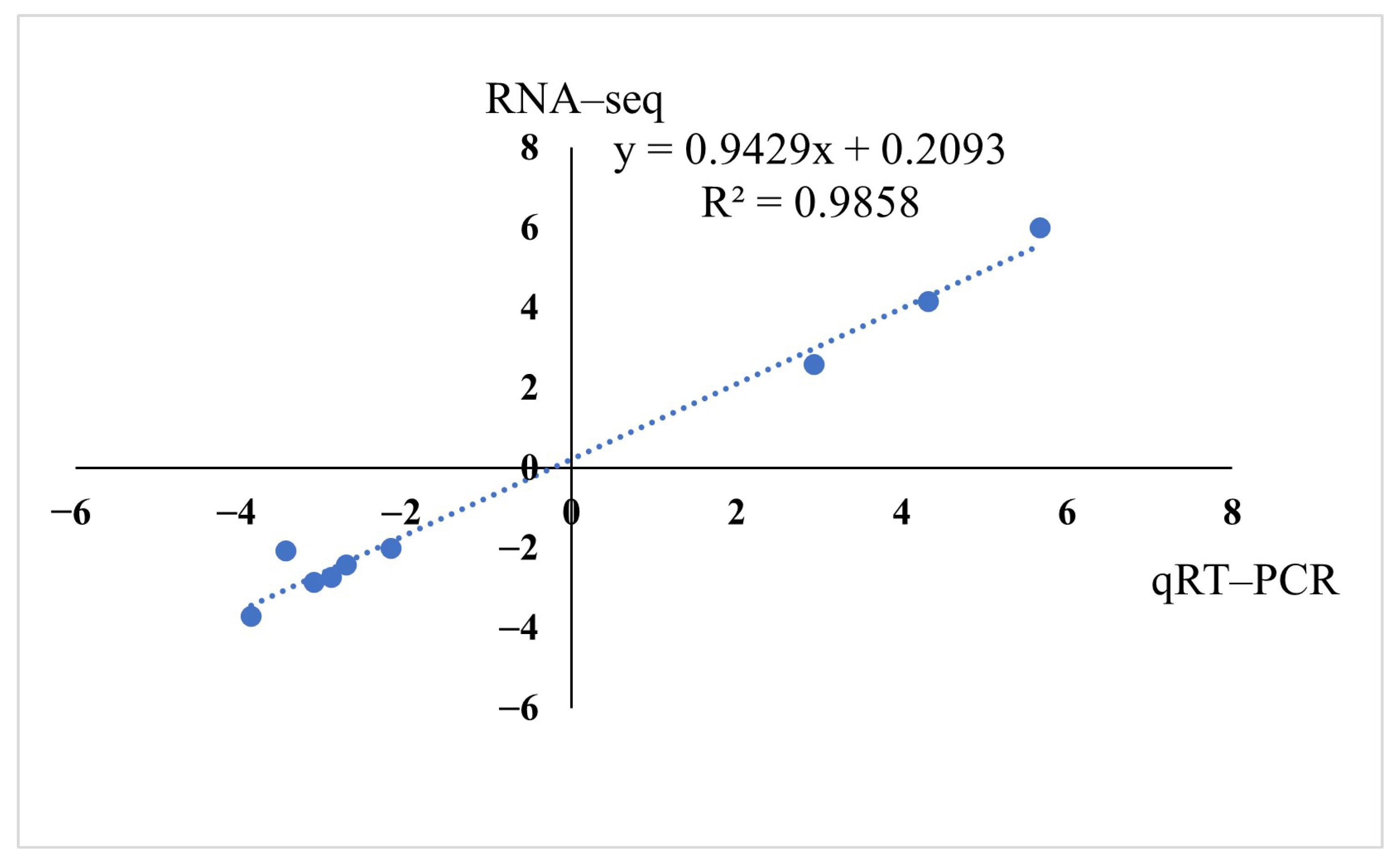
2.4. Validation of RNA-Seq Results by Quantitative Real-Time PCR (qRT-PCR)
3. Results
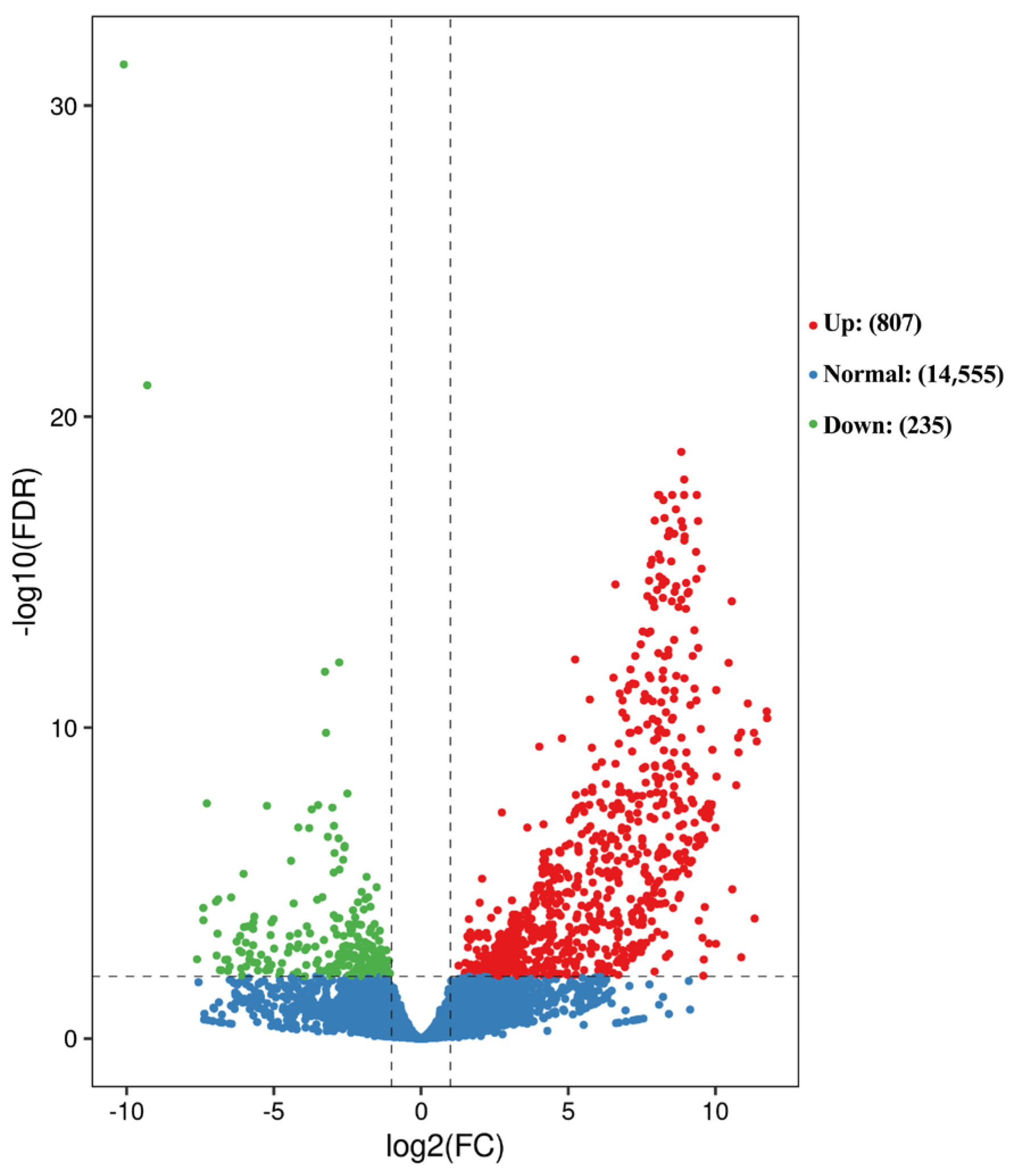
3.1. Transcriptome Sequencing
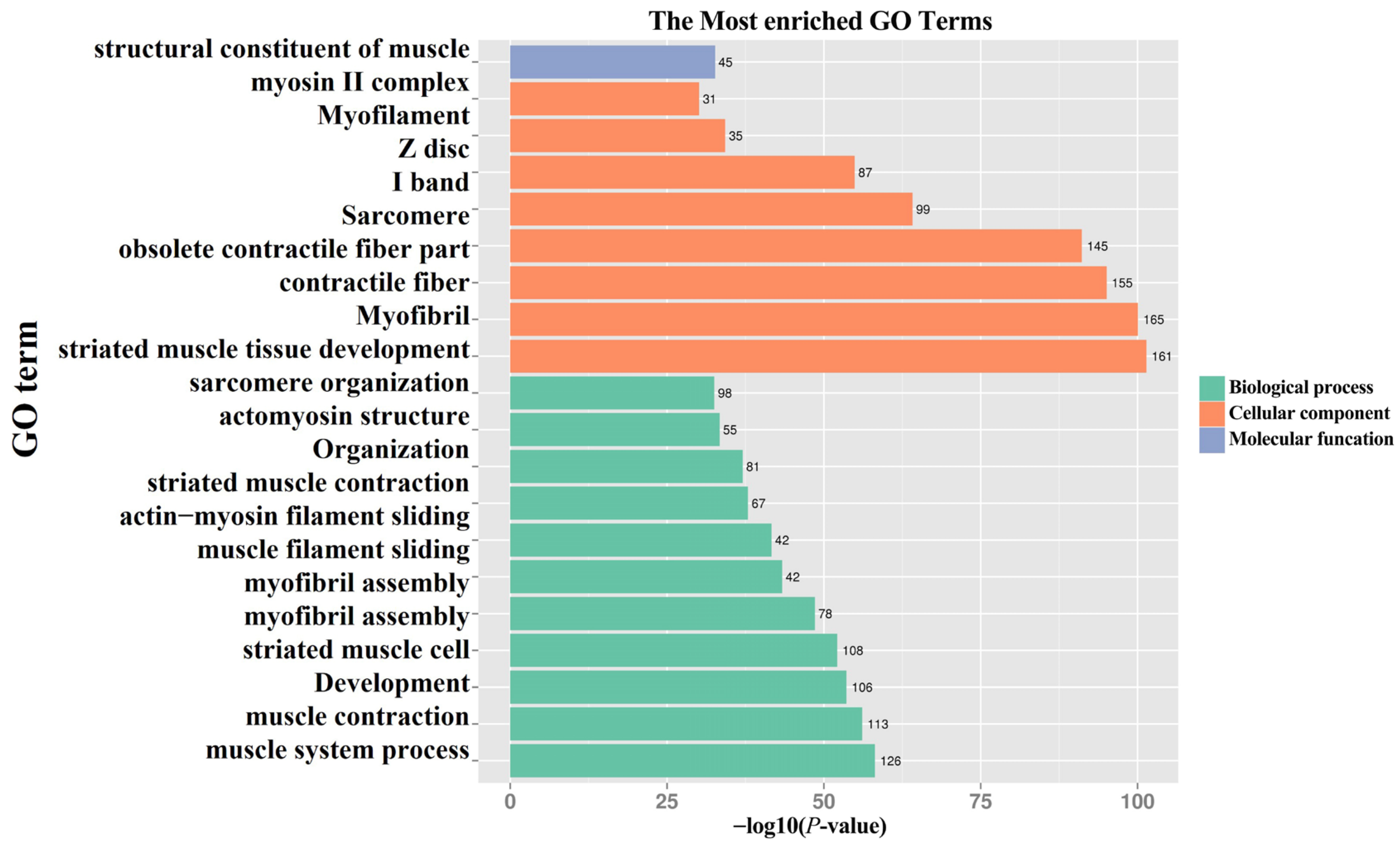
3.2. GO and KEGG Enrichment Analysis of Differentially Expressed Genes
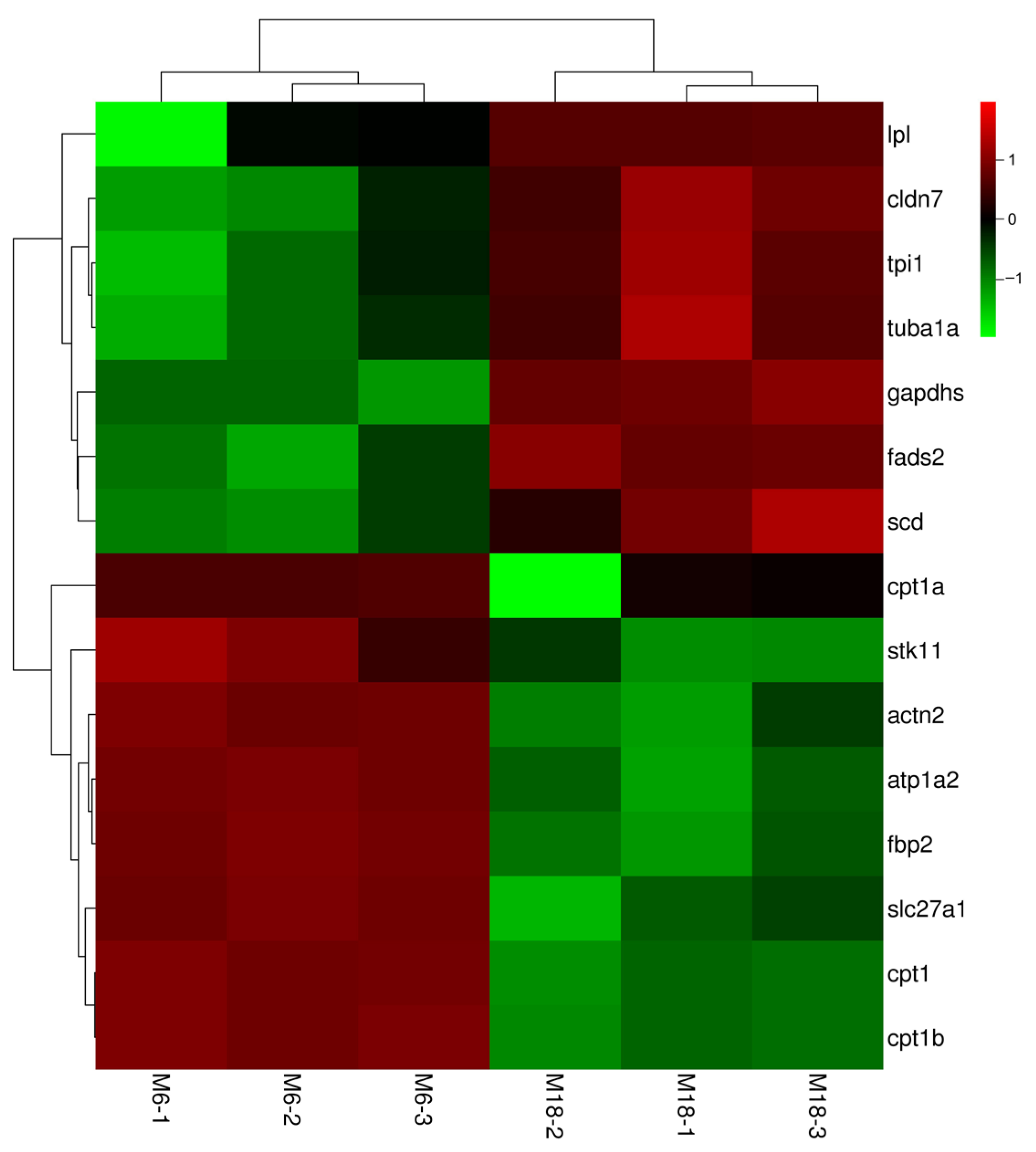
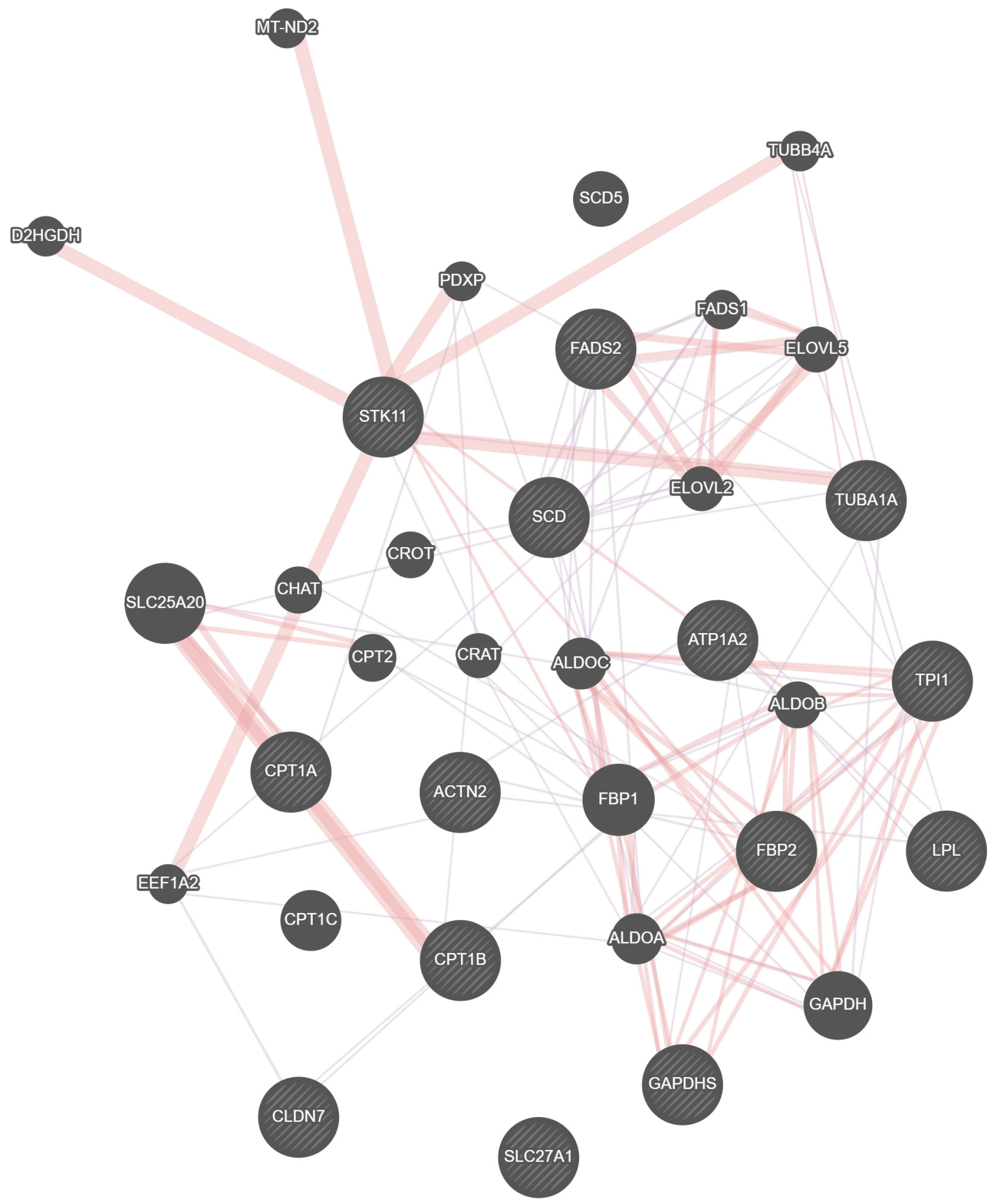
3.3. Important Candidate Genes Regulating the Growth and Development of Adipose Tissue in the Bighead’s Eye Socket
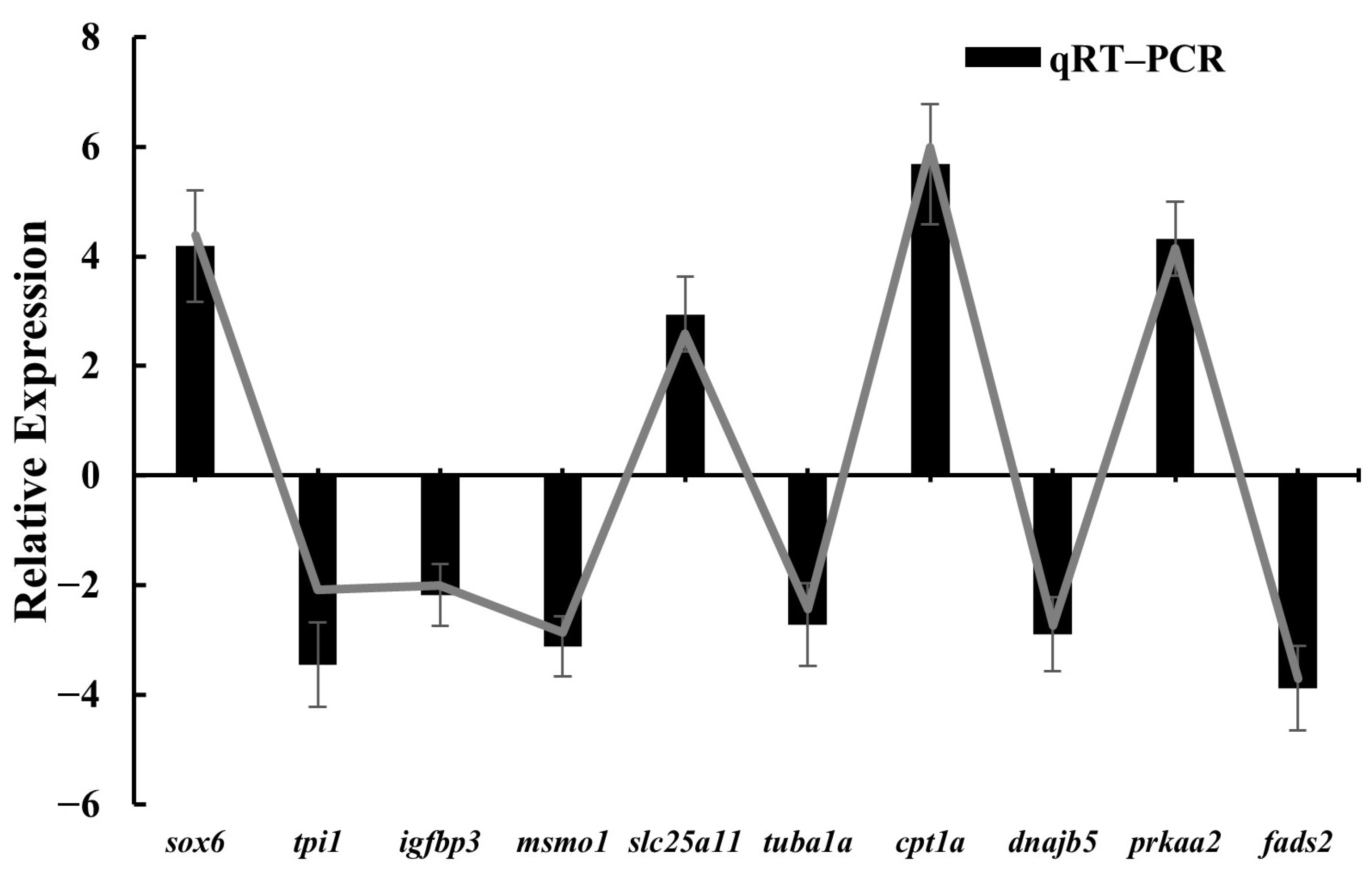
3.4. Validation of RNA-Seq Results by qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample ID | Raw Database | Clean Database | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|
| M6-1 | 6,871,532,810 | 6,846,104,587 | 96.87 | 91.91 | 46.97 |
| M6-2 | 7,485,026,392 | 7,468,839,798 | 96.80 | 91.73 | 47.73 |
| M6-3 | 7,616,530,578 | 7,563,379,410 | 96.79 | 91.79 | 46.35 |
| M18-1 | 6,431,992,260 | 6,382,998,824 | 96.53 | 91.19 | 45.37 |
| M18-2 | 6,100,950,998 | 6,090,612,188 | 97.03 | 92.31 | 48.38 |
| M18-3 | 5,914,123,710 | 5,903,172,099 | 96.83 | 91.79 | 46.60 |

References
- Tao, X.; Liang, Y.; Yang, X.M.; Pang, J.; Zhong, Z.; Chen, X.; Yang, Y.; Zeng, K.; Kang, R.; Lei, Y.; et al. Transcriptomic profiling in muscle and adipose tissue identifies genes related to growth and lipid deposition. PLoS ONE 2017, 12, e0184120. [Google Scholar] [CrossRef]
- Rule, D.C.; Thornton, J.H.; McGilliard, A.D.; Beitz, D.C. Effect of adipose-tissue site, animal size, and fasting on lipolysis in bovine adipose-tissue invitro. Int. J. Biochem. 1992, 24, 789–793. [Google Scholar] [CrossRef]
- Tian, J.J.; Lei, C.X.; Ji, H.; Zhou, J.S.; Xie, J. Dietary arachidonic acid decreases the expression of transcripts related to adipocyte development and chronic inflammation in the adipose tissue of juvenile grass carp, Ctenopharyngodon idella. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 30, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, C. Adipogenesis in fish. J. Exp. Biol. 2018, 221 (Suppl. S1), jeb161588. [Google Scholar] [CrossRef]
- Svetlana, M.; Zinaida, N.; Svetlana, P.; Alexey, V.; Denis, E.; Nina, N. Age-specific lipid and fatty acid profiles of atlantic salmon juveniles in the varzuga river. Int. J. Mol. Sci. 2016, 17, 1050. [Google Scholar] [CrossRef] [PubMed]
- Howe, P.R.; Downing, J.A.; Grenyer, B.F.; Grigonis-Deane, E.M.; Bryden, W.L. Tuna fishmeal as a source of DHA for n-3 PUFA enrichment of pork, chicken, and eggs. Lipids 2002, 37, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Tacon, A.G.J.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Xu, H.G.; Dong, X.J.; Ai, Q.H.; Mai, K.S.; Xu, W.; Zhang, Y.J.; Zuo, R.T. Regulation of tissue LC-PUFA contents, Δ6 fatty acyl desaturase (FADS2) gene expression and the methylation of the putative FADS2 gene promoter by different dietary fatty acid profiles in Japanese seabass (Lateolabrax japonicus). PLoS ONE 2014, 9, e87726. [Google Scholar] [CrossRef]
- Betancor, M.B.; Almaida-Pagán, P.F.; Hernández, A.; Tocher, D.R. Effects of dietary fatty acids on mitochondrial phospholipid compositions, oxidative status and mitochondrial gene expression of zebrafish at different ages. Fish Physiol. Biochem. 2015, 41, 1187–1204. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Saari, N.; Jahurul, H.A.; Abbas, K.A. Pufas in fish: Extraction, fractionation, importance in health. Compr. Rev. Food Sci. Food Saf. 2010, 8, 59–74. [Google Scholar] [CrossRef]
- Zhao, R.X. Current status of research on docosahexaenoic acid (DHA) mechanism in Japan. Mod. Fish. Inf. 1991, 8, 12–14. [Google Scholar]
- Sawada, T.; Takahashi, K.; Hatano, M. Molecular-species analysis of fish oil triglyceride by light-scattering mass detector equipped liquid-chromatography.2. triglyceride composition of tuna and bonito orbital fats. Nippon. Suisan Gakkaishi 1993, 59, 285–290. [Google Scholar] [CrossRef]
- Qian, X.; Ba, Y.; Zhuang, Q.F.; Zhong, G.F. RNA-Seq technology and its application in fish transcriptomics. Omics J. Integr. Biol. 2014, 18, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Ahi, E.P.; Verta, J.P.; Kurko, J.; Ruokolainen, A.; Singh, P.; Debes, P.V.; Primmer, C.R. Gene co-expression patterns in Atlantic salmon adipose tissue provide a molecular link among seasonal changes, energy balance and age at maturity. Mol. Ecol. 2024, 34, e17313. [Google Scholar] [CrossRef]
- Abasubong, K.P.; Adjoumani, J.J.Y.; Li, X.F.; Liu, W.B.; Jiang, G.Z. Dietary supplementation of glycyrrhetinic acid benefit growth performance and lipid metabolism in blunt snout bream (Megalobrama amblycephala) juveniles. Aquac. Nutr. 2021, 27, 407–416. [Google Scholar] [CrossRef]
- Tan, P.; Wabike, E.E.; Qin, G.; Lou, B.; Xu, D.; Chen, R.; Wang, L. Effects of dietary n-3 long-chain polyunsaturated fatty acids (n-3 LC-PUFAs) on growth performance, body composition and subcutaneous adipose tissue transcriptome analysis of juvenile yellow drum (Nibea albiflora). Aquac. Nutr. 2021, 27, 556–567. [Google Scholar] [CrossRef]
- Kohno, H.; Yamaguchi, N.; Ohdoi, C.; Nakajima, S.; Odashima, S.; Tanaka, T. Modifying effect of tuna orbital oil rich in docosahexaenoic acid and vitamin D-3 on azoxymethane-induced colonic aberrant crypt foci in rats. Oncol. Rep. 2000, 7, 1069–1074. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, P.; Pang, H.; Chen, T.; Zhang, G. Expression analysis of mRNA decay of maternal genes during bombyx mori maternal-to-zygotic transition. Int. J. Mol. Sci. 2019, 20, 5651. [Google Scholar] [CrossRef]
- Ramayo-Caldas, Y.; Mach, N.; Esteve-Codina, A.; Corominas, J.; Castelló, A.; Ballester, M.; Estellé, J.; Ibáñez-Escriche, N.; Fernández, A.I.; Pérez-Enciso, M.; et al. Liver transcriptome profile in pigs with extreme phenotypes of intramuscular fatty acid composition. BMC Genom. 2012, 13, 547. [Google Scholar] [CrossRef]
- Xing, K.; Zhu, F.; Zhai, L.; Liu, H.; Wang, Y.; Wang, Z.; Chen, S.; Hou, Z.; Wang, C. Integration of transcriptome and whole genomic resequencing data to identify key genes affecting swine fat deposition. PLoS ONE 2015, 10, e0122396. [Google Scholar] [CrossRef]
- Ohea, E.K.; Leveille, G.A. Significance of adipose tissue and liver as sites of fatty acid synthesis in pig and efficiency of utilization of various substrates for lipogenesis. J. Nutr. 1969, 99, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Haynie, K.R.; Vandanmagsar, B.; Wicks, S.E.; Zhang, J.; Mynatt, R.L. Inhibition of carnitine palymitoyltransferase1b induces cardiac hypertrophy and mortality in mice. Diabetes Obes. Metab. 2014, 16, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zou, X.; Zhao, M.; Guan, Q.Q.; Xuan, Z.Y.; Liu, L.S.; Gao, Z.X. Integrated transcriptome and metabolome analysis of liver reveals unsynchronized growth mechanisms in blunt-snout bream (Megalobrama amblycephala). BMC Genom. 2025, 26, 30. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, H.; Shi, B.; Jiang, Y.; Liu, X.; Hu, J. Transcriptional Regulation of the Bovine Fatty Acid Transport Protein 1 Gene by Kruppel-Like Factors 15. Animals 2019, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Xie, L.; Ma, J.E.W.; Zhang, L.; Chao, Z.; Chen, S.; Nie, Q.; Lin, Z.; Zhang, X. Lower expression of slc27a1 enhances intramuscular fat deposition in chicken via down-regulated fatty acid oxidation mediated by cpt1a. Front. Physiol. 2017, 8, 449. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Y.; Hua, Z.; Wu, A.; Pan, X.; Yang, J.; Wang, X. Muscle transcriptome analysis provides new insights into the growth gap between fast-and slow-growing Sinocyclocheilus grahami. Front. Genet. 2023, 14, 1217952. [Google Scholar] [CrossRef]
- Bange, E.; Marmarelis, M.E.; Hwang, W.T.; Yang, Y.X.; Thompson, J.C.; Rosenbaum, J.; Bauml, J.M.; Ciunci, C.; Alley, E.W.; Cohen, R.B.; et al. Impact of kras and tp53 co-mutations on outcomes after first-line systemic therapy among patients with stk11-mutated advanced non-small-cell lung cancer. JCO Precis. Oncol. 2019, 3, 1–11. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, H.; Wu, L.; Ru, H.; Wei, N.; Yao, F.; Li, Y. Copper and zinc treatments alter the thyroid endocrine system in zebrafish embryos/larvae. Toxics 2022, 10, 756. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Xu, H.; Xing, R.; Pan, Y.; Li, W.; Cui, J.; Zhang, H.; Lu, Y. Decreased fructose-1,6-bisphosphatase-2 expression promotes glycolysis and growth in gastric cancer cells. Mol. Cancer 2013, 12, 110. [Google Scholar] [CrossRef]
- Tsai, S.H.; Chang, E.Y.C.; Chang, Y.C.; Hee, S.W.; Tsai, Y.C.; Chang, T.J.; Chuang, L.M. Knockdown of RyR3 Enhances Adiponectin Expression Through an atf3-Dependent Pathway. Endocrinology 2013, 154, 1117–1129. [Google Scholar] [CrossRef]
- Ji, S.; Sun, J.; Bian, C.; Huang, X.; Chang, Z.; Yang, M.; Lu, R.-H.; Ji, H. cAMP-dependent protein kinase A in grass carp Ctenopharyngodon idella: Molecular characterization, gene structure, tissue distribution and mRNA expression in endoplasmic reticulum stress-induced adipocyte lipolysis. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 250, 110479. [Google Scholar] [CrossRef]
- Sepe, A.; Tchkonia, T.; Thomou, T.; Zamboni, M.; Kirkland, J.L. Aging and Regional Differences in Fat Cell Progenitors—A Mini-Review. Gerontology 2011, 57, 66–75. [Google Scholar] [CrossRef]
- Wang, Z.X.; Shang, P.; Li, Q.G.; Wang, L.Y.; Chamba, Y.Z.; Zhang, B.; Zhang, H.; Wu, C.X. iTRAQ-based proteomic analysis reveals key proteins affecting muscle growth and lipid deposition in pigs. Sci. Rep. 2017, 7, 46717. [Google Scholar] [CrossRef]
- Yang, J.; Li, Z.H.; Gan, X.D.; Gang, Z.; Gao, J.J.; Xiong, C.L.; Qiu, X.P.; Wang, X.B.; Yin, Z.; Zheng, F. Deletion of Pr130 Interrupts Cardiac Development in Zebrafish. Int. J. Mol. Sci. 2016, 17, 1746. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.L.; Lancaster, P.A.; DeSilva, U.; Horn, G.W.; Krehbiel, C.R. Coordinated gene expression between skeletal muscle and intramuscular adipose tissue in growing beef cattle. J. Anim. Sci. 2015, 93, 4302–4311. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, W.; Hammels, I.; Jenke, B.; Binczek, E.; Schmidt-Soltau, I.; Brodesser, S.; Thevis, M. Obesity resistance and deregulation of lipogenesis in Delta 6-fatty acid desaturase (FADS2) deficiency. EMBO Rep. 2014, 15, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Wang, C.; Lv, H.; Yu, J. The role of fatty acid desaturase 2 in multiple tumor types revealed by bulk and single-cell transcriptomes. Lipids Health Dis. 2023, 22, 25. [Google Scholar] [CrossRef]
- Garrido, D.; Kabeya, N.; Betancor, M.B.; Pérez, J.A.; Acosta, N.G.; Tocher, D.R.; Rodríguez, C.; Monroig, Ó. Functional diversification of teleost Fads2 fatty acyl desaturases occurs independently of the trophic level. Sci. Rep. 2019, 9, 11199. [Google Scholar] [CrossRef]
- Paton, C.M.; Ntambi, J.M. Biochemical and physiological function of stearoyl-CoA desaturase. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, 28–37. [Google Scholar] [CrossRef]
- Liu, P.; Ji, H.; Li, C.; Tian, J.; Wang, Y.; Yu, P. Ontogenetic development of adipose tissue in grass carp (Ctenopharyngodon idellus). Fish Physiol. Biochem. 2015, 41, 867–878. [Google Scholar] [CrossRef]
- Imrie, D.; Sadler, K.C. White adipose tissue development in zebrafish is regulated by both developmental time and fish size. Dev. Dyn. 2010, 239, 3013–3023. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Luo, Q.; Long, H.; Hu, Z.; Que, T.; Zhang, X.A.; Li, Z.; Wang, G.; Yi, L.; Liu, Z.; et al. Alpha-enolase as a potential cancer prognostic marker promotes cell growth, migration, and invasion in glioma. Mol. Cancer 2014, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.H.; Wang, G.S.; Ren, P.; Zhang, L.N.; Ai, Q.H.; Sun, Y.Z.; Han, F.; Wang, Z.Y. Integrated transcriptomics and metabolomics unveil key regulators of feed efficiency in Larimichthys crocea on fishmeal-free diets. Aquac. Nutr. 2025, 2147842. [Google Scholar] [CrossRef]
- Du, A.; Zhao, F.; Liu, Y.; Xu, L.; Chen, K.; Sun, D.; Han, B. Genetic polymorphisms of PKLR gene and their associations with milk production traits in Chinese Holstein cows. Front. Genet. 2022, 13, 1002706. [Google Scholar] [CrossRef]
- Ding, X.; Wang, L.; Chen, M.; Wu, Y.; Ge, S.; Li, J.; Fan, X.; Lin, M. Sperm-specific glycolysis enzyme glyceraldehyde-3-phosphate dehydrogenase regulated by transcription factor SOX10 to promote uveal melanoma tumorigenesis. Front. Cell Dev. Biol. 2021, 9, 610683. [Google Scholar] [CrossRef]

| Primers | Sequence (5′-3′) | Tm (°C) |
|---|---|---|
| Hyn-tpi1-qpcr-1F | CGGAATCACGGAGAAAGTTGT | 59 |
| Hyn-tpi1-qpcr-1R | AGGCTCATAAGCAAGGACCAC | |
| Hyn-igfbp3-qPCR-1F | TAAGGACGCCGTTAGAGAGC | 55 |
| Hyn-igfbp3-qPCR-1R | AGTTTGGAATGCGGAAGC | |
| Hyn-msmo1-qpcr-1F | TATTTCAGTTCCTCCCTTTCA | 55 |
| Hyn-msmo1-qpcr-1R | GCATGGGAGTCCCAGTCATAG | |
| Hyn-slc25a11-qPCR-1F | AGGCGAGTCACCTTGCTACA | 58 |
| Hyn-slc25a11-qPCR-1R | AATGGATTTGGGGCGAAGTTTTT | |
| Hyn-tuba1a-qPCR-1F | CGTGGTGCCCAAAGATGTG | 60 |
| Hyn-tuba1a-qPCR-1R | GGGAGGCTGGTAGTTGATGC | |
| Hyn-cpt1a-qPCR-1F | TTTTACGACGGACGGTTGC | 58 |
| Hyn-cpt1a-qPCR-1R | CTGCTTGTTCTTCCCACGAC | |
| Hyn-dnajb5-qPCR-1F | CTACGATGTTCTGACCGACCC | 59 |
| Hyn-dnajb5-qPCR-1R | CCACCCTTGTTACGGCTGA | |
| Hyn-prkaa2-qPCR-1F | ACAGCCCTAAGGCACGATG | 58 |
| Hyn-prkaa2-qPCR-1R | ACGGGTTCACCACTTTCCA | |
| Hyn-sox6-qPCR-1F | ACGGAGGTGAGGATGGATT | 56 |
| Hyn-sox6-qPCR-1R | GGAGGTTTGTTGTGGAGCA | |
| Hyn-fads2-qPCR-1F | AGCACGACTTCGGTCATCTATC | 58 |
| Hyn-fads2-qPCR-1R | GCACAGTTCCACTACAAACG | |
| Hynβ-actin-qPCR-F | TATCCTATTGAGCACGGTATTG | 57 |
| Hynβ-actin-qPCR-R | CCTGTTGGCTTTGGGATTC |
| Regulation | Name of Pathway | Pathway ID | Genes |
|---|---|---|---|
| up | Fatty acid metabolism | ko01212 | hadh, cpt1b, cpt1a, acsl1 |
| Tight junction | ko04530 | actn2, actn3, prkaa2, stk11 | |
| PPAR signaling pathway | ko03320 | slc27a1, sorbs1, adipoq | |
| Glycolysis/Gluconeogenesis | ko00010 | fbp2, gapdh, aldoa, pgm5 | |
| Cardiac muscle contraction | ko04260 | ryr3, atp1a2, actc1, myh1, myl3 | |
| Adrenergic signaling in cardiomyocytes | ko04261 | ppp2r3a, tpm2, cacna1s | |
| down | Fatty acid metabolism | ko01212 | hadha, hadhb, fads2 |
| Tight junction | ko04530 | tuba1a, tuba1c, cldn7 | |
| PPAR signaling pathway | ko03320 | scd, apoa1, lpl | |
| Glycolysis/Gluconeogenesis | ko00010 | fbp1, hk2, eno1, eno4, gapdhs, tpi1, pklr, ldhb, aldoc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Lei, Q.; Liu, J.; Sun, Z.; Yu, X.; Guo, X.; Tong, J. Transcriptome Profiling Reveals Stage-Specific Regulation of Lipid Metabolism in Orbital Fat of Bighead Carp (Hypophthalmichthys nobilis). Animals 2025, 15, 2602. https://doi.org/10.3390/ani15172602
Wang J, Lei Q, Liu J, Sun Z, Yu X, Guo X, Tong J. Transcriptome Profiling Reveals Stage-Specific Regulation of Lipid Metabolism in Orbital Fat of Bighead Carp (Hypophthalmichthys nobilis). Animals. 2025; 15(17):2602. https://doi.org/10.3390/ani15172602
Chicago/Turabian StyleWang, Junru, Qi Lei, Jun Liu, Zhiruo Sun, Xiaomu Yu, Xusheng Guo, and Jingou Tong. 2025. "Transcriptome Profiling Reveals Stage-Specific Regulation of Lipid Metabolism in Orbital Fat of Bighead Carp (Hypophthalmichthys nobilis)" Animals 15, no. 17: 2602. https://doi.org/10.3390/ani15172602
APA StyleWang, J., Lei, Q., Liu, J., Sun, Z., Yu, X., Guo, X., & Tong, J. (2025). Transcriptome Profiling Reveals Stage-Specific Regulation of Lipid Metabolism in Orbital Fat of Bighead Carp (Hypophthalmichthys nobilis). Animals, 15(17), 2602. https://doi.org/10.3390/ani15172602







