Nutritional Intervention with Antimicrobial Peptides Improves Growth Performance, Muscle Quality, Antioxidant Capacity, and Immune Function of Crucian Carp (Carassius auratus) Through TLR4/NF-κB Signaling Pathway
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Composition and Design
2.2. Feeding and Management
2.3. Tissue Collection
2.4. Muscle Quality Analysis
2.5. Antioxidant Capacity and Immune Indicators
2.6. Real-Time qPCR
2.7. Western Blot
2.8. Computation
2.9. Regression Modeling
3. Statistical Analysis
4. Results
4.1. Growth Performance
4.2. Visceral Organs Index
4.3. Muscle Quality
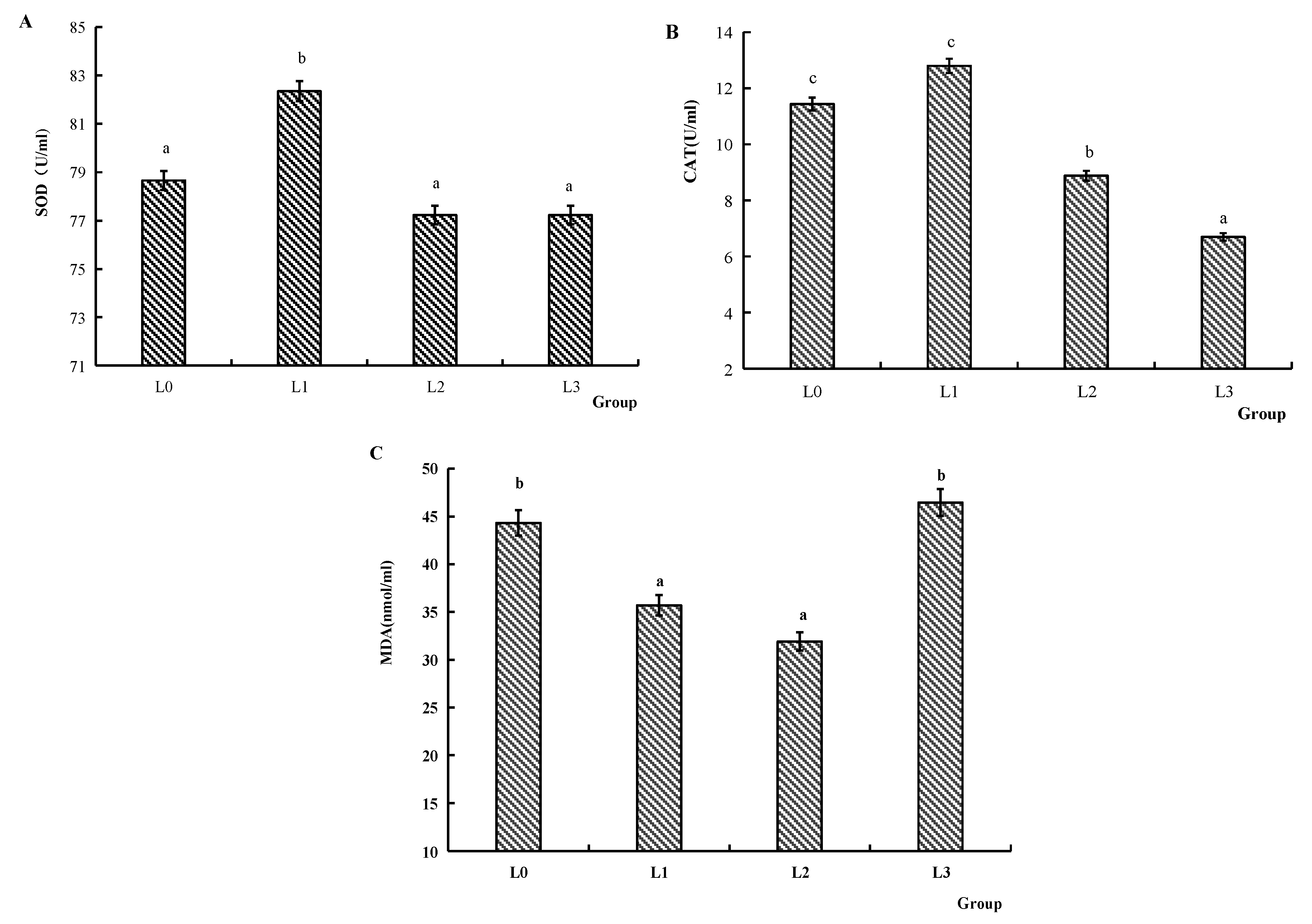
4.4. Antioxidant Abilities and Immune Function
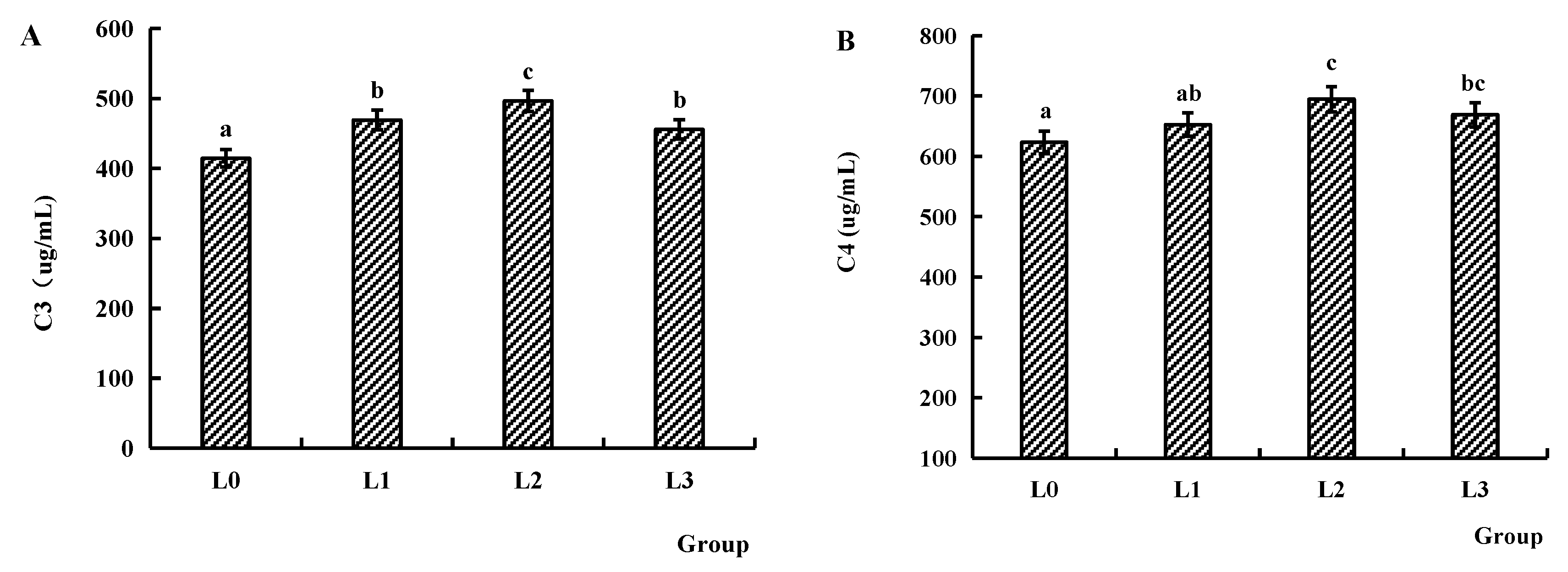
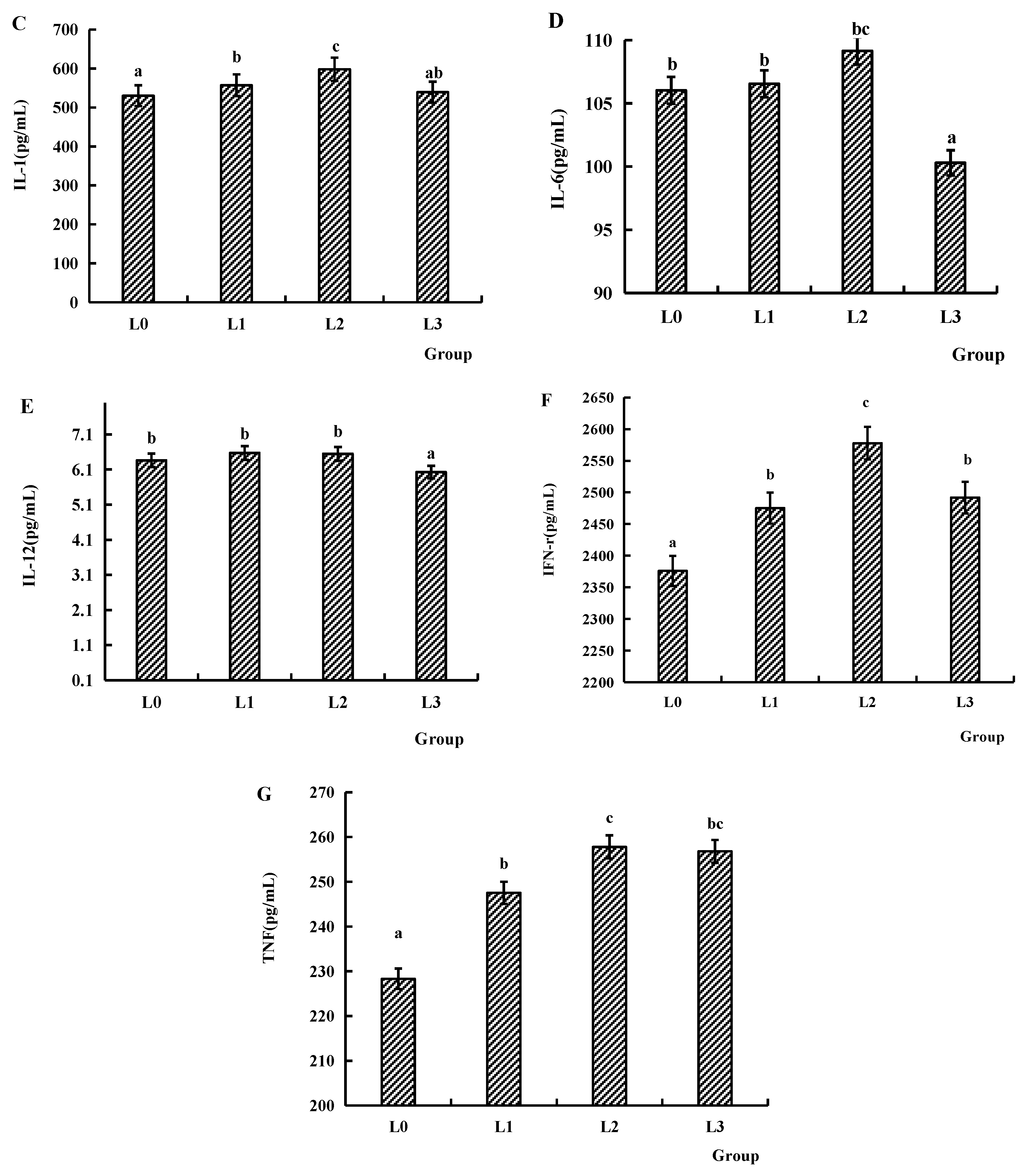
4.5. Immune Factors of Serum
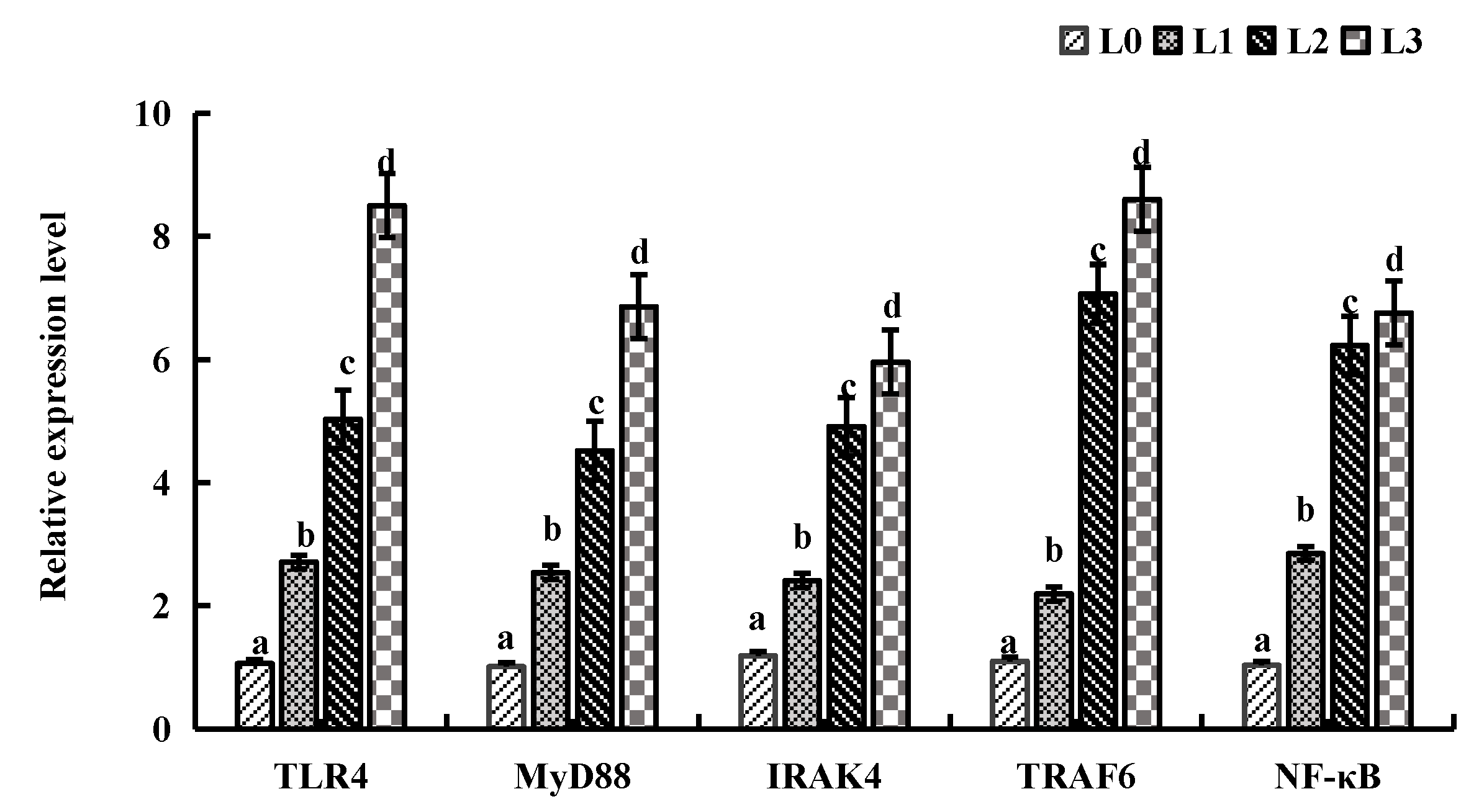
4.6. Key Gene Expression in Signaling Pathway
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, L.; Ramos, F. Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. Int. J. Antimicrob. Agents 2018, 52, 135–143. [Google Scholar] [CrossRef]
- Bao, L.S.; Chen, Y.H.; Li, H.H.; Zhang, J.S.; Wu, P.; Ye, K.; Ai, H.L.; Chu, W.Y. Dietary Ginkgo biloba leaf extract alters immune-related gene expression and disease resistance to Aeromonas hydrophila in common carp Cyprinus carpio. Fish Shellfish Immunol. 2019, 94, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.; Lymbery, A.; Song, S.K.; Hosseini Shekarabi, P. Adjuvant effects of medicinal herbs and probiotics for fish vaccines. Rev. Aquac. 2019, 11, 1325–1341. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Y.X.; Xie, K.; Zhang, J.Z.; Wang, Y.; Hu, Y.; Zhong, L. Sanguisnarine improves intestinal health in grass carp fed high-fat diets: Involvement of antioxidant, physical and immune barrier, and intestinal microbiota. Antioxidants 2023, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.B.; Hu, J.; Lv, Q.J.; Liu, L.J.; Wen, L.F.; Yang, X.K.; Zhao, H.H. The effects of Natucin C-Natucin P mixture on blood biochemical parameters, antioxidant activity and non-specific immune responses in tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2016, 55, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Noga, E.J.; Ullal, A.J.; Corrales, J.; Fernandes, J.M. Application of antimicrobial polypeptide host defenses to aquaculture: Exploitation of downregulation and upregulation responses. Comp. Biochem. Physiol. Part D Genom. Proteom. 2011, 6, 44–54. [Google Scholar] [CrossRef]
- Semreen, M.H.; El-Gamal, M.I.; Abdin, S.; Alkhazraji, H.; Kamal, L.; Hammad, S.; El-Awady, F.; Waleed, D.; Kourbaj, L. Recent updates of marine antimicrobial peptides. Saudi Pharm. J. 2018, 26, 396–409. [Google Scholar] [CrossRef]
- Ganz, T. The role of antimicrobial peptides in innate immunity. Integr. Comp. Biol. 2003, 43, 300–304. [Google Scholar] [CrossRef]
- Silveira, R.F.; Roque-Borda, C.A.; Vicente, E.F. Antimicrobial peptides as a feed additive alternative to animal production, food safety and public health implications: An overview. Anim. Nutr. 2021, 7, 896–904. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Huang, Y.; He, H.Q.; Deng, Y.J.; Deng, C.X.; Wen, L.F. Effects of Antimicrobial Peptides and Chinese Herbal Medicine Additives on Growth performance, Serum Biochemical indices andintestinal Morphology of Broilers. Chin. J. Anim. Nutr. 2023, 35, 230–239. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Chen, R.Y.; Yu, J.; Ding, G.H.; Seah, R.W.X.; Chen, J. Antimicrobial peptide hepcidin contributes to restoration of the intestinal flora after Aeromonas hydrophila infection in Acrossocheilus fasciatus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263, 109486. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Wen, L.; Wu, C.; Yao, Z.; Yan, Z.; Li, R.; Chen, L.; Chen, F.; Xie, Z.; et al. The effect of the antimicrobial peptide plectasin on the growth performance, intestinal health, and immune function of yellow-feathered chickens. Front. Vet. Sci. 2021, 8, 688611. [Google Scholar] [CrossRef]
- Daneshmand, A.; Kermanshahi, H.; Sekhavati, M.H.; Javadmanesh, A.; Ahmadian, M.; Alizadeh, M.; Aldawoodi, A. Effects of clfchimera peptide on intestinal morphology, integrity, microbiota, and immune cells in broiler chickens challenged with necrotic enteritis. Sci. Rep. 2020, 10, 17704. [Google Scholar] [CrossRef]
- Chen, C.; Shi, J.; Wang, D.; Kong, P.; Wang, Z.; Liu, Y. Antimicrobial peptides as promising antibiotic adjuvants to combat drug-resistant pathogens. Crit. Rev. Microbiol. 2023, 50, 267–284. [Google Scholar] [CrossRef]
- Dong, X.Q.; Zhang, D.M.; Chen, Y.K.; Wang, Q.J.; Yang, Y.Y. Effects of antimicrobial peptides (AMPs) on blood biochemical parameters, antioxidase activity, and immune function in the common carp (Cyprinus carpio). Fish Shellfish Immunol. 2015, 47, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, J.; Feng, Y.; Ye, Q.; Wen, L.; Xu, G.; Zou, J. Effects of compound antimicrobial peptides on the growth performance, antioxidant and immune responses and disease resistance of grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol. 2020, 107, 163–170. [Google Scholar] [CrossRef]
- Wang, J.; Wilson, A.E.; Su, B.; Dunham, R.A. Functionality of dietary antimicrobial peptides in aquatic animal health: Multiple meta-analyses. Anim. Nutr. 2023, 12, 200–214. [Google Scholar] [CrossRef]
- Dong, X.Q.; Zhang, D.M.; Chen, Y.K.; Wang, Q.J.; Li, B.; Yang, Y.Y.; Ju, X. Effects of antimicrobial peptides on growth performance, viscerosomatic index, and muscle composition of Jian carp (Cyprinus carpio). Chin. J. Anim. Sci. 2015, 51, 47–51. [Google Scholar] [CrossRef]
- Sari, D.P.; Rostini, I.; Nurhayati, A.; Pratama, R.I. Meat quality of fresh Bonylip Barb and all-female hybrid Bonylip Barb (Osteochilus hasselti Valenciennes, 1842) fish based on organoleptic, physical and chemical characteristics. Int. J. Fish. Aquat. Stud. 2018, 6, 282–290. [Google Scholar]
- Thiex, N. Evaluation of analytical methods for the determination of moisture, crude protein, crude fat, and crude fiber in distillers dried grains with solubles. J. AOAC Int. 2009, 92, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.D.; Xie, S.L.; Zhou, A.G.; Zhang, C.N.; Wen, L.F.; Xu, G.H.; Zou, J.X. Effects of mixed antimicrobial peptide on the growth performance, antioxidant and immune responses and disease resistance of Pengze crucian carp (Carassius auratus var. Pengze). Fish Shellfish Immunol. 2021, 114, 112–118. [Google Scholar] [CrossRef]
- Rashidian, G.; Moghaddam, M.M.; Mirnejad, R.; Azad, Z.M. Supplementation of zebrafish (Danio rerio) diet using a short antimicrobial peptide: Evaluation of growth performance, immunomodulatory function, antioxidant activity, and disease resistance. Fish Shellfish Immunol. 2021, 119, 42–50. [Google Scholar] [CrossRef]
- Lin, X.; Chen, W.; Lin, S.; Luo, L. Effects of dietary cecropin on growth, non-specific immunity and disease resistance of tilapia (O reochromis niloticus× O. aureus). Aquac. Res. 2015, 46, 2999–3007. [Google Scholar] [CrossRef]
- Gyan, W.R.; Yang, Q.; Tan, B.; Jan, S.S.; Jiang, L.; Chi, S.; Dong, X.; Liu, H.; Shuang, Z. Effects of antimicrobial peptides on growth, feed utilization, serum biochemical indices and disease resistance of juvenile shrimp, Litopenaeus vannamei. Aquac. Res. 2020, 51, 1222–1231. [Google Scholar] [CrossRef]
- Jan, S.S.; Yang, D.Q.; Thammasena, R. Effect of antimicrobial peptides on the growth and immunity of swamp eels. Aquac. Fish Health 2021, 10, 137–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, K.; Zhu, Y.; Liu, Y.; Huang, H.; Zhou, H.; Chen, D.; Ma, B.; Ge, H.; Zhang, W.; et al. Effects of microencapsulated antimicrobial peptides on the growth, immunity, infection resistance, gut microbiota, and gene transcriptome in Manila clam (Ruditapes philippinarum) larvae. Fish Shellfish Immunol. 2025, 165, 110542. [Google Scholar] [CrossRef]
- Li, S.; Chi, S.; Cheng, X.; Wu, C.; Xu, Q.; Qu, P.; Gao, W.; Liu, Y. Effects of antimicrobial peptides on the growth performance, antioxidant and intestinal function in juvenile largemouth bass, Micropterus salmoides. Aquac. Rep. 2020, 16, 100252. [Google Scholar] [CrossRef]
- Sun, H. Effects on Fish Gut Microbial Community Structure and Symbiotic Bacterial Colonization Under Different Habitats While Dailyfeeding Antimicrobial Peptide. Ph.D. Thesis, Xiamen University, Xiamen, China, 2022. [Google Scholar] [CrossRef]
- Zheng, H.; Ji, J.X.; Zhou, L.S.; Li, L.Y.; Ma, J.W.; Xiao, S.J.; Guo, Y.M. Establishment of Regression Models of muscleColor Score on Colorimetric Value, Marbling Score and Intramuscular Fat Content in Pigs. Acta Agric. Univ. Jiangxiensis 2019, 41, 24–131. [Google Scholar] [CrossRef]
- Domaradzki, P.; Stanek, P.; Litwińczuk, Z.; Skałecki, P.; Florek, M. Slaughter value and muscle quality of suckler calves: A review. Musclescience 2017, 134, 135–149. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Myoglobin chemistry and musclecolor. Annu. Rev. Food Sci. Technol. 2013, 4, 9–99. [Google Scholar] [CrossRef]
- Pascual Guzmán, A.; Trocino, A.; Susta, L.; Barbut, S. Comparing three textural measurements of chicken breast fillets affected by severe wooden breast and spaghetti meat. Ital. J. Anim. Sci. 2021, 20, 465–471. [Google Scholar] [CrossRef]
- Yang, C.; Li, X.L.; Liao, X.G.; Li, S.B.; Yang, J.H. A Review of factors influencing intramuscular fat content in porcine meat. Agric. Sci. Technol. 2017, 18, 142–146. [Google Scholar] [CrossRef]
- Zhang, M.M.; Luo, X.; Zhang, Y.M.; Mao, Y.W.; Yang, X.Y.; Lei, H.M.; Han, M.S.; Liang, R.R. A review of recent research on the effect and underlying mechanism of calcium salts on meat color. J. Food Sci. 2019, 40, 327–333. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Yonar, S.M. Growth performance, haematological changes, immune response, antioxidant activity and disease resistance in rainbow trout (Oncorhynchus mykiss) fed diet supplemented with ellagic acid. Fish Shellfish Immunol. 2019, 95, 391–398. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Liu, X.; Wen, L.; Zou, J. Effects of dietary antimicrobial peptides on intestinal morphology, antioxidant status, immune responses, microbiota and pathogen disease resistance in grass carp Ctenopharyngodon idellus. Microb. Pathog. 2022, 165, 105386. [Google Scholar] [CrossRef]
- Ting, C.H.; Pan, C.Y.; Chen, Y.C.; Lin, Y.C.; Chen, T.Y.; Rajanbabu, V.; Chen, J.Y. Impact of Tilapia hepcidin 2-3 dietary supplementation on the gut microbiota profile and immunomodulation in the grouper (Epinephelus lanceolatus). Sci. Rep. 2019, 9, 19047. [Google Scholar] [CrossRef]
- Salinas, I.; Magadán, S. Omics in fish mucosal immunity. Dev. Comp. Immunol. 2017, 75, 99–108. [Google Scholar] [CrossRef]
- Rojo-Cebreros, A.H.; Ibarra-Castro, L.; Martínez-Brown, J.M. Immunostimulation and trained immunity in marine fish larvae. Fish Shellfish Immunol. 2018, 80, 15–21. [Google Scholar] [CrossRef]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K. Main components of fish immunity: An overview of the fish immune system. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Zhang, T.; Wen, B.; Huo, X.; Ren, J.; Chen, X. Dietary supplementation of Adonis-derived astaxanthin enhances antioxidant capacity and immune response in Litopenaeus vannamei. Comp. Immunol. Rep. 2025, 9, 200233. [Google Scholar] [CrossRef]
- Chen, C.W.; Pei, M.T.; Tan, F.X.; Yang, D.Q. The effect of antimicrobial peptides on the growth performance and immunity of eel. J. Aquac. 2023, 36, 48–53. [Google Scholar] [CrossRef]
- Boshra, H.; Li, J.; Sunyer, J.O. Recent advances on the complement system of teleost fish. Fish Shellfish Immunol. 2006, 20, 239–262. [Google Scholar] [CrossRef]
- Merle, N.S.; Noe, R.; Halbwachs-Mecarelli, L.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement system part II: Role in immunity. Front. Immunol. 2015, 6, 257. [Google Scholar] [CrossRef]
- Meng, X.Z. Identification and Expression Analysis of Three Genes Involved in Complement System from Grass Carp. Ph.D. Thesis, Shanghai Ocean University, Shanghai, China, 2019. [Google Scholar]
- Cai, W. Efficient Induction of Antimicrobial Peptides from Hermetia Illucens Linnaeus and Its Preliminary Study on Growth, Immune Effects and Mechanism in Common Carp (Cyprinus carpio). Master’s Thesis, Sichuan Agriculture University, Chengdu, China, 2024. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Lei, L.; Xu, J.; Qin, Y.; Gao, Q.; Zou, J. Characterisation of IL-15 and IL-2Rβ in grass carp: IL-15 upregulates cytokines and transcription factors of type 1 immune response and NK cell activation. Fish Shellfish Immunol. 2020, 107, 104–117. [Google Scholar] [CrossRef]
- Guo, Y.F. Functional Identification of Interleukin 6 and Its Receptors on Grass Carp. Ph.D. Thesis, University of Electronic Science and Technology of China, Chengdu, China, 2017. [Google Scholar]
- Li, C.L. The Comparative Study on the Inducible Expression of Interleukin 12 in Grass Carp Macrophages and Dendritic-like Cells. Ph.D. Thesis, University of Electronic Science and Technology of China, Chengdu, China, 2020. [Google Scholar] [CrossRef]
- Ding, Y.C. Study on the Immunomodulatory Activity of Phosvitin-Derived Antibacterial Peptide Pt5 in Zebrafish. Ph.D. Thesis, Ocean University of China, Qingdao, China, 2013. [Google Scholar]
- Li, L.; Zou, J.; Su, J.G.; Liu, L.H.; Zhang, S.; Nie, P. Interferon (IFN) system in fish: Research progress and contributions from China. J. Fish. China 2023, 47, 153–168. [Google Scholar] [CrossRef]
- Secombes, C.J.; Zou, J. Evolution of interferons and interferon receptors. Front. Immunol. 2017, 8, 209. [Google Scholar] [CrossRef]
- Zou, J.; Carrington, A.; Collet, B.; Dijkstra, J.M.; Yoshiura, Y.; Bols, N.; Secombes, C. Identification and bioactivities of IFN-γ in rainbow trout Oncorhynchus mykiss: The first Th1-type cytokine characterized functionally in fish. J. Immunol. 2005, 175, 2484–2494. [Google Scholar] [CrossRef]
- Grayfer, L.; Garcia, E.G.; Belosevic, M. Comparison of macrophage antimicrobial responses induced by type II interferons of the goldfish (Carassius auratus L.). J. Biol. Chem. 2010, 285, 23537–23547. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Lv, M.; Qiu, X.; Wang, X.; Zhang, A.; Yang, K.; Zhou, H. IFN-γ manipulates NOD1-mediated interaction of autophagy and Edwardsiella piscicida to augment intracellular clearance in fish. J. Immunol. 2021, 207, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Sun, D.F.; Yu, X.; Tang, Z.T.; Xu, D.; Sun, G. Effects of Antimicrobial Peptides on Growth Performance, Immune Functions, Serum Antioxidant Functions and Intestinal Development of Quail. J. Sichuan Agric. Univ. 2022, 40, 260–285. [Google Scholar] [CrossRef]
- Lan, Y.; Chen, C.X.; Wang, Q.K.; Wang, Q. Research progress on mechanism of action of immune factors of fish and the related techniques. Jiangsu Agric. Sci. 2024, 52, 1–8. Available online: http://www.jsnykx.cn/oa/DArticle.aspx?id=202404001&type=view (accessed on 20 July 2025).
- Lu, J.H.; Lan, L.L.; Quan, J.Q.; Zhao, G.Y.; Sun, J.; Jiang, C.P.; Liu, Z. Histopathological Changes and Gene Expression Dynamics of TLR Signaling Pathway in Oncorhynchus mykiss Infected with Ichthyophthirius Multifiliis. J. Agric. Biotechnol. 2022, 30, 739–750. [Google Scholar] [CrossRef]
- Carmody, R.J.; Chen, Y.H. Nuclear factor-kappaB: Activation and regulation during toll-like receptor signaling. Cell Mol. Immunol. 2007, 4, 31–41. Available online: https://www.researchgate.net/publication/6458184 (accessed on 20 July 2025).
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basilio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell type-specific roles of NF-kappaB linking inflammation and thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Tseng, C.C.; Chu, T.W.; Danata, R.H.; Risjani, Y.; Shih, H.T.; Hu, S.Y. Hepcidin-expressing fish eggs as a novel food supplement to modulate immunity against pathogenic infection in zebrafish (Danio rerio). Sustainability 2020, 12, 4057. [Google Scholar] [CrossRef]
- Pan, C.Y.; Lee, S.C.; Rajanbabu, V.; Lin, C.H.; Chen, J.Y. Insights into the antibacterial and immunomodulatory functions of tilapia hepcidin (TH) 2–3 against Vibrio vulnificus infection in mice. Dev. Comp. Immunol. 2012, 36, 166–173. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Zhang, H.; Du, X.; Cao, Z.; Wu, Y.; Chun, S.L.; Sun, Y. Antibacterial activity and mechanisms of TroHepc2-22, a derived peptide of hepcidin2 from golden pompano (Trachinotus ovatus). Int. J. Mol. Sci. 2023, 24, 9251. [Google Scholar] [CrossRef]
- Raheem, N.; Straus, S.K. Mechanisms of action for antimicrobial peptides with antibacterial and antibiofilm functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.R.; Jhong, J.H.; Wang, Z.; Chen, S.; Wan, Y.; Horng, J.T.; Lee, T.Y. Characterization and identification of natural antimicrobial peptides on different organisms. Int. J. Mol. Sci. 2020, 21, 986. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, Z.Y.; Xia, B.B.; Zhang, Y.W.; Liu, X.L.; Yu, Y.; Tang, N.; Tong, X.M.; Wang, M.; Ye, X.; et al. Identification of antimicrobial peptides from the human gut microbiome using deep learning. Nat. Biotechnol. 2022, 40, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.X.; Aminov, R.; Franco, O.L.; Fuente-Nunez, C.D.L.; Wang, G.S.; Wang, J.H. Antimicrobial peptides and their druggability, bio-safety, stability, and resistance. Front. Microbiol. 2024, 15, 1425952. [Google Scholar] [CrossRef] [PubMed]


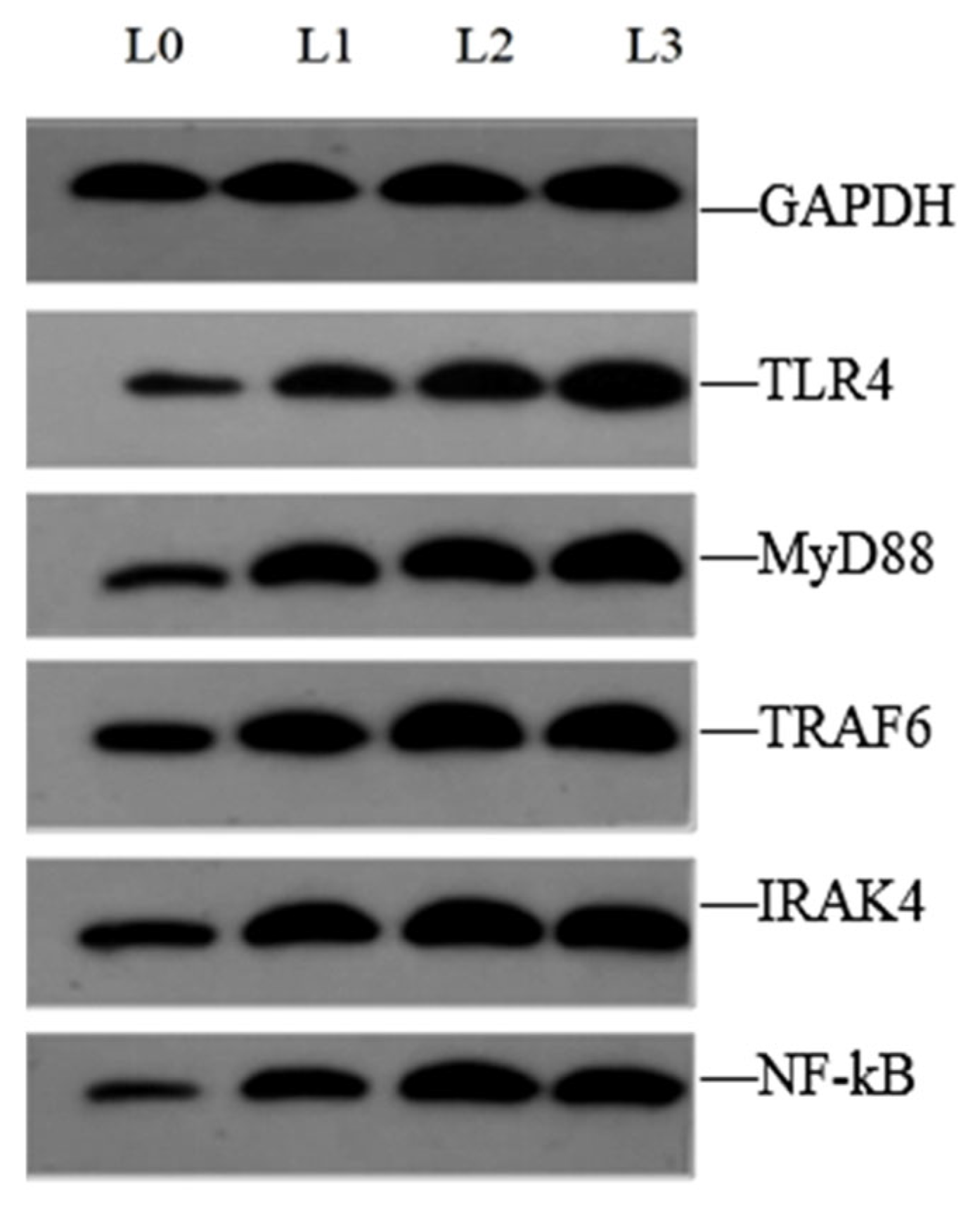
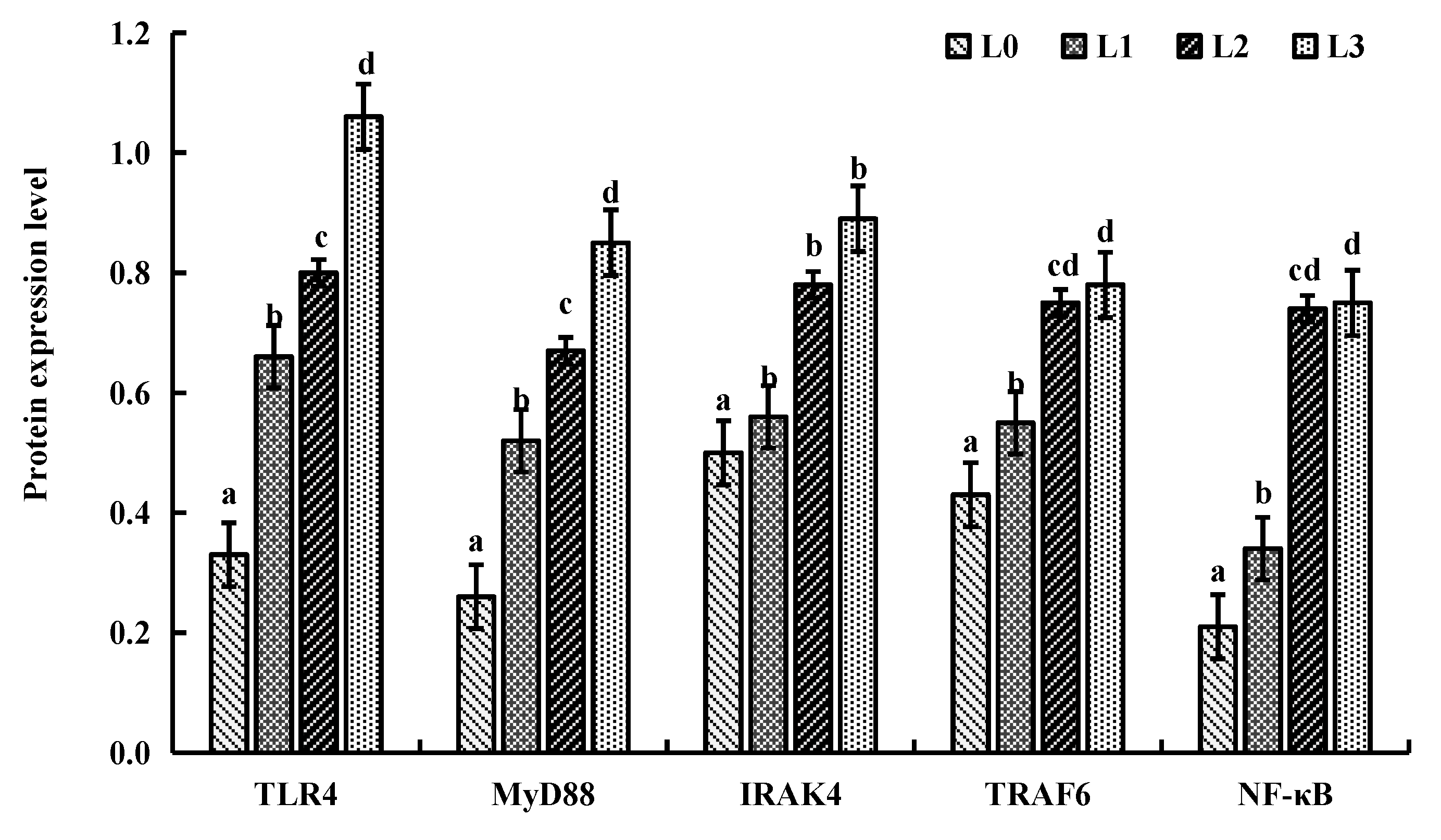
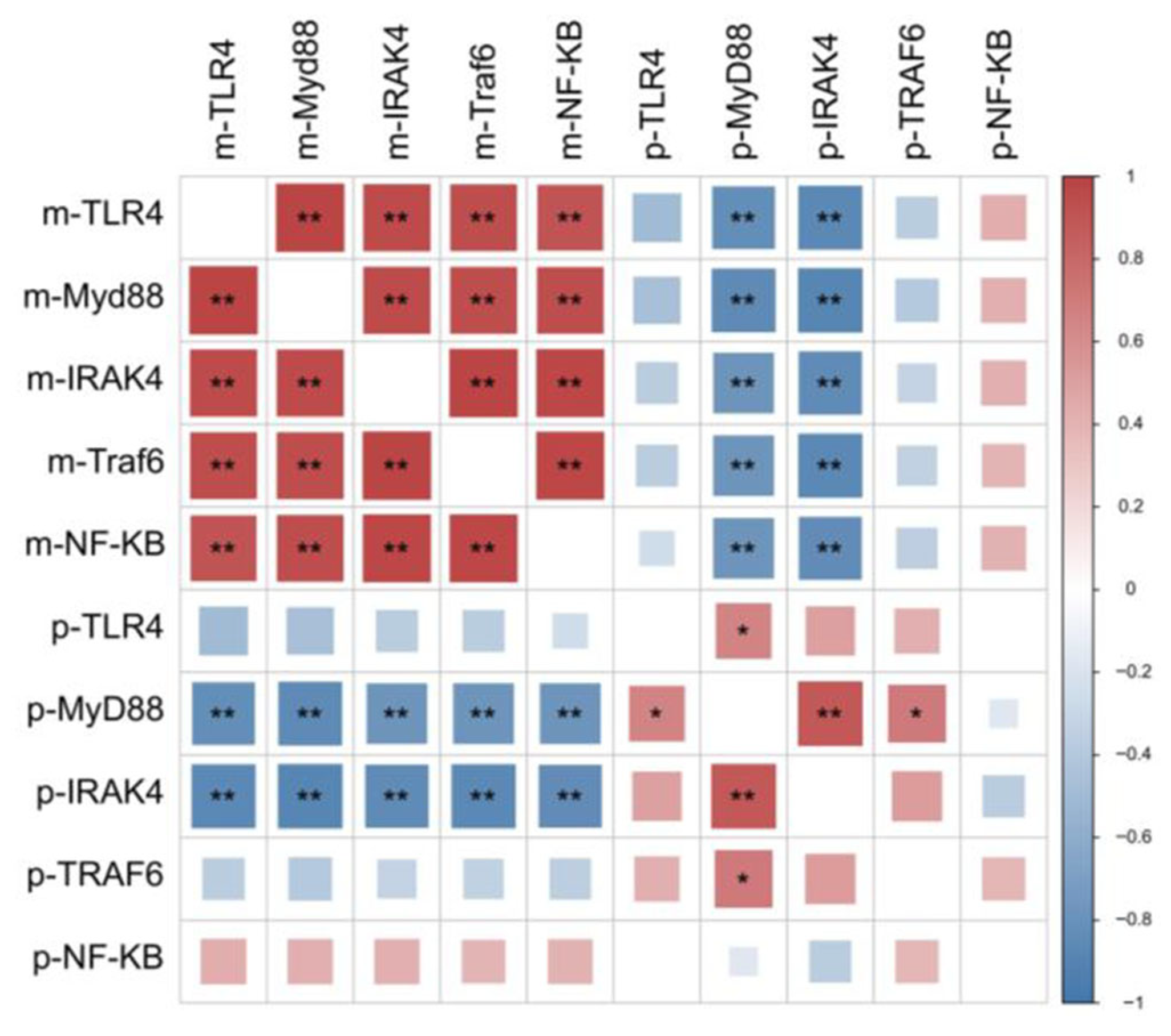
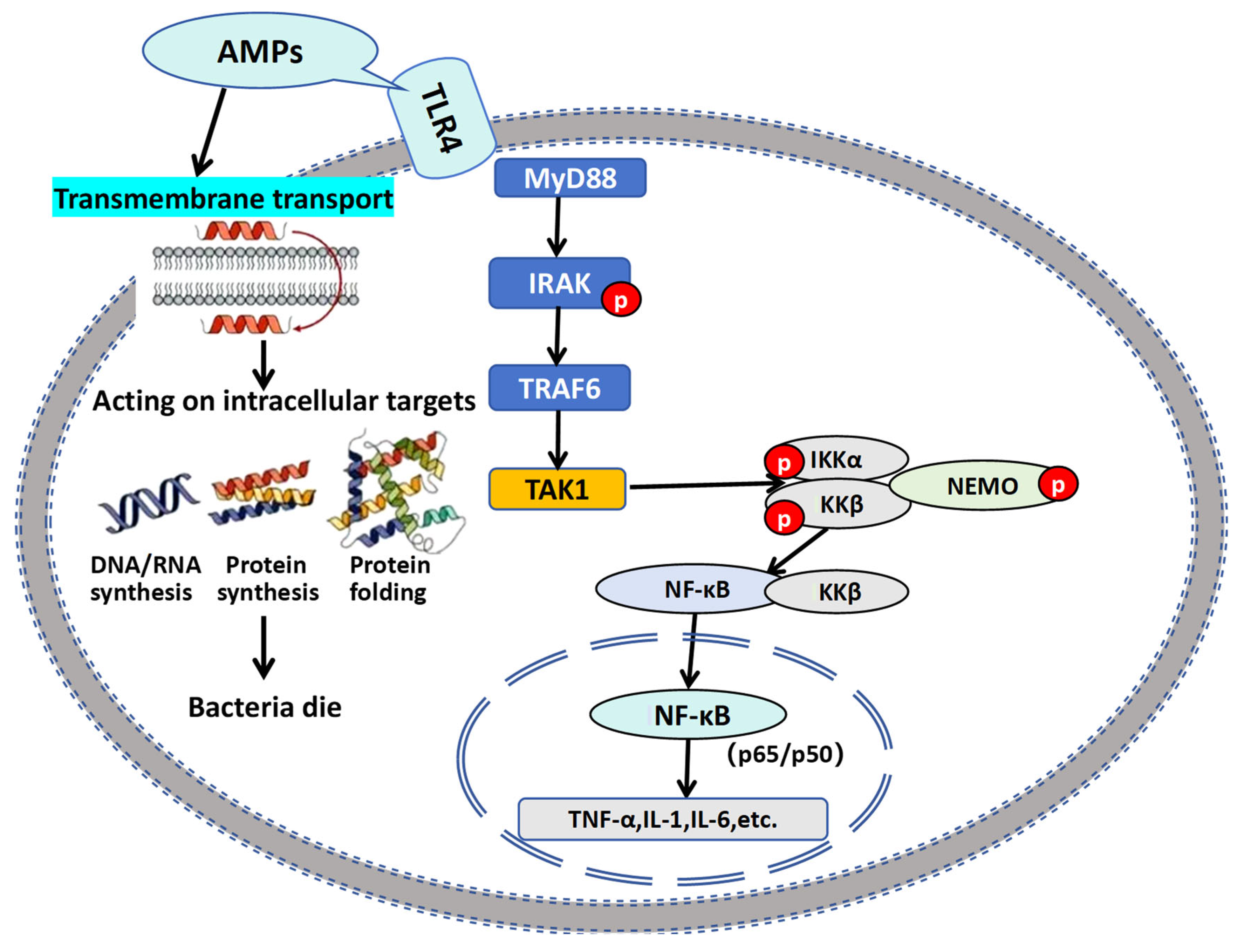
| Ingredients | L0 | L1 | L2 | L3 |
|---|---|---|---|---|
| Antimicrobial peptides | 0 | 0.2 | 0.4 | 0.6 |
| Fish meal | 160 | 160 | 160 | 160 |
| Soybean meal | 340 | 340 | 340 | 340 |
| Corn oil | 40 | 40 | 40 | 40 |
| Wheat bran | 100 | 100 | 100 | 100 |
| flour | 169 | 169 | 169 | 169 |
| Corn protein powder | 140 | 140 | 140 | 140 |
| Ca (H2PO4) 2 | 30 | 30 | 30 | 30 |
| Premix a | 10 | 10 | 10 | 10 |
| Lys | 2 | 2 | 2 | 2 |
| Met | 5 | 5 | 5 | 5 |
| Choline chloride (50%) | 3 | 3 | 3 | 3 |
| VC (35%) | 1 | 1 | 1 | 1 |
| Proximate composition (%) | ||||
| Dry matter | 92.69 | 92.59 | 92.23 | 92,11 |
| Crude protein | 32.45 | 32.03 | 32.09 | 32.16 |
| Crude lipid | 6.65 | 6.74 | 6.85 | 6.57 |
| Ash | 8.77 | 8.71 | 8.73 | 8.86 |
| Calcium | 1.27 | 1.24 | 1.26 | 1.28 |
| Total phosphorus | 1.50 | 1.51 | 1.49 | 1.51 |
| Energy (MJ/kg) | 18.57 | 18.63 | 18.57 | 18.58 |
| Gene | ID | Primer Orientation | Size | Primer Sequence |
|---|---|---|---|---|
| TLR4 | 132145260 | Forward | 141 | GCGAGTCAGAACCTCAGCCAATG |
| TLR4 | 132145260 | Reverse | 141 | TCCCAAACCCTTGCTCCCTCTC |
| MyD88 | 403145 | Forward | 85 | AGGTGCAAGAGGATGGTGGTAGTC |
| MyD88 | 403145 | Reverse | 85 | GGCTGAGTGCGAACTTGGTCTG |
| IRAK4 | 393132 | Forward | 88 | GGCTTTGGCGTCGTCTTCAGAG |
| IRAK4 | 393132 | Reverse | 88 | CTTCAAGTGAGCTGTCGTCCATAGG |
| TRAF6 | 554561 | Forward | 110 | TACGAGTGCCCTATCTGTCTGATGG |
| TRAF6 | 554561 | Reverse | 110 | TTCTGCCCTGTGTCCCTGATGG |
| NF-κB | 122880105 | Forward | 99 | AGGCGGTAAGGAAGTTGCGAATG |
| NF-κB | 122880105 | Reverse | 99 | CAGGCTGGTCTGAAGTGTGGTTC |
| Parameters | Group | ||||
|---|---|---|---|---|---|
| L0 | L1 | L2 | L3 | p-Value | |
| Initial weight (g) | 15.50 ± 0.50 | 15.50 ± 0.50 | 15.50 ± 0.50 | 15.50 ± 0.50 | - |
| Final weight (g) | 29.42 ± 0.32 b | 30.63 ± 0.54 c | 29.99 ± 0.53 bc | 28.22 ± 0.23 a | 0.001 |
| WGR (%) | 89.83 ± 2.07 b | 97.63 ± 3.50 c | 93.46 ± 3.45 bc | 82.04 ± 1.49 a | 0.001 |
| SGR (%) | 1.31 ± 0.03 b | 1.39 ± 0.04 c | 1.35 ± 0.04 bc | 1.22 ± 0.02 a | 0.001 |
| Condition factor | 2.59 ± 0.08 a | 2.70 ± 0.01 ab | 2.82 ± 0.11 b | 2.71 ± 0.06 ab | 0.038 |
| FCR | 1.32 ± 0.01 a | 1.29 ± 0.01 a | 1.33 ± 0.01 a | 1.42 ± 0.05 b | 0.002 |
| Survival (%) | 100 | 100 | 100 | 100 | - |
| Parameters | Group | ||||
|---|---|---|---|---|---|
| L0 | L1 | L2 | L3 | p-Value | |
| Visceral weight (g) | 3.09 ± 0.16 | 2.79 ± 0.57 | 2.45 ± 0.31 | 2.73 ± 0.78 | 0.550 |
| Hepatopancreas weight (g) | 0.66 ± 0.05 | 0.68 ± 0.09 | 0.54 ± 0.08 | 0.71 ± 0.23 | 0.467 |
| Spleen weight (g) | 0.08 ± 0.02 | 0.06 ± 0.01 | 0.06 ± 0.005 | 0.08 ± 0.02 | 0.214 |
| Kidney weight (g) | 0.15 ± 0.01 | 0.13 ± 0.04 | 0.11 ± 0.01 | 0.18 ± 0.04 | 0.178 |
| Intestines weight (g) | 0.99 ± 0.19 | 0.72 ± 0.13 | 0.73 ± 0.17 | 0.93 ± 0.28 | 0.298 |
| Intestines length (cm) | 24.14 ± 0.42 | 22.47 ± 3.07 | 20.85 ± 0.99 | 23.02 ± 4.18 | 0.527 |
| Visceral index (%) | 10.27 ± 0.71 | 9.47 ± 1.88 | 8.32 ± 0.80 | 9.65 ± 2.70 | 0.600 |
| Hepatopancreas index (%) | 2.20 ± 0.12 | 2.31 ± 0.29 | 1.84 ± 0.23 | 2.54 ± 0.80 | 0.349 |
| Spleen index (%) | 0.28 ± 0.07 | 0.21 ± 0.36 | 0.21 ± 0.01 | 0.28 ± 0.07 | 0.259 |
| Kidney index (%) | 0.50 ± 0.01 | 0.46 ± 0.12 | 0.38 ± 0.03 | 0.62 ± 0.16 | 0.094 |
| Parameters | Group | ||||
|---|---|---|---|---|---|
| L0 | L1 | L2 | L3 | p-Value | |
| Lightness (L*) | 44.81 ± 3.54 | 42.61 ± 0.67 | 42.54 ± 1.06 | 44.99 ± 0.82 | 0.291 |
| Redness (a*) | 3.35 ± 1.01 a | 5.09 ± 0.36 b | 3.44 ± 0.22 a | 2.23 ± 0.68 a | 0.004 |
| Yellowness (b*) | 10.22 ± 0.96 | 10.10 ± 0.56 | 10.11 ± 0.76 | 9.80 ± 0.18 | 0.890 |
| Shear force (N) | 9.92 ± 0.93 a | 10.32 ± 0.35 a | 9.93 ± 0.94 a | 12.70 ± 0.45 b | 0.004 |
| Parameters | Group | ||||
|---|---|---|---|---|---|
| L0 | L1 | L2 | L3 | p-Value | |
| Water (%) | 9.25 ± 1.09 | 8.28 ± 0.83 | 8.51 ± 1.47 | 8.33 ± 0.40 | 0.651 |
| Crude protein (%) | 64.63 ± 1.50 b | 65.51 ± 1.87 b | 66.24 ± 1.40 b | 56.77 ± 1.95 a | <0.001 |
| Crude fat (%) | 8.33 ± 0.56 d | 6.92 ± 0.20 c | 5.51 ± 0.34 b | 3.49 ± 0.12 a | <0.001 |
| Crude ash (%) | 7.99 ± 1.26 | 8.49 ± 0.39 | 7.84 ± 0.18 | 8.58 ± 0.63 | 0.557 |
| Ca (%) | 0.32 ± 0.06 | 0.34 ± 0.05 | 0.30 ± 0.05 | 0.28 ± 0.04 | 0.499 |
| P (%) | 0.40 ± 0.03 | 0.41 ± 0.02 | 0.39 ± 0.03 | 0.38 ± 0.02 | 0.401 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Jiang, D.; Qu, G.; Wang, G. Nutritional Intervention with Antimicrobial Peptides Improves Growth Performance, Muscle Quality, Antioxidant Capacity, and Immune Function of Crucian Carp (Carassius auratus) Through TLR4/NF-κB Signaling Pathway. Animals 2025, 15, 2554. https://doi.org/10.3390/ani15172554
Dong X, Jiang D, Qu G, Wang G. Nutritional Intervention with Antimicrobial Peptides Improves Growth Performance, Muscle Quality, Antioxidant Capacity, and Immune Function of Crucian Carp (Carassius auratus) Through TLR4/NF-κB Signaling Pathway. Animals. 2025; 15(17):2554. https://doi.org/10.3390/ani15172554
Chicago/Turabian StyleDong, Xiaoqing, Dan Jiang, Guijuan Qu, and Guiqin Wang. 2025. "Nutritional Intervention with Antimicrobial Peptides Improves Growth Performance, Muscle Quality, Antioxidant Capacity, and Immune Function of Crucian Carp (Carassius auratus) Through TLR4/NF-κB Signaling Pathway" Animals 15, no. 17: 2554. https://doi.org/10.3390/ani15172554
APA StyleDong, X., Jiang, D., Qu, G., & Wang, G. (2025). Nutritional Intervention with Antimicrobial Peptides Improves Growth Performance, Muscle Quality, Antioxidant Capacity, and Immune Function of Crucian Carp (Carassius auratus) Through TLR4/NF-κB Signaling Pathway. Animals, 15(17), 2554. https://doi.org/10.3390/ani15172554





