Dietary Supplementation with Gotu Kola (Centella asiatica) Extract Enhanced Innate Immune Responses, Modulated Immune-Related Gene Expression, and Improved Gut Microbiota in Giant Freshwater Prawn (Macrobrachium rosenbergii)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Preparation of C. Asiatica Extract and Experiment Diets
2.3. Prawn Cultivation and Tissue Collection
2.4. Gene Expression Based on RT-qPCR
2.4.1. RNA Extraction
2.4.2. cDNA Synthesis and Gene Expression Analysis
2.5. Immune Assay from Prawn Hemolymph
2.5.1. Sample Preparation
2.5.2. Total Protein Analysis
2.5.3. Lysozyme Activity
2.5.4. Phenoloxidase (PO) Activity
2.6. Metagenomic Analysis of Gut Microbiota Based on 16S rRNA Sequencing
2.7. Statistical Analysis
3. Results
3.1. Effect of C. asiatica Crude Extract Supplementation on Growth Performance of Giant Freshwater Prawns
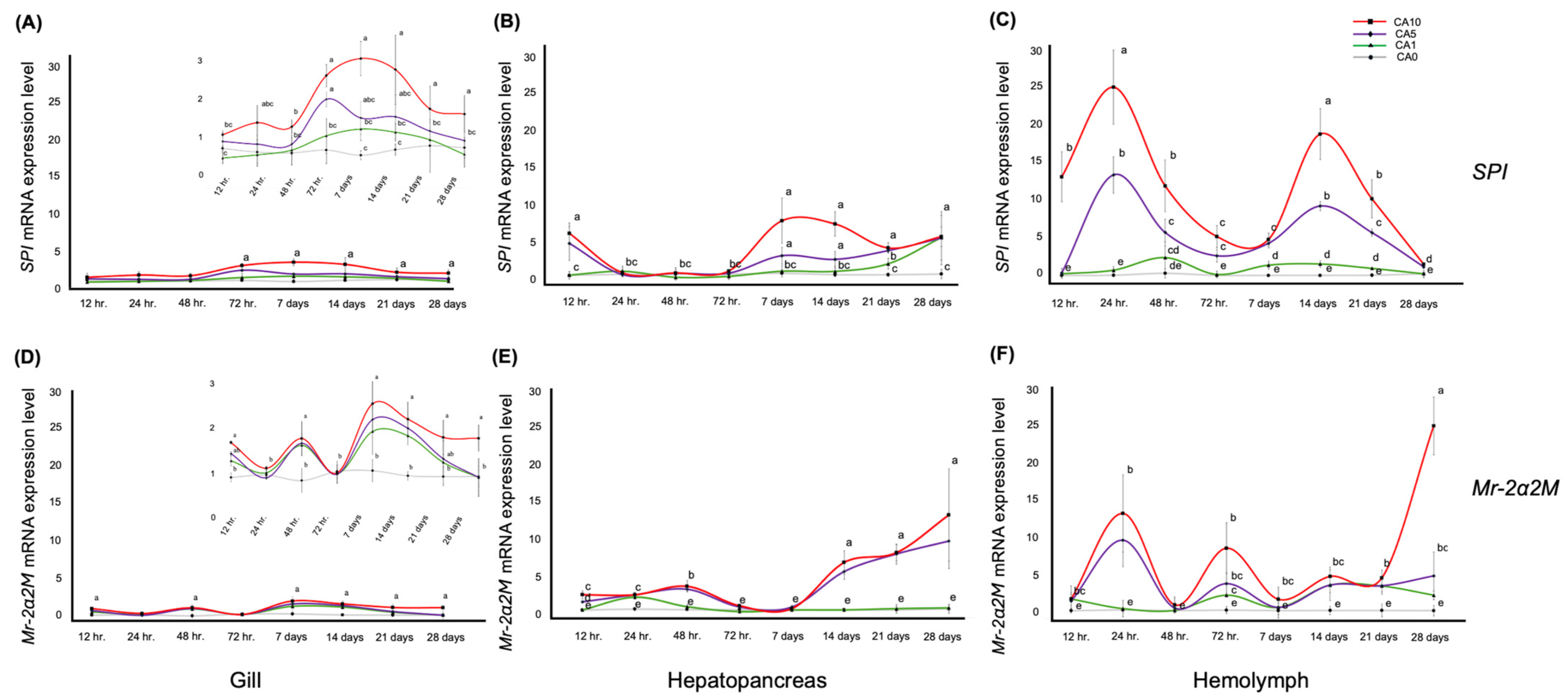
3.2. Mr-2α2M and SPI Gene Expression Levels in the Tissues over a Feeding Period of 12 h to 28 Days
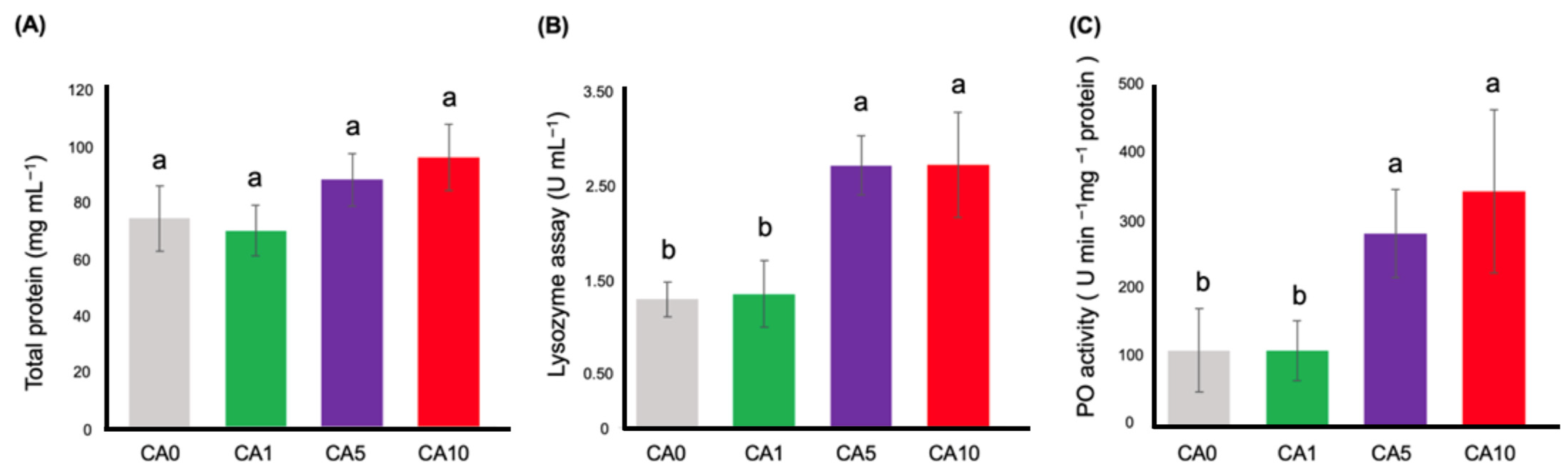
3.3. Innate Immune Parameters Assay
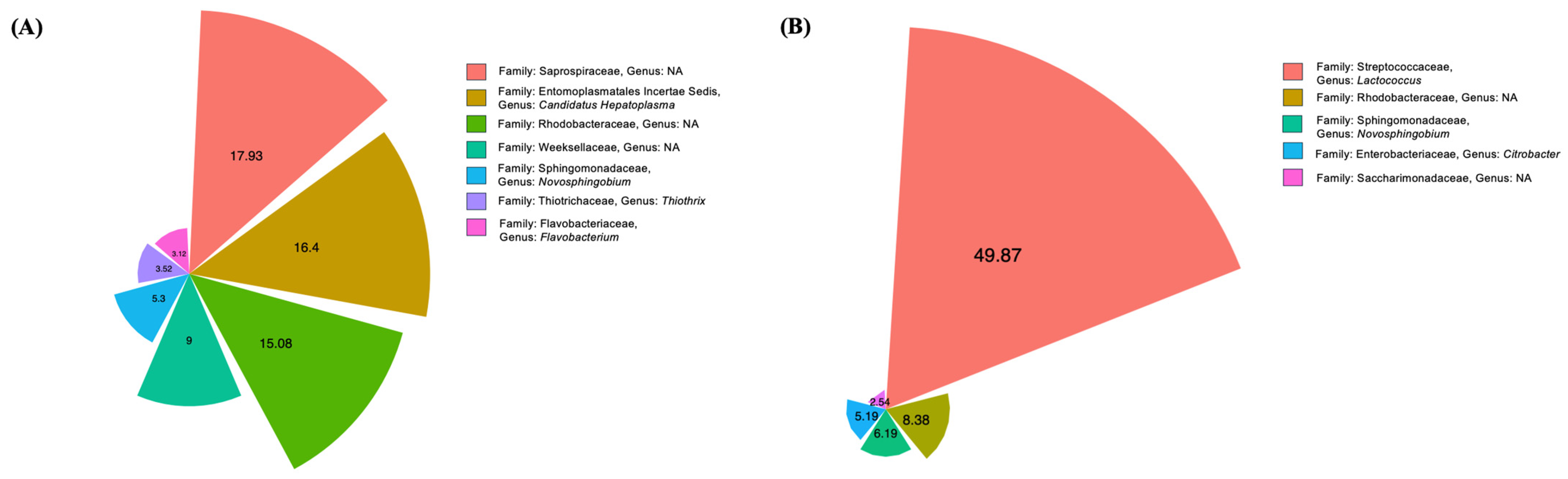
3.4. Intestine Microbiome Analysis Based on 16S rRNA Gene Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IW | Initial body weight |
| FW | Final body weight |
| AWG | Average weight gain |
| ADG | Average daily gain |
| SGR | Specific growth rate |
| IP | Intermolt period |
| EP | Experimental period |
| n | Number of prawns per tank |
| m | Total number of molts per tank |
| pro-PO | Prophenoloxidase |
References
- Pillai, B.R.; Ponzoni, R.W.; Das Mahapatra, K.; Panda, D. Genetic Improvement of Giant Freshwater Prawn Macrobrachium rosenbergii: A Review of Global Status. Rev. Aquac. 2022, 14, 1285–1299. [Google Scholar] [CrossRef]
- De Almeida Marques, H.L.; Moraes-Valenti, P.M.C. Current Status and Prospects of Farming the Giant River Prawn (Macrobrachium rosenbergii (De Man 1879) and the Amazon River Prawn Macrobrachium amazonicum (Heller 1862) in Brazil. Aquac. Res. 2012, 43, 984–992. [Google Scholar] [CrossRef]
- Menasveta, P.; Piyatiratitivokul, S. A Comparative Study on Larviculture Techniques for the Giant Freshwater Prawn, Macrobrachium rosenbergii (De Man). Aquaculture 1980, 20, 239–249. [Google Scholar] [CrossRef]
- Haslawati, B.; Saadiah, I.; Siti-Dina, R.P.; Othman, M.; Latif, M.T. Environmental Assessment of Giant Freshwater Prawn, Macrobrachium rosenbergii Farming Through Life Cycle Assessment. Sustainability 2022, 14, 14776. [Google Scholar] [CrossRef]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef]
- Kwang, J. Oral Vaccination of Baculovirus-Expressed VP28 Displays Enhanced Protection Against White Spot Syndrome Virus in Penaeus monodon. PLoS ONE 2011, 6, e26428. [Google Scholar]
- Lakshmi, B.; Viswanath, B.; Sai Gopal, D.V.R. Probiotics as Antiviral Agents in Shrimp Aquaculture. J. Pathog. 2013, 2013, 424123. [Google Scholar] [CrossRef]
- Orhan, I.E. Centella asiatica (L.) Urban: From Traditional Medicine to Modern Medicine with Neuroprotective Potential. Evid. Based Complement. Altern. Med. 2012, 2012, 946259. [Google Scholar] [CrossRef]
- Kamol, P.; Nukool, W.; Pumjaroen, S.; Inthima, P.; Kongbangkerd, A.; Suphrom, N.; Buddhachat, K. Harnessing Postharvest Light Emitting Diode (LED) Technology of Centella asiatica (L.) Urb. to Improve Centelloside Content by Up-Regulating Gene Expressions in the Triterpenoid Pathway. Heliyon 2024, 10, e23639. [Google Scholar] [CrossRef]
- Seevaratnam, V.; Banumathi, P.; Premalatha, M.R.; Sundaram, S.P.; Arumugam, T. Functional Properties of Centella asiatica (L.): A Review. Int. J. Pharm. Pharm. Sci. 2012, 4, 8–14. [Google Scholar]
- Agme-Ghodke, V.; Agmea, R.N.; Sagarb, A.D. Analysis of Bioactive Compounds in Leaves Extract of Centella asiatica by Using HRLC-MS and IR Techniques. J. Chem. Pharm. Res. 2016, 8, 122–125. [Google Scholar]
- Jagtap, N.S.; Khadabadi, S.S.; Ghorpade, D.S.; Banarase, N.B.; Naphade, S.S. Antimicrobial and Antifungal Activity of Centella asiatica (L.) Urban, Umbeliferae. Res. J. Pharm. Technol. 2009, 2, 328–330. [Google Scholar]
- Chen, H.; Hua, X.M.; Ze, B.C.; Wang, B.; Wei, L. The Anti-Inflammatory Effects of Asiatic Acid in Lipopolysaccharide-Stimulated Human Corneal Epithelial Cells. Int. J. Ophthalmol. 2017, 10, 179. [Google Scholar]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Use of Asiatic pennywort Centella asiatica aqueous extract as a bath treatment to control columnaris in Nile tilapia. J. Aquat. Anim. Health. 2010, 22, 14–20. [Google Scholar] [CrossRef]
- Srichaiyo, N.; Tongsiri, S.; Hoseinifar, S.H.; Dawood, M.A.O.; Jaturasitha, S.; Esteban, M.Á.; Ringø, E.; Van Doan, H. The Effects Gotu Kola (Centella asiatica) Powder on Growth Performance, Skin Mucus, and Serum Immunity of Nile Tilapia (Oreochromis niloticus) Fingerlings. Aquac. Rep. 2020, 16, 100239. [Google Scholar] [CrossRef]
- Ibrahim, W.N.W.; Huzmi, H.; Lee, K.L.; Low, C.F.; Aznan, A.S.; Iberahim, N.A.; Najiah, M. In Vitro and In Vivo Characterisations of Centella asiatica Extract Against Vibrio alginolyticus Infection in Whiteleg Shrimp, Penaeus vannamei. Songklanakarin J. Sci. Technol. 2023, 45, 189–196. [Google Scholar]
- Kulkarni, A.; Krishnan, S.; Anand, D.; Kokkattunivarthil Uthaman, S.; Otta, S.K.; Karunasagar, I.; Kooloth Valappil, R. Immune Responses and Immunoprotection in Crustaceans with Special Reference to Shrimp. Rev. Aquac. 2021, 13, 431–459. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, Q. Innate Immune Responses Against Viral Pathogens in Macrobrachium. Dev. Comp. Immunol. 2021, 117, 103966. [Google Scholar] [CrossRef]
- Ho, P.Y.; Cheng, C.H.; Cheng, W. Identification and Cloning of the α2-Macroglobulin of Giant Freshwater Prawn Macrobrachium rosenbergii and Its Expression in Relation with the Molt Stage and Bacteria Injection. Fish Shellfish Immunol. 2009, 26, 459–466. [Google Scholar] [CrossRef]
- Aspán, A.; Sturtevant, J.; Smith, V.J.; Söderhäll, K. Purification and Characterization of a Prophenoloxidase Activating Enzyme from Crayfish Blood Cells. Insect Biochem. 1990, 20, 709–718. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An Improvement of the 2−ΔΔCT Method for Quantitative Real-Time Polymerase Chain Reaction Data Analysis. Biostat. Bioinforma. Biomath. 2013, 3, 71–85. [Google Scholar]
- Likittrakulwong, W.; Na-Nakorn, U.; Poompuang, S.; Koonawootrittriron, S.; Srisapoome, P. Molecular Identification and Expression Profiling of a Novel Alpha2-Macroglobulin Gene in Giant Freshwater Prawn (Macrobrachium rosenbergii, De Man). Agric. Nat. Resour. 2017, 51, 25–35. [Google Scholar] [CrossRef]
- Tewpair, P.; Areechon, N.; Srisapoome, P. Characterization of Complementary cDNA (cDNA) and Expression Analysis of Serine Proteinase Inhibitor (SPI) from Giant Freshwater Prawn (Macrobrachium rosenbergii, de Man). In Proceedings of the 46. Kasetsart University Annual Conference, Bangkok, Thailand, 29 January–1 February 2008. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Helal, R.; Melzig, M.F. Determination of Lysozyme Activity by a Fluorescence Technique in Comparison with the Classical Turbidity Assay. Die Pharm. 2008, 63, 415–419. [Google Scholar]
- Franssens, V.; Simonet, G.; Breugelmans, B.; Van Soest, S.; Van Hoef, V.; Vanden Broeck, J. The Role of Hemocytes, Serine Protease Inhibitors and Pathogen-Associated Patterns in Prophenoloxidase Activation in the Desert Locust, Schistocerca gregaria. Peptides 2008, 29, 235–241. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R Language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Foster, Z.S.; Sharpton, T.J.; Grünwald, N.J. Metacoder: An R Package for Visualization and Manipulation of Community Taxonomic Diversity Data. PLoS Comput. Biol. 2017, 13, e1005404. [Google Scholar] [CrossRef]
- Ugyen, U.; Singanusong, R.; Phinyo, M.; Changtor, P.; Chaijamrus, S.; Thongsook, T. Extraction of Heat-Stabilised Defatted Rice Bran Protein by Solid-State Fermentation Using Heterofermentative Microbes From Traditional Asian Starters. Food Technol. Biotechnol. 2023, 61, 523–535. [Google Scholar] [CrossRef]
- Ajayi, O.A.; Olumide, M.D.; Chioma, G.O. Efficiency of Centella asiatica (Gotu Kola) Leaf Meal as Feed Additive in Broiler Chicken Diet. Niger. J. Anim. Prod. 2020, 47, 153–160. [Google Scholar] [CrossRef]
- Maneewan, C.; Mekbungwan, A.; Charerntantanakul, W.; Yamauchi, K.; Yamauchi, K.E. Effects of Dietary Centella asiatica (L.) Urban on Growth Performance, Nutrient Digestibility, Blood Composition in Piglets Vaccinated with Mycoplasma hyopneumoniae. Anim. Sci. J. 2014, 85, 569–574. [Google Scholar] [CrossRef]
- Prasad, A.; Dhawan, S.S.; Mathur, A.K.; Prakash, O.; Gupta, M.M.; Verma, R.K.; Lal, R.K.; Mathur, A. Morphological, Chemical and Molecular Characterization of Centella asiatica Germplasms for Commercial Cultivation in the Indo-Gangetic Plains. Nat. Prod. Commun. 2014, 9, 779–784. [Google Scholar] [CrossRef]
- Ng, J.J.Y.; Yusoff, N.A.H.; Elias, N.A.; Norhan, N.A.-S.; Harun, N.A.; Abdullah, F.; Ishak, A.N.; Hassan, M. Phytotherapy Use for Disease Control in Aquaculture: A Review of the Last 5 Years. Aquac. Int. 2024, 32, 2687–2712. [Google Scholar] [CrossRef]
- Kawamura, G.; Yong, A.S.K.; Hsein Loong, A.; Doison, A.; Ooi, S.; Lim, L.-S. Malaysian Herbs as Feeding Attractants and Enhancers for the Giant Freshwater Prawn (Macrobrachium rosenbergii) and the Whiteleg Shrimp (Litopenaeus vannamei). Borneo J. Mar. Sci. Aquac. 2019, 3, 57–67. [Google Scholar] [CrossRef]
- AftabUddin, S.; Siddique, M.A.M.; Romkey, S.S.; Shelton, W.L. Antibacterial Function of Herbal Extracts on Growth, Survival and Immunoprotection in the Black Tiger Shrimp Penaeus monodon. Fish Shellfish Immunol. 2017, 65, 52–58. [Google Scholar] [CrossRef]
- El-Desouky, H.; El-Asely, A.; Shaheen, A.A.; Abbass, A. Effects of Zingiber officinalis and Cyanodon dactylon on the Growth Performance and Immune Parameters of Macrobrachium rosenbergii. World J. Fish Mar. Sci. 2012, 4, 301–307. [Google Scholar]
- Poongodi, R.; Bhavan, P.S.; Muralisankar, T.; Radhakrishnan, S. Growth Promoting Potential of Garlic, Ginger, Turmeric and Fenugreek on the Freshwater Prawn Macrobrachium rosenbergii. Int. J. Pharm. Bio Sci. 2012, 3, 914–926. [Google Scholar]
- Muralisankar, T.; Bhavan, P.S.; Radhakrishnan, S.; Santhanam, P. Dietary Supplement of Medicinal Herbal Leaf Powder on Growth Performance, Digestive Enzymes Activities, Energy Utilization and Vitamin Levels of the Freshwater Prawn Macrobrachium rosenbergii. Proc. Zool. Soc. 2018, 71, 265–271. [Google Scholar] [CrossRef]
- Perazzolo, L.M.; Gargioni, R.; Ogliari, P.; Barracco, M.A.A. Evaluation of Some Hemato-Immunological Parameters in the Shrimp Farfantepenaeus paulensis Submitted to Environmental and Physiological Stress. Aquaculture 2002, 214, 19–33. [Google Scholar] [CrossRef]
- Lorenzon, S.; Martinis, M.; Ferrero, E.A. Ecological Relevance of Hemolymph Total Protein Concentration in Seven Unrelated Crustacean Species from Different Habitats Measured Predictively by a Density-Salinity Refractometer. J. Mar. Sci. 2011, 2011, 153654. [Google Scholar] [CrossRef]
- Sung, H.H.; Kou, G.H.K.; Song, Y.L. Vibriosis Resistance Induced by Glucan Treatment in Tiger Shrimp (Penaeus monodon). Fish Pathol. 1994, 29, 11–17. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, J.C. The Virulence of Enterococcus to Freshwater Prawn Macrobrachium rosenbergii and Its Immune Resistance Under Ammonia Stress. Fish Shellfish Immunol. 2002, 12, 97–109. [Google Scholar] [CrossRef]
- Söderhäll, K.; Cerenius, L. Crustacean Immunity. Annu. Rev. Fish Dis. 1992, 2, 3–23. [Google Scholar] [CrossRef]
- Shen, W.-Y.; Fu, L.-L.; Li, W.-F.; Zhu, Y.-R. Effect of Dietary Supplementation with Bacillus subtilis on the Growth, Performance, Immune Response and Antioxidant Activities of the Shrimp (Litopenaeus vannamei). Aquac. Res. 2010, 41, 1691–1698. [Google Scholar] [CrossRef]
- Chang, Z.Q.; Ge, Q.Q.; Sun, M.; Wang, Q.; Lv, H.Y.; Li, J. Immune Responses by Dietary Supplement with Astragalus Polysaccharides in the Pacific White Shrimp, Litopenaeus vannamei. Aquac. Nutr. 2018, 24, 702–711. [Google Scholar] [CrossRef]
- Javahery, S.; Noori, A.; Hoseinifar, S.H. Growth Performance, Immune Response, and Digestive Enzyme Activity in Pacific White Shrimp, Penaeus vannamei Boone, 1931, Fed Dietary Microbial Lysozyme. Fish Shellfish Immunol. 2019, 92, 528–535. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chang, P.S.; Chen, H.Y. Differential Time-Series Expression of Immune-Related Genes of Pacific White Shrimp Litopenaeus vannamei in Response to Dietary Inclusion of β-1,3-Glucan. Fish Shellfish Immunol. 2008, 24, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ji, X.; Wang, X.; Li, T.; Wang, H.; Zeng, Q. Identification and Characterization of Differentially Expressed Genes in Hepatopancreas of Oriental River Prawn Macrobrachium nipponense Under Nitrite Stress. Fish Shellfish Immunol. 2019, 87, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines 2020, 8, 468. [Google Scholar] [CrossRef]
- Ning, J.; Liu, Y.; Gao, F.; Song, C.; Cui, Z. Two Alpha-2 Macroglobulin from Portunus trituberculatus Involved in the Prophenoloxidase System, Phagocytosis and Regulation of Antimicrobial Peptides. Fish Shellfish Immunol. 2019, 89, 574–585. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, X.; Tran, N.T.; Huang, Y.; Gong, Y.; Zhang, Y.; Li, S. Innate Immune Responses and Metabolic Alterations of Mud Crab (Scylla paramamosain) in Response to Vibrio parahaemolyticus Infection. Fish Shellfish Immunol. 2019, 87, 166–177. [Google Scholar] [CrossRef]
- Zhao, Y.R.; Xu, Y.H.; Jiang, H.S.; Xu, S.; Zhao, X.F.; Wang, J.X. Antibacterial Activity of Serine Protease Inhibitor 1 from Kuruma Shrimp Marsupenaeus japonicus. Dev. Comp. Immunol. 2014, 44, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Apitanyasai, K.; Chang, C.C.; Ng, T.H.; Ng, Y.S.; Liou, J.H.; Lo, C.F.; Wang, H.C. Penaeus vannamei Serine Proteinase Inhibitor 7 (LvSerpin7) Acts as an Immune Brake by Regulating the ProPO System in AHPND-Affected Shrimp. Dev. Comp. Immunol. 2020, 106, 103600. [Google Scholar] [CrossRef] [PubMed]
- Fraune, S.; Zimmer, M. Host-Specificity of Environmentally Transmitted Mycoplasma-Like Isopod Symbionts. Environ. Microbiol. 2008, 10, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zang, Y.; Song, K.; Ma, Y.; Dai, T.; Serwadda, A. A Meta-Transcriptomics Survey Reveals Changes in the Microbiota of the Chinese Mitten Crab Eriocheir sinensis Infected with Hepatopancreatic Necrosis Disease. Front. Microbiol. 2017, 8, 732. [Google Scholar] [CrossRef]
- Knupp, C.; Faisal, M.; Brenden, T.O.; Soto, E.; LaFrentz, B.R.; Griffin, M.J.; Wiens, G.D.; Cavender, W.; Van Vliet, D.; Loch, T.P. Ultraviolet Light Differentially Reduces Viability of Fish- and Fish Farm-Associated Flavobacteria (Families Flavobacteriaceae and Weeksellaceae). N. Am. J. Aquac. 2023, 85, 311–323. [Google Scholar] [CrossRef]
- Lightner, D.V. A Review of the Diseases of Cultured Penaeid Shrimps and Prawns with Emphasis on Recent Discoveries and Developments. In Proceedings of the First International Conference on the Culture of Penaeid Prawns/Shrimps, Iloilo City, Philippines, 4-7 December 1984; Taki, Y., Primavera, J.H., Llobrera, J.A., Eds.; Aquaculture Department, Southeast Asian Fisheries Development Center: Iloilo City, Philippines, 1985; pp. 79–103. [Google Scholar]
- Adel, M.; El-Sayed, A.-F.M.; Yeganeh, S.; Dadar, M.; Giri, S.S. Effect of Potential Probiotic Lactococcus lactis Subsp. lactis on Growth Performance, Intestinal Microbiota, Digestive Enzyme Activities, and Disease Resistance of Litopenaeus vannamei. Probiotics Antimicrob. Proteins 2017, 9, 150–156. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Cai, X.; Ai, Y.; Long, H.; Ren, W.; Huang, A.; Zhang, X.; Xie, Z.-Y. Dietary Supplementation of Astaxanthin is Superior to Its Combination with Lactococcus lactis in Improving the Growth Performance, Antioxidant Capacity, Immunity and Disease Resistance of White Shrimp (Litopenaeus vannamei). Aquac. Rep. 2022, 24, 101124. [Google Scholar] [CrossRef]

| Gene Name | Primer Name | Primer Sequences 5′–3′ | Annealing Temperature (°C) | Melting Temperature (°C) | Accession Number | Reference |
|---|---|---|---|---|---|---|
| Mr-2α2M | Mr-2α2M forward | GATATGAAGTTGATGGAAA | 60 | 78.4 | ABK60046.1 | Likittrakulwong et al., 2017 [22] |
| Mr-2α2M reverse | GTGAACTCTGGCTGGTAGTAA | |||||
| SPI | SPI forward | CACTTTAAGCCCGTGTGCGGTAAT | 58 | 84.5 | FJ429307.1 | Tewpair et al., 2008 [23] |
| SPI reverse | TGAACATCTTCGAGTGGGAACACC | |||||
| ß-actin | ß-actin forward | TTCACCATCGGCATTGAGAGGTTC | 60 | 85.1 | KY038927.1 | Likittrakulwong et al., 2017 [22] |
| ß-actin reverse | CACGTCGCACTTCATGATGGAGTT |
| Parameter | Groups | |||
|---|---|---|---|---|
| CA0 | CA1 | CA5 | CA10 | |
| Initial body weight (g) | 2.84 ± 0.47 | 2.71 ± 0.45 | 2.98 ± 0.52 | 2.85 ± 0.34 |
| Final body weight (g) | 4.01 ± 0.58 | 4.06 ± 0.75 | 4.35 ± 0.83 | 4.32 ± 0.81 |
| Average weight gain (g) | 1.17 ± 0.142 | 1.35 ± 0.124 | 1.38 ± 0.131 | 1.47 ± 0.192 |
| Average daily gain (g day−1) | 0.041 ± 0.010 | 0.048 ± 0.010 | 0.049 ± 0.013 | 0.052 ± 0.01 |
| Specific growth rate (% day−1) | 1.23 ± 0.02 c | 1.41 ± 0.02 b | 1.42 ± 0.01 b | 1.48 ± 0.02 a |
| Survival rate (%) | 64.44 ± 5.09 | 66.67 ± 3.33 | 64.44 ± 5.09 | 65.55 ± 1.92 |
| Intermolt period (Days) | 7.33 ± 0.57 | 6.66 ± 0.57 | 7.33 ± 0.57 | 7.66 ± 0.57 |
| Feeding Day | Gene | Organ | CA0 | CA1 | CA5 | CA10 |
|---|---|---|---|---|---|---|
| 12 h. | SPI | Gill | 1.00 ± 0.06 b | 0.76 ± 0.14 ab | 1.18 ± 0.27 ab | 1.35 ± 0.05 ab |
| Hepatopancreas | 1.00 ± 0.47 b | 1.03 ± 0.46 b | 4.98 ± 2.13 a | 6.25 ± 1.30 a | ||
| Hemolymph | 1.00 ± 0.37 b | 1.20 ± 0.215 b | 1.30 ± 0.62 b | 13.51 ± 3.15 a | ||
| Mr-2α2M | Gill | 1.00 ± 0.11 c | 1.42 ± 0.13 b | 1.60 ± 0.06 b | 1.88 ± 0.04 a | |
| Hepatopancreas | 1.00 ± 0.05 c | 0.99 ± 0.06 c | 2.05 ± 0.16 b | 3.00 ± 0.53 a | ||
| Hemolymph | 1.00 ± 0.28 b | 2.50 ± 0.83 a | 2.25 ± 0.87 a | 2.53 ± 1.66 a | ||
| 24 h. | SPI | Gill | 0.91 ± 0.28 a | 0.84 ± 0.29 a | 1.11 ± 0.22 a | 1.65 ± 1.23 a |
| Hepatopancreas | 1.13 ± 0.26 a | 1.50 ± 0.67 a | 1.07 ± 0.24 a | 1.31 ± 0.41 a | ||
| Hemolymph | 1.03 ± 0.2 c | 1.66 ± 0.48 c | 13.74 ± 2.32 b | 24.88 ± 4.7 a | ||
| Mr-2α2M | Gill | 1.06 ± 0.20 a | 1.12 ± 0.05 a | 0.99 ± 0.04 a | 1.23 ± 0.05 a | |
| Hepatopancreas | 1.11 ± 0.22 b | 2.68 ± 0.26 a | 2.98 ± 0.14 a | 2.99 ± 0.33 a | ||
| Hemolymph | 1.11 ± 0.31 b | 1.27 ± 1.06 b | 10.03 ± 3.40 a | 13.47 ± 4.95 a | ||
| 48 h. | SPI | Gill | 0.89 ± 0.04 b | 0.96 ± 0.38 b | 1.11 ± 0.16 b | 1.55 ± 0.17 a |
| Hepatopancreas | 1.15 ± 0.27 a | 0.75 ± 0.34 a | 1.25 ± 0.72 a | 1.30 ± 0.41 a | ||
| Hemolymph | 1.26 ± 0.59 c | 3.26 ± 1.99 bc | 6.44 ± 1.72 b | 12.36 ± 3.3 a | ||
| Mr-2α2M | Gill | 0.92 ± 0.30 b | 1.81 ± 0.26 a | 1.86 ± 0.24 a | 1.98 ± 0.43 a | |
| Hepatopancreas | 1.04 ± 0.15 b | 1.42 ± 0.34 b | 3.71 ± 0.25 a | 4.10 ± 0.76 a | ||
| Hemolymph | 1.02 ± 0.32 a | 1.05 ± 0.36 a | 1.35 ± 0.38 a | 1.75 ± 1.04 a | ||
| 72 h. | SPI | Gill | 0.96 ± 0.34 b | 1.32 ± 0.44 b | 2.24 ± 0.19 a | 2.84 ± 0.18 a |
| Hepatopancreas | 1.16 ± 0.33 a | 0.87 ± 0.39 a | 1.26 ± 0.30 a | 1.55 ± 0.12 a | ||
| Hemolymph | 1.01 ± 0.18 b | 1.12 ± 0.42 b | 3.49 ± 0.81 a | 5.93 ± 1.42 a | ||
| Mr-2α2M | Gill | 1.15 ± 0.26 a | 1.09 ± 0.10 a | 1.08 ± 0.23 a | 1.11 ± 0.13 a | |
| Hepatopancreas | 1.04 ± 0.13 a | 0.77 ± 0.10 a | 1.39 ± 0.30 a | 1.55 ± 0.21 a | ||
| Hemolymph | 1.08 ± 0.46 c | 2.99 ± 0.24 b | 4.47 ± 1.37 ab | 9.02 ± 3.24 a | ||
| 7 days | SPI | Gill | 0.83 ± 0.11 c | 1.49 ± 0.29 b | 1.77 ± 0.41 b | 3.27 ± 0.43 a |
| Hepatopancreas | 1.25 ± 0.42 b | 1.51 ± 0.68 b | 3.44 ± 1.06 a | 7.83 ± 2.82 a | ||
| Hemolymph | 1.01 ± 0.16 c | 2.30 ± 0.52 b | 5.07 ± 0.6 a | 5.56 ± 0.83 a | ||
| Mr-2α2M | Gill | 1.17 ± 0.28 b | 2.16 ± 0.58 a | 2.46 ± 0.32 a | 2.86 ± 0.56 a | |
| Hepatopancreas | 1.02 ± 0.10 a | 0.98 ± 0.20 a | 1.37 ± 0.21 a | 1.17 ± 0.05 a | ||
| Hemolymph | 1.02 ± 0.51 b | 1.42 ± 1.34 a | 1.43 ± 0.56 a | 2.47 ± 1.56 a | ||
| 4 days | SPI | Gill | 0.97 ± 0.14 c | 1.41 ± 0.22 bc | 1.80 ± 0.55 ab | 2.99 ± 0.88 a |
| Hepatopancreas | 1.09 ± 0.23 b | 1.49 ± 0.66 b | 2.98 ± 1.01 a | 7.43 ± 1.55 a | ||
| Hemolymph | 1.01 ± 0.17 d | 2.45 ± 0.41 c | 9.79 ± 0.58 b | 18.92 ± 3.23 a | ||
| Mr-2α2M | Gill | 1.04 ± 0.11 b | 2.05 ± 0.23 a | 2.24 ± 0.16 a | 2.47 ± 0.44 a | |
| Hepatopancreas | 1.03 ± 0.22 b | 0.99 ± 0.11 b | 6.02 ± 1.01 a | 7.22 ± 1.56 a | ||
| Hemolymph | 1.03 ± 0.53 b | 4.28 ± 1.98 a | 4.29 ± 0.97 a | 5.41 ± 1.22 a | ||
| 21 days | SPI | Gill | 1.07 ± 0.66 b | 1.22 ± 0.29 b | 1.44 ± 0.22 b | 2.00 ± 0.57 a |
| Hepatopancreas | 1.08 ± 0.22 c | 2.44 ± 0.67 b | 4.08 ± 0.23 a | 4.42 ± 0.66 a | ||
| Hemolymph | 1.03 ± 0.22 d | 1.89 ± 0.11 c | 6.42 ± 0.44 b | 10.70 ± 2.44 a | ||
| Mr-2α2M | Gill | 1.02 ± 0.23 b | 1.38 ± 0.55 ab | 1.47 ± 0.45 ab | 2.01 ± 0.43 a | |
| Hepatopancreas | 1.02 ± 0.44 b | 1.17 ± 0.55 b | 8.33 ± 1.32 a | 8.52 ± 1.05 a | ||
| Hemolymph | 1.03 ± 0.82 b | 4.15 ± 1.01 a | 4.20 ± 0.76 a | 5.22 ± 1.01 a | ||
| 28 days | SPI | Gill | 1.02 ± 0.26 b | 0.85± 0.32 b | 1.2 ± 0.31 b | 1.87± 0.46 a |
| Hepatopancreas | 1.16 ± 0.6 b | 5.71 ± 2.78 a | 5.65 ± 3.31 a | 5.84 ± 1.23 a | ||
| Hemolymph | 1.19 ± 0.45 b | 1.20 ± 0.18 b | 2.09 ± 0.2 a | 2.41± 0.11 a | ||
| Mr-2α2M | Gill | 1.02 ± 0.26 b | 1.00 ± 0.18 b | 1.00 ± 0.48 b | 1.99 ± 0.33 a | |
| Hepatopancreas | 1.16 ± 0.6 b | 1.26 ± 0.37 b | 10.02 ± 3.59 a | 13.46 ± 6.06 a | ||
| Hemolymph | 1.01 ± 0.66 c | 2.99 ± 0.53 b | 5.47 ± 3.06 b | 24.711 ± 3.71 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Changtor, P.; Pinmuang, D.; Nasalingkhan, C.; Yimtragool, N. Dietary Supplementation with Gotu Kola (Centella asiatica) Extract Enhanced Innate Immune Responses, Modulated Immune-Related Gene Expression, and Improved Gut Microbiota in Giant Freshwater Prawn (Macrobrachium rosenbergii). Animals 2025, 15, 2507. https://doi.org/10.3390/ani15172507
Changtor P, Pinmuang D, Nasalingkhan C, Yimtragool N. Dietary Supplementation with Gotu Kola (Centella asiatica) Extract Enhanced Innate Immune Responses, Modulated Immune-Related Gene Expression, and Improved Gut Microbiota in Giant Freshwater Prawn (Macrobrachium rosenbergii). Animals. 2025; 15(17):2507. https://doi.org/10.3390/ani15172507
Chicago/Turabian StyleChangtor, Phanupong, Donlaya Pinmuang, Channarong Nasalingkhan, and Nonglak Yimtragool. 2025. "Dietary Supplementation with Gotu Kola (Centella asiatica) Extract Enhanced Innate Immune Responses, Modulated Immune-Related Gene Expression, and Improved Gut Microbiota in Giant Freshwater Prawn (Macrobrachium rosenbergii)" Animals 15, no. 17: 2507. https://doi.org/10.3390/ani15172507
APA StyleChangtor, P., Pinmuang, D., Nasalingkhan, C., & Yimtragool, N. (2025). Dietary Supplementation with Gotu Kola (Centella asiatica) Extract Enhanced Innate Immune Responses, Modulated Immune-Related Gene Expression, and Improved Gut Microbiota in Giant Freshwater Prawn (Macrobrachium rosenbergii). Animals, 15(17), 2507. https://doi.org/10.3390/ani15172507





