Population Dynamics and Reintroduction Strategies for the Alpine Marmot in Romania
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Statistical Analysis
3. Results
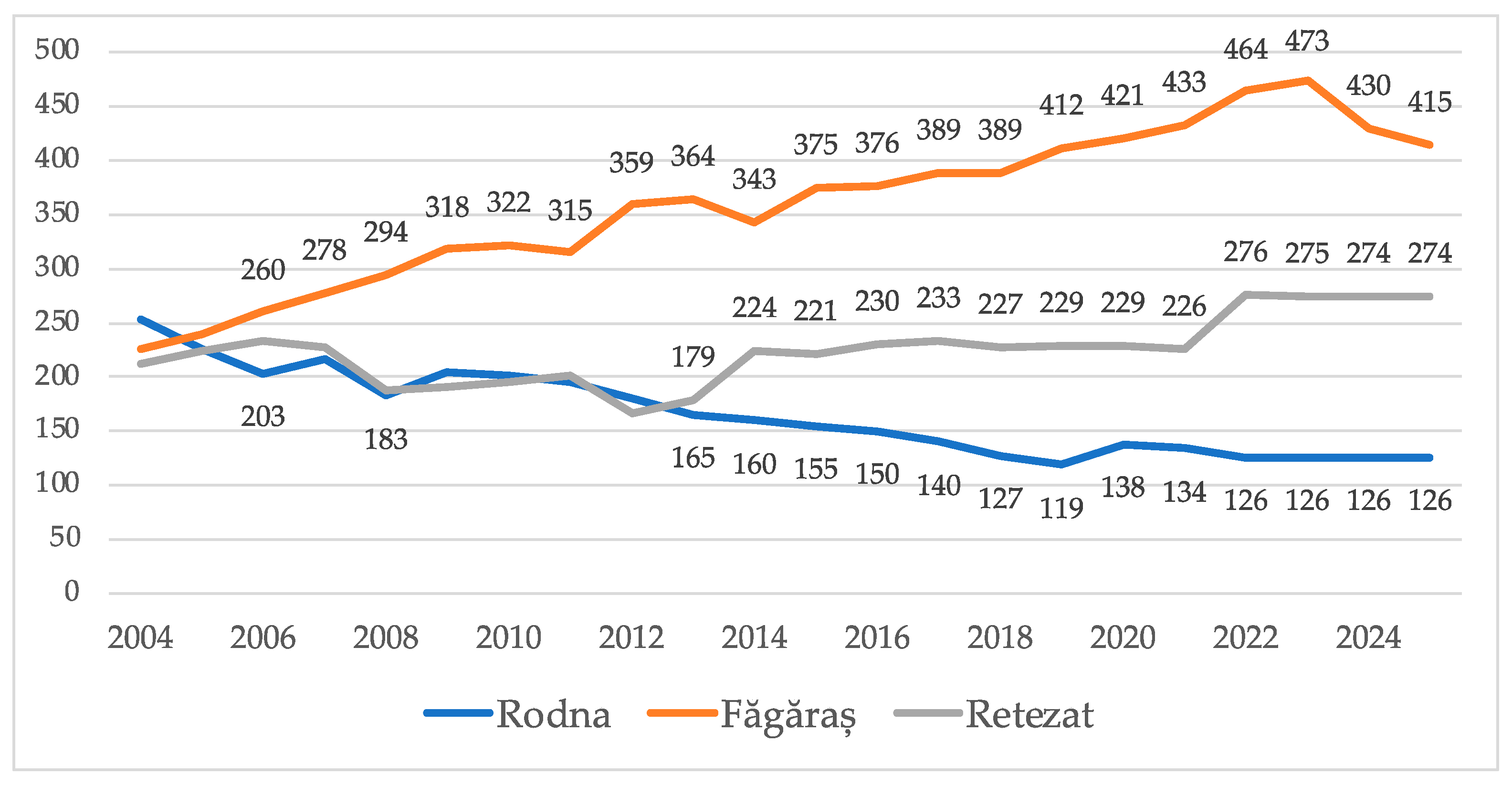
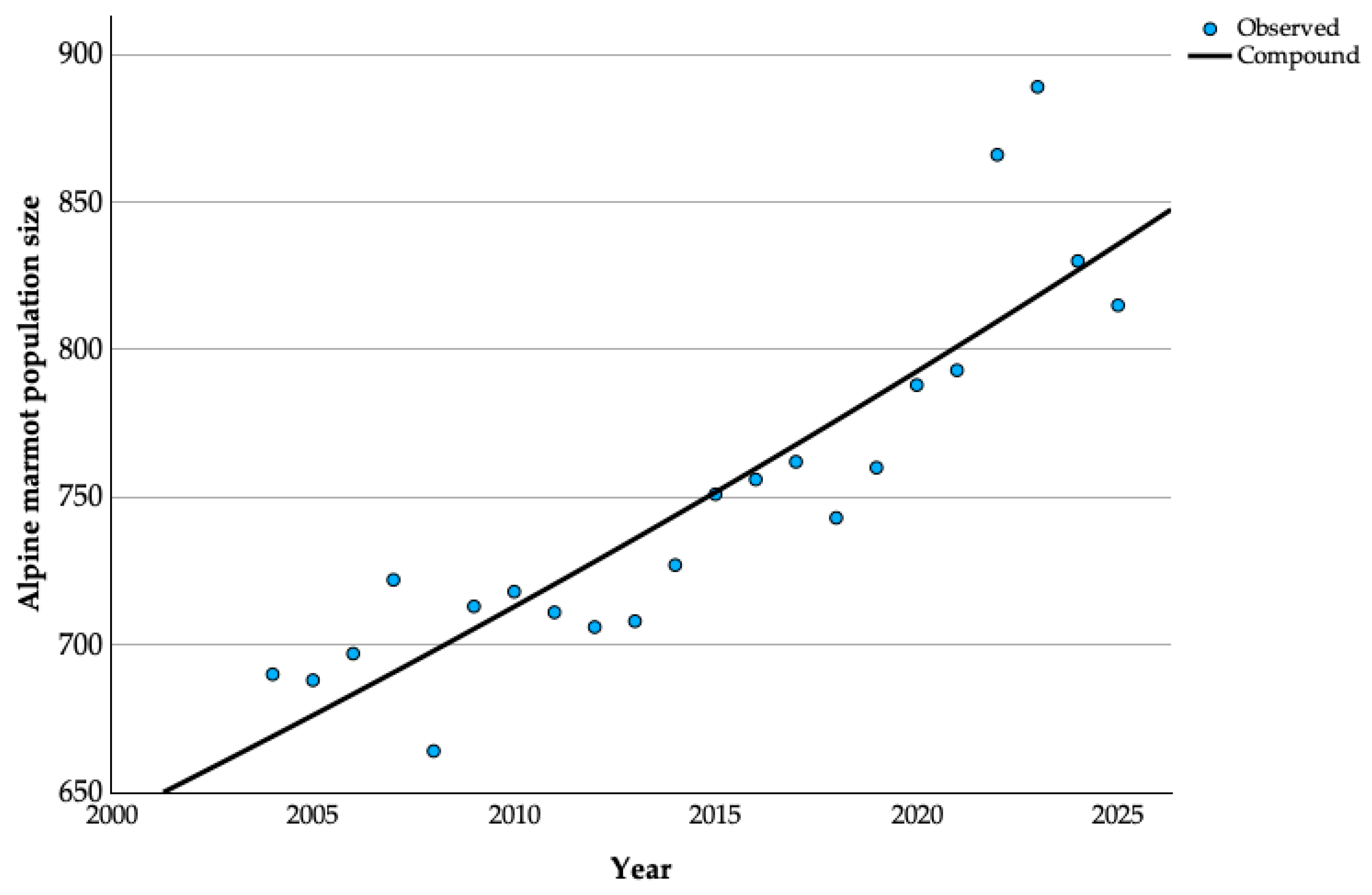
3.1. Alpine Marmot Population Growth in the Past Two Decades
3.2. Relationship Between Natural Resources and the Marmot Population
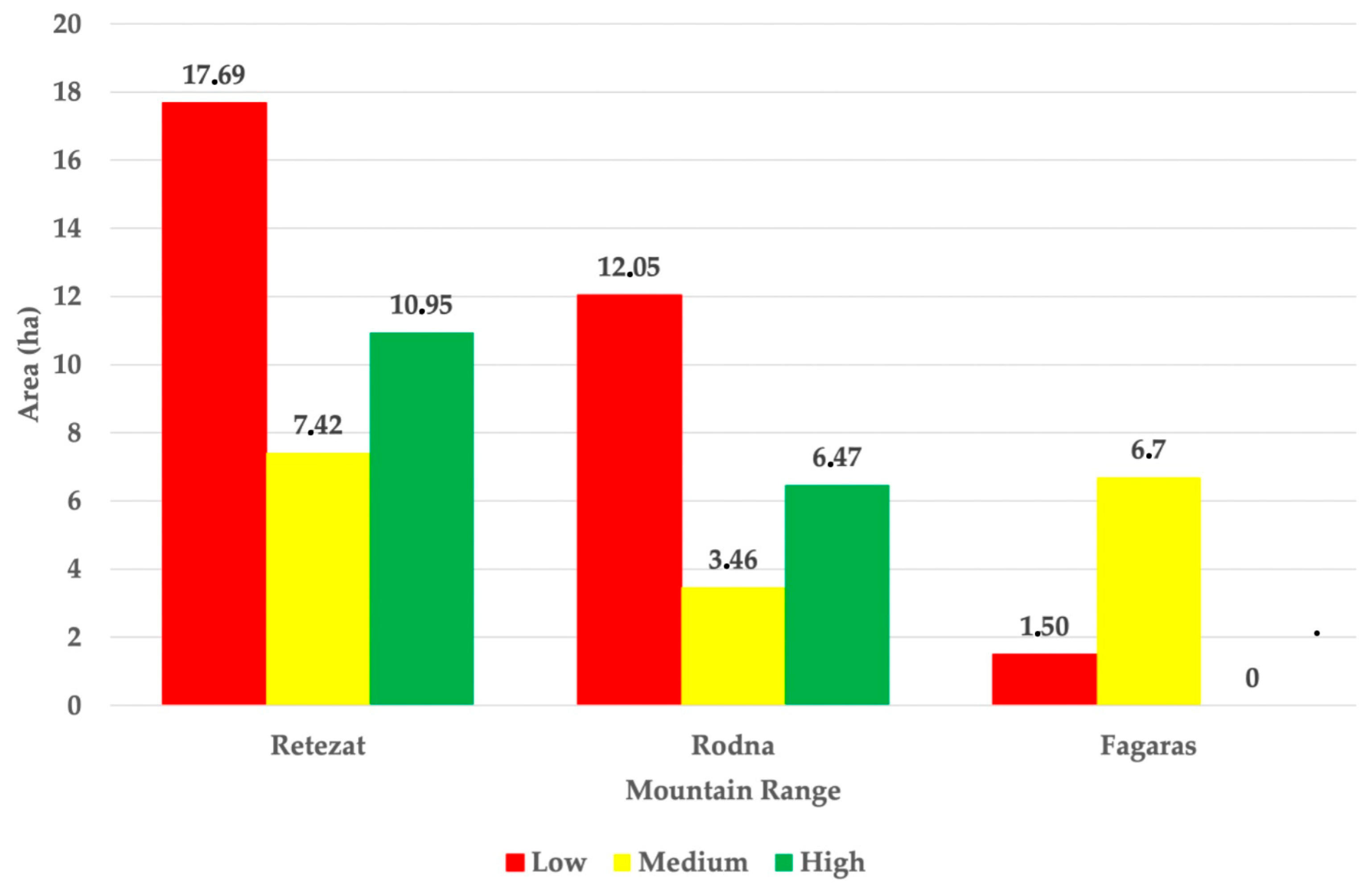
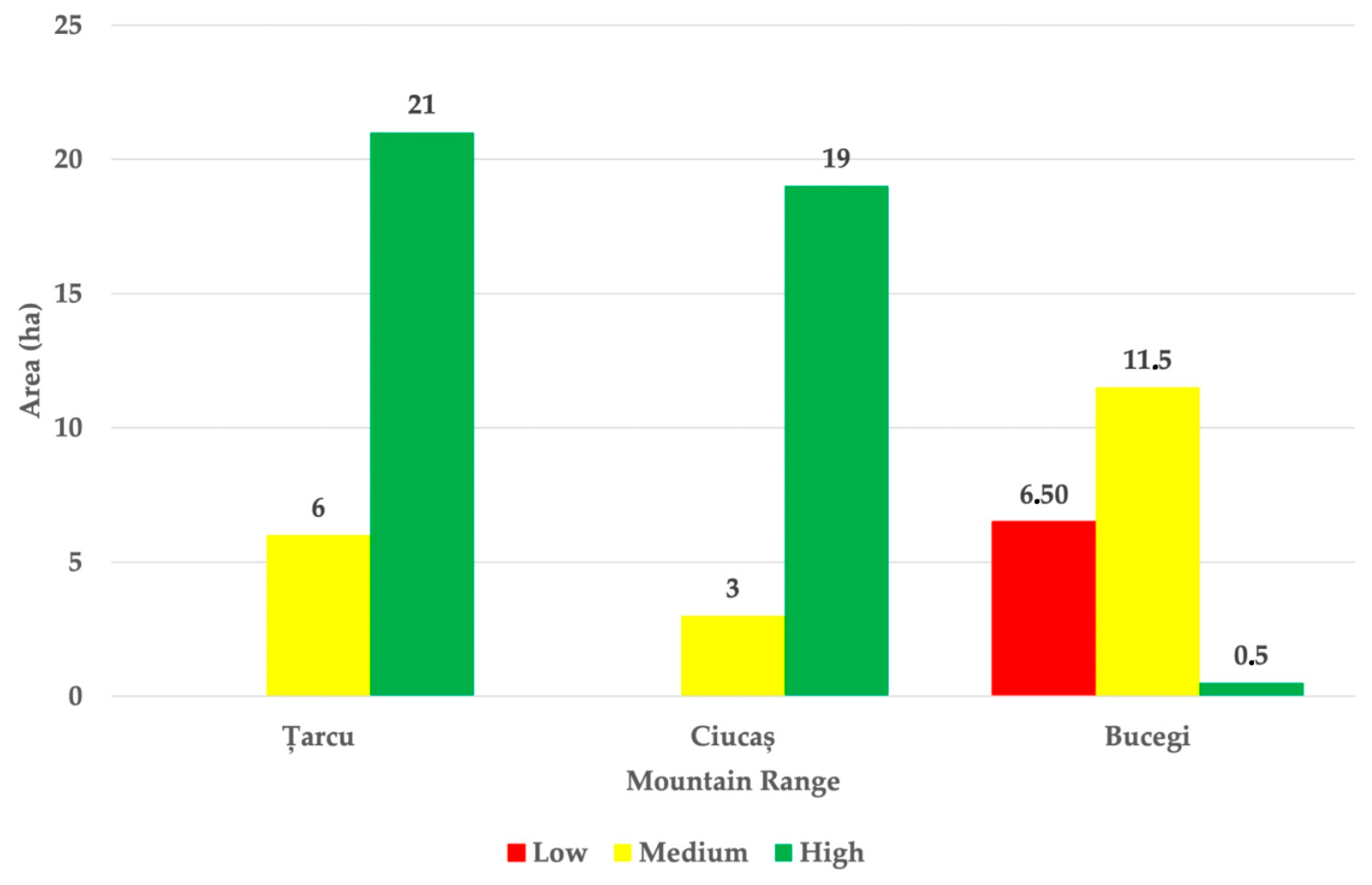
3.3. Habitat Suitability Index of the Already Colonized Regions
3.4. Ecological Diagnostic Key of the Possible Introduction Sites of the Alpine Marmot
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harrison, R.G.; Bogdanowicz, S.M.; Hoffmann, R.S.; Yensen, E.; Sherman, P.W. Phylogeny and evolutionary history of the ground squirrels (Rodentia: Marmotinae). J. Mamm. Evol. 2003, 10, 249–276. [Google Scholar] [CrossRef]
- Armitage, K.B. The evolution, ecology, and systematics of marmots. Oecologia Mont. 2000, 9, 1–18. [Google Scholar]
- Polly, P.D.; Cardini, A.; Davis, E.B.; Steppan, S.J. Marmot Evolution and Global Change in the Past 10 Million Years. Evolution of the Rodents: Advances in Phylogeny, Functional Morphology and Development; Cambridge University Press: Cambridge, UK, 2015; pp. 246–276. [Google Scholar]
- Ballová, Z.; Šibík, J. Microhabitat Utilization of the Tatra Marmot (Marmota marmota latirostris) in the Western Carpathian Mountains, Europe. Arct Antarct. Alp. Res. 2015, 47, 169–183. [Google Scholar] [CrossRef]
- Barash, D.P. Marmots: Social Behavior and Ecology; Standford University Press: Redwood City, CA, USA, 1989. [Google Scholar]
- Forti, A.; Byloos, C.; Arseni, M.; Volcan, G.; Dorigatti, E.; Donini, V.; Partel, P.; Marchesini, G. Late summer spatial behaviour of Alpine marmots Marmota marmota in the Eastern Italian Alps: Effects of intrinsic and extrinsic drivers. Mamm. Res. 2024, 70, 21–34. [Google Scholar] [CrossRef]
- Ruf, T.; Geiser, F. Daily torpor and hibernation in birds and mammals. Biol. Rev. 2015, 90, 891–926. [Google Scholar] [CrossRef]
- Milsom, W.K.; Zimmer, M.B.; Harris, M.B. Regulation of cardiac rhythm in hibernating mammals. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1999, 124, 383–391. [Google Scholar] [CrossRef]
- Blumstein, D.T. Social Effects on Emergence from Hibernation in Yellow-Bellied Marmots. J. Mammal. 2009, 90, 1184–1187. [Google Scholar] [CrossRef]
- Ruf, T.; Bieber, C. Why hibernate? Predator avoidance in the edible dormouse. Mamm. Res. 2023, 68, 1–11. [Google Scholar] [CrossRef]
- Stephens, P.A.; Frey-roos, F.; Arnold, W.; Sutherland, W.J. Model complexity and population predictions. The alpine marmot as a case study. J. Anim. Ecol. 2002, 71, 343–361. [Google Scholar] [CrossRef]
- Chibowski, P.; Zegarek, M.; Zarzycka, A.; Suska-Malawska, M. Ecosystem engineers in the extreme: The modest impact of marmots on vegetation cover and plant nitrogen and phosphorus content in a cold, extremely arid mountain environment. Ecol. Evol. 2023, 13, e9948. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tu, Y.; Wang, D.; Wang, B.; Li, Z.; Wang, J.; Zhang, X.; Shi, Z.; Yang, X. Research progress on the impact of small rodent disturbance on grassland ecosystem. Ecol. Front. 2025, 45, 1388–1396. [Google Scholar] [CrossRef]
- Valkó, O.; Tölgyesi, C.; Kelemen, A.; Bátori, Z.; Gallé, R.; Rádai, Z.; Bragina, T.M.; Bragin, Y.A.; Deák, B. Steppe Marmot (Marmota bobak) as ecosystem engineer in arid steppes. J. Arid Environ. 2021, 184, 104244. [Google Scholar] [CrossRef]
- Barrio, I.C.; Herrero, J.; Bueno, C.G.; López, B.C.; Aldezabal, A.; Campos-Arceiz, A.; García-González, R. The successful introduction of the alpine marmot Marmota marmota in the Pyrenees, Iberian Peninsula, Western Europe. Mamm. Rev. 2013, 43, 142–155. [Google Scholar] [CrossRef]
- Noyes, D.H.; Holmes, W.G. Behavioral Responses of Free-Living Hoary Marmots to a Model Golden Eagle. J. Mammal. 1979, 60, 408–411. [Google Scholar] [CrossRef]
- Pedrini, P.; Sergio, F. Regional conservation priorities for a large predator: Golden eagles (Aquila chrysaetos) in the Alpine range. Biol. Conserv. 2002, 103, 163–172. [Google Scholar] [CrossRef]
- Poudel, B.S. The Effects of Pastoralism on the Behaviour of the Himalayan Marmot (Marmota himalayana) in High Altitude Rangelands in Nepal. Ph.D. Thesis, Charles Sturt University, Bathurst, Australia, 2016. [Google Scholar]
- Poudel, B.S.; Spooner, P.G.; Matthews, A. Pastoralist disturbance effects on Himalayan marmot foraging and vigilance activity. Ecol. Res. 2016, 31, 93–104. [Google Scholar] [CrossRef]
- Herrero, J.; García-González, R.; García-Serrano, A.; Garcia-Gonzalez, R.; Garda-Serrano, A. Altitudinal Distribution of Alpine Marmot (Marmota marmota) in the Pyrenees, Spain/France. Arct. Alp. Res. 1994, 26, 328–331. [Google Scholar] [CrossRef]
- Glad, A.; Mallard, F. Alpine marmot (Marmota marmota) distribution evolution under climate change: The use of species distribution models at a local scale in the western Pyrenees massif (France). Ecol. Inform. 2022, 69, 101646. [Google Scholar] [CrossRef]
- Galluzzi, M.; Armanini, M.; Ferrari, G.; Zibordi, F.; Scaravelli, D.; Chirici, G.; Nocentini, S.; Models, A. Habitat Suitability Models, for ecological study of the alpine marmot in the central Italian Alps. Ecol. Inform. 2017, 37, 10–17. [Google Scholar] [CrossRef]
- Preleuthner, M.; Pinsker, W.; Kruckenhauser, L.; Miller, W.J.; Prosl, H. Alpine marmots in Austria. Acta Theriol. 1995, 3, 87–100. [Google Scholar] [CrossRef]
- López, B.C.; Pino, J.; López, A. Explaining the successful introduction of the alpine marmot in the Pyrenees. Biol. Invasions 2010, 12, 3205–3217. [Google Scholar] [CrossRef]
- Geacu, S.; Dumitrascu, M. Alpine marmot populations after four decades of living in the glacial areas of the Fagaras, Rodna and Retezat Mountains, Romania. J. Environ. Biol. 2017, 38, 703–711. [Google Scholar] [CrossRef]
- Filipascu, A. The Alpine Marmot in the Wilderness if Our Ancestors; Editura Știintifica: Bucharest, Romania, 1969. [Google Scholar]
- Ramousse, R.; Le Berre, M. Management of Alpine Marmot populations. Oecologia Mont. 1993, 2, 23–29. [Google Scholar]
- Hume, I.D.; Beiglböck, C.; Ruf, T.; Frey-Roos, F.; Bruns, U.; Arnold, W. Seasonal changes in morphology and function of the gastrointestinal tract of free-living alpine marmots (Marmota marmota). J. Comp. Physiol. B 2002, 172, 197–207. [Google Scholar]
- Garin, I.; Aldezabal, A.; Herrero, J.; Garcia-Serrano, A.; Remon, J.L. Diet selection of the alpine Marmot (Marmota m. marmota L.) in the Pyrénées. Rev. Ecol. 2008, 63, 383–390. [Google Scholar] [CrossRef]
- Armitage, K.B. Alarm responses of the yellow-bellied marmot. In Marmot. Biology; Cambridge University Press: Cambridge, UK, 2014; pp. 195–210. [Google Scholar]
- Chapron, G.; Kaczensky, P.; Linnell, J.D.C.; Von Arx, M.; Huber, D.; Andrén, H.; López-Bao, J.V.; Adamec, M.; Álvares, F.; Anders, O.; et al. Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 2014, 346, 35–51. [Google Scholar] [CrossRef]
- Werner, J.R. The Endangered Vancouver Island Marmot: Allee Effects and Reintroduction Success. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2006. [Google Scholar]
- Brashares, J.S.; Werner, J.R.; Sinclair, A.R.E. Social ‘meltdown’ in the demise of an island endemic: Allee effects and the Vancouver Island marmot. J. Anim. Ecol. 2010, 79, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Minea, G.; Mititelu-Ionuș, O.; Gyasi-Agyei, Y.; Ciobotaru, N.; Rodrigo-Comino, J. Impacts of Grazing by Small Ruminants on Hillslope Hydrological Processes: A Review of European Current Understanding. Water Resour. Res. 2022, 58, e2021WR030716. [Google Scholar] [CrossRef]
- Parlamentul României. Legea 407/2006. Legea Vânătorii și a Protecției Fondului Cinegetic; Monitorul Oficial 944/2006; Parlamentul României: Bucharest, Romania, 2006.
- Ministerul Mediului, Apelor și Pădurilor. Instrucțiuni din 2 Noiembrie 2022 Pentru Evaluarea Efectivelor Unor Specii de Faună Cinegetică Admise la Vânătoare și Pentru Reglementarea Modului de Stabilire a Cotelor de Recoltă Pentru Acestea; ORDIN nr. 2.847/2022; MONITORUL OFICIAL 1094/2022; Ministerul Mediului, Apelor și Pădurilor: Bucharest, Romania, 2022.
- Wang, S.L.; Hou, F.J. Burrow characteristics and ecological significance of Marmota himalayana in the northeastern Qinghai-Tibetan Plateau. Ecol. Evol. 2021, 11, 9100–9109. [Google Scholar] [CrossRef] [PubMed]
- Hardalau, D.; Fedorca, M.; Popovici, D.-C.; Ionescu, G.; Fedorca, A.; Mirea, I.; Daniel, I.; Ionescu, O. Insights in Managing Ungulates Population and Forest Sustainability in Romania. Diversity 2025, 17, 194. [Google Scholar] [CrossRef]
- Ministerul Mediului, Apelor și Pădurilor. Ordin nr. 393/2002 pentru aprobarea cheilor de bonitare și a densităților optime pentru specii de vânat; Ministerul Mediului, Apelor și Pădurilor: Bucharest, Romania, 2002.
- Almasan, H. Bonitatea Fondurilor de Vânătoare și Efectivele la Principalele Specii de Vânat; Redacția de Propaganda Tehnica Agricola: Bucharest, Romania, 1989. [Google Scholar]
- Borgo, A.; Vettorazzo, E.; Martino, N. Dynamics of the colonization process in reintroduced populations of the alpine marmot. Ethol. Ecol. Evol. 2009, 21, 317–323. [Google Scholar] [CrossRef]
- Viviano, A.; Mori, E. Aliens in their native country: The case of the Alpine marmot Marmota marmota (Linnaeus, 1758) (Mammalia, Rodentia) in the Apennine ridge. Biogeographia 2021, 36, 1–8. [Google Scholar] [CrossRef]
- Giari, C.; Corlatti, L.; Morocutti, E.; Storch, I.; Zenth, F. Captures do not affect escape response to humans in Alpine marmot. Wildlife Biol. 2024, 2024, e01292. [Google Scholar] [CrossRef]
- Aryal, A.; Brunton, D.; Ji, W.; Rothman, J.; Coogan, S.C.P.; Adhikari, B.; Su, J.; Raubenheimer, D. Habitat, diet; macronutrient, and fiber balance of Himalayan marmot (Marmota himalayana) in the Central Himalaya, Nepal. J. Mammal. 2015, 96, 308–316. [Google Scholar] [CrossRef]
- Turnock, B.Y.; Litt, A.R.; Vore, J.M.; Hammond, C.A.M. Habitat characteristics of the hoary marmot: Assessing distribution limitations in Montana. Ecosphere 2017, 8, e01977. [Google Scholar] [CrossRef]
- Řičánková, V.P.; Riegert, J.; Semančíková, E.; Hais, M.; Čejková, A.; Prach, K. Habitat preferences in gray marmots (Marmota baibacina). Acta Theriol. 2014, 59, 317–324. [Google Scholar] [CrossRef]
- Haas, M.; Tomíková, K.; Janiga, M.; Abduakassov, A.; Ballová, Z.K. Indication of air pollution based on the comparison of mercury and other elements in the faeces of marmots from the Western Carpathians and the Dzungarian Alatau. Environ. Sci. Pollut. Res. 2025, 32, 3617–3628. [Google Scholar] [CrossRef]
- Armstrong, D.P.; Reynolds, M.H. Modelling Reintroduced Populations: The State of the Art and Future Directions. In Reintroduction Biology: Integrating Science and Management; John Wiley and Sons: Hoboken, NJ, USA, 2012; pp. 165–222. [Google Scholar]
- Lloyd, N.A.; Hostetter, N.J.; Jackson, C.L.; Converse, S.J.; Moehrenschlager, A. Optimizing release strategies: A stepping-stone approach to reintroduction. Anim. Conserv. 2019, 22, 105–115. [Google Scholar] [CrossRef]
- Singer, F.J.; Williams, E.; Miller, M.W.; Zeigenfuss, L.C. Population growth, fecundity, and survivorship in recovering populations of bighorn sheep. Restor. Ecol. 2000, 8, 75–84. [Google Scholar] [CrossRef]
- Haig, S.; Ballou, J.; Derrickson, S. Management Options for Preserving Genetic Diversity: Reintroduction of Guam Rails to the Wild. Conserv. Biol. 1990, 4, 290–300. [Google Scholar] [CrossRef]
- Bichet, C.; Sauzet, S.; Averty, L.; Dupont, P.; Ferrandiz-Rovira, M.; Ferrari, C.; Figueroa, I.; Tafani, M.; Rézouki, C.; López, B.C.; et al. Multiple geographic origins and high genetic differentiation of the Alpine marmots reintroduced in the Pyrenees. Conserv. Genet. 2016, 17, 1157–1169. [Google Scholar] [CrossRef]
- Uchida, K.; Blumstein, D.T. Habituation or sensitization? Long-term responses of yellow-bellied marmots to human disturbance. Behav. Ecol. 2021, 32, 668–678. [Google Scholar] [CrossRef]
- Popov, I.; Moiseev, A.; Iurmanov, A.; Romanov, A.; Karpov, E.; Orlova, K.; Tereshchenko, N.; Emets, E.; Lebedev, Y.; Gnedenko, A. Impact of tourism on pristine habitats at the Avachinsky Pass (Kamchatka), a World Heritage Site. Geogr. Environ. Sustain. 2021, 17, 18–25. [Google Scholar] [CrossRef]
- Morgan, A.; Monclús, R.; Nelson, J.; Foli, E.; Chunwang, L.; Blumstein, D.T. How do humans impact yellow-bellied marmots? An integrative analysis. Appl. Anim. Behav. Sci. 2021, 245, 105495. [Google Scholar] [CrossRef]
- Dumitru, L.M.; Stoian, A.C.; Darie, L.; Ciobotaru, E. Brown bear maulings on domestic animals in Romania- Preliminary study. Sci. Papers. Ser. D Anim. Sci. 2017, 60, 343. [Google Scholar]
- Stăncioiu, P.T.; Dutcă, I.; Bălăcescu, M.C.; Ungurean, Ş.V. Coexistence with Bears in Romania: A Local Community Perspective. Sustainability 2019, 11, 7167. [Google Scholar] [CrossRef]
- Miller, K.; Ritchie, E.; Weston, A. Free-Ranging Dogs and Wildlife Conservation. In Free-Ranging Dogs & Wildlife Conservation; Gompper, M., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 286–304. [Google Scholar]
- Wang, Z.; Kang, Y.; Wang, Y.; Tan, Y.; Yao, B.; An, K.; Su, J. Himalayan Marmot (Marmota himalayana) Redistribution to High Latitudes under Climate Change. Animals 2023, 13, 2736. [Google Scholar] [CrossRef] [PubMed]
- Mainini, B.; Neuhaus, P.; Ingold, P. Behaviour of marmots marmota marmota under the influence of different hiking activities. Biol. Conserv. 1993, 64, 161–164. [Google Scholar] [CrossRef]

| Variable | Code | Type | Min. | Max. | Mean | Type |
|---|---|---|---|---|---|---|
| Altitude | ALT | Numerical | 1600 | 2324 | 2006 | Numerical |
| Exposition | EXP | Categorical (8 levels) | Ordinal | |||
| Slope | SL | Numerical | 10 | 40 | 25.32 | Numerical |
| Territory size | TS | Numerical | 0.3 | 6.47 | 1.95 | Numerical |
| Active entrance | AE | Numerical | 0 | 50 | 8.18 | Numerical |
| Abandoned entrance | ABE | Numerical | 0 | 30 | 4.56 | Numerical |
| Anthropic factor | AF | Categorical (3 levels) | Ordinal | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gridan, A.; Sîrbu, G.; Baciu, I.; Ionescu, G.; Ionescu, O.; Hardalau, D. Population Dynamics and Reintroduction Strategies for the Alpine Marmot in Romania. Animals 2025, 15, 2496. https://doi.org/10.3390/ani15172496
Gridan A, Sîrbu G, Baciu I, Ionescu G, Ionescu O, Hardalau D. Population Dynamics and Reintroduction Strategies for the Alpine Marmot in Romania. Animals. 2025; 15(17):2496. https://doi.org/10.3390/ani15172496
Chicago/Turabian StyleGridan, Alexandru, George Sîrbu, Iulia Baciu, Georgeta Ionescu, Ovidiu Ionescu, and Darius Hardalau. 2025. "Population Dynamics and Reintroduction Strategies for the Alpine Marmot in Romania" Animals 15, no. 17: 2496. https://doi.org/10.3390/ani15172496
APA StyleGridan, A., Sîrbu, G., Baciu, I., Ionescu, G., Ionescu, O., & Hardalau, D. (2025). Population Dynamics and Reintroduction Strategies for the Alpine Marmot in Romania. Animals, 15(17), 2496. https://doi.org/10.3390/ani15172496









