Refinement of the Lipopolysaccharide-Induced Synovitis Model in Equine Middle Carpal Joints
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
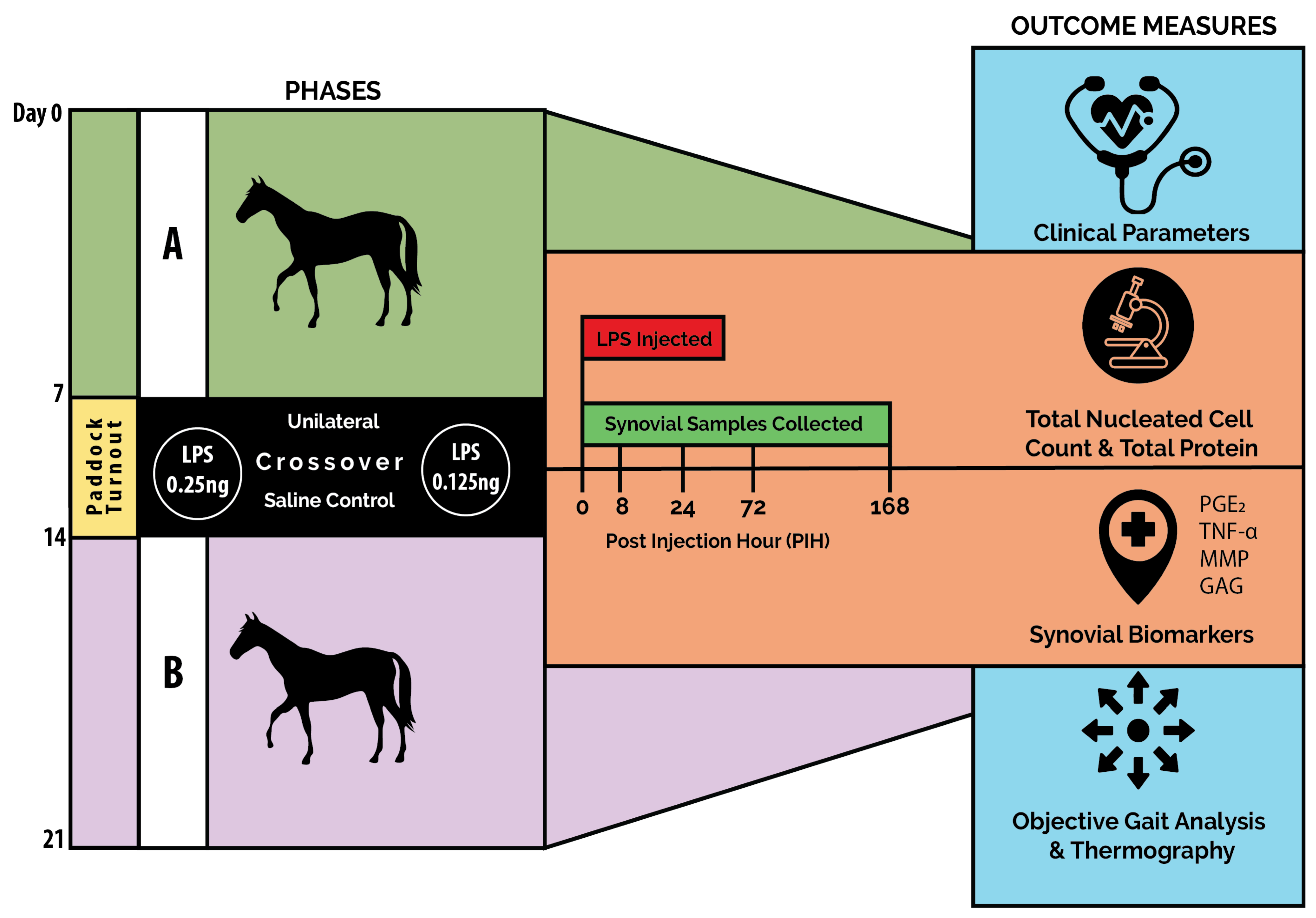
2.1. Study Design
2.2. Experimental Animals
2.3. Experimental Protocol
2.3.1. Preparation of LPS
2.3.2. Induction of Inflammation
2.4. Clinical Evaluations
2.4.1. Welfare Monitoring
2.4.2. Clinical Measurements
2.4.3. Lameness Evaluation
2.4.4. Thermography
2.5. Synovial Fluid Analysis
Synovial Fluid Biomarker Analysis
2.6. Statistical Analysis
3. Results
3.1. Welfare Monitoring
3.2. Clinical Measurements
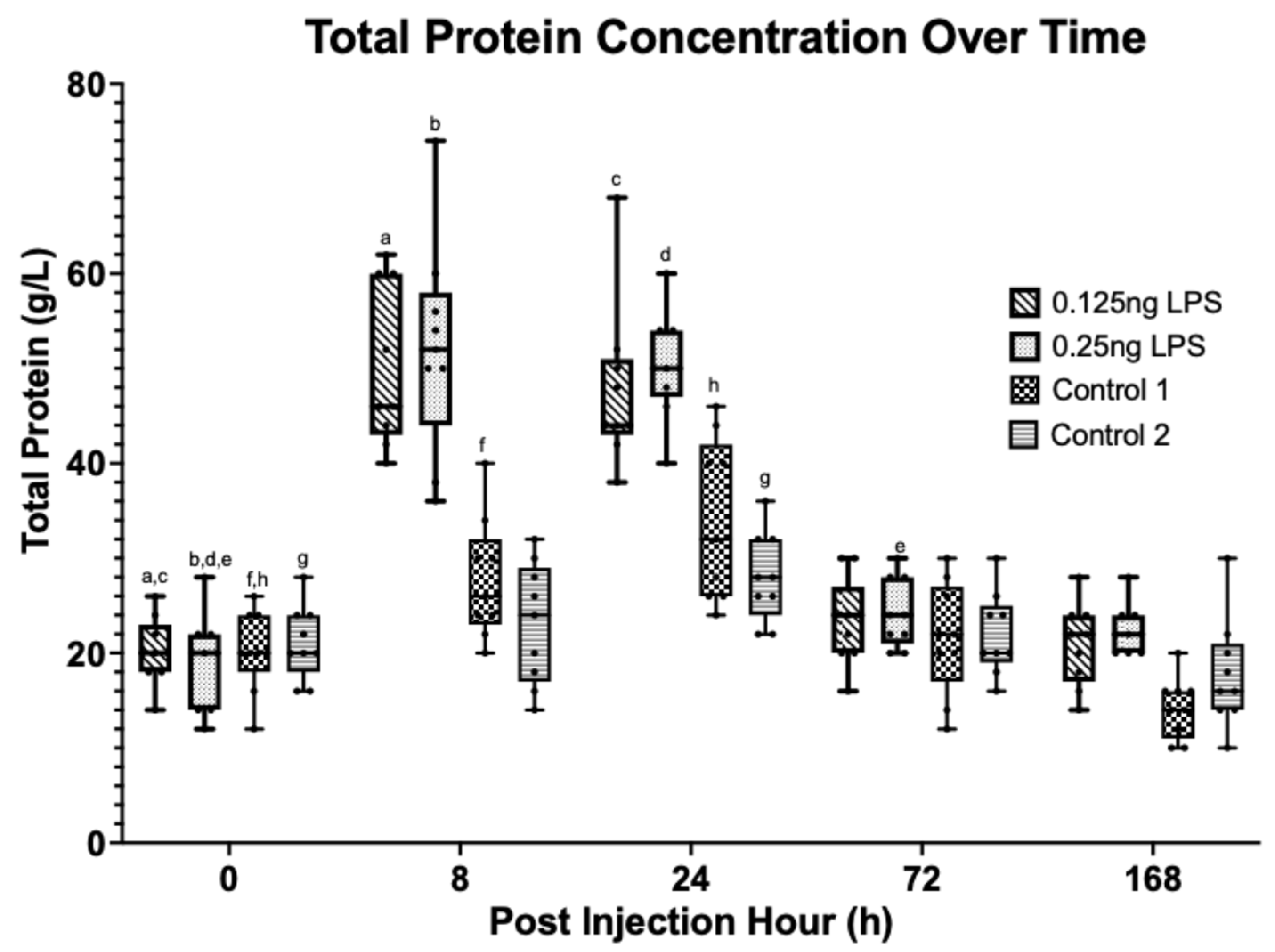
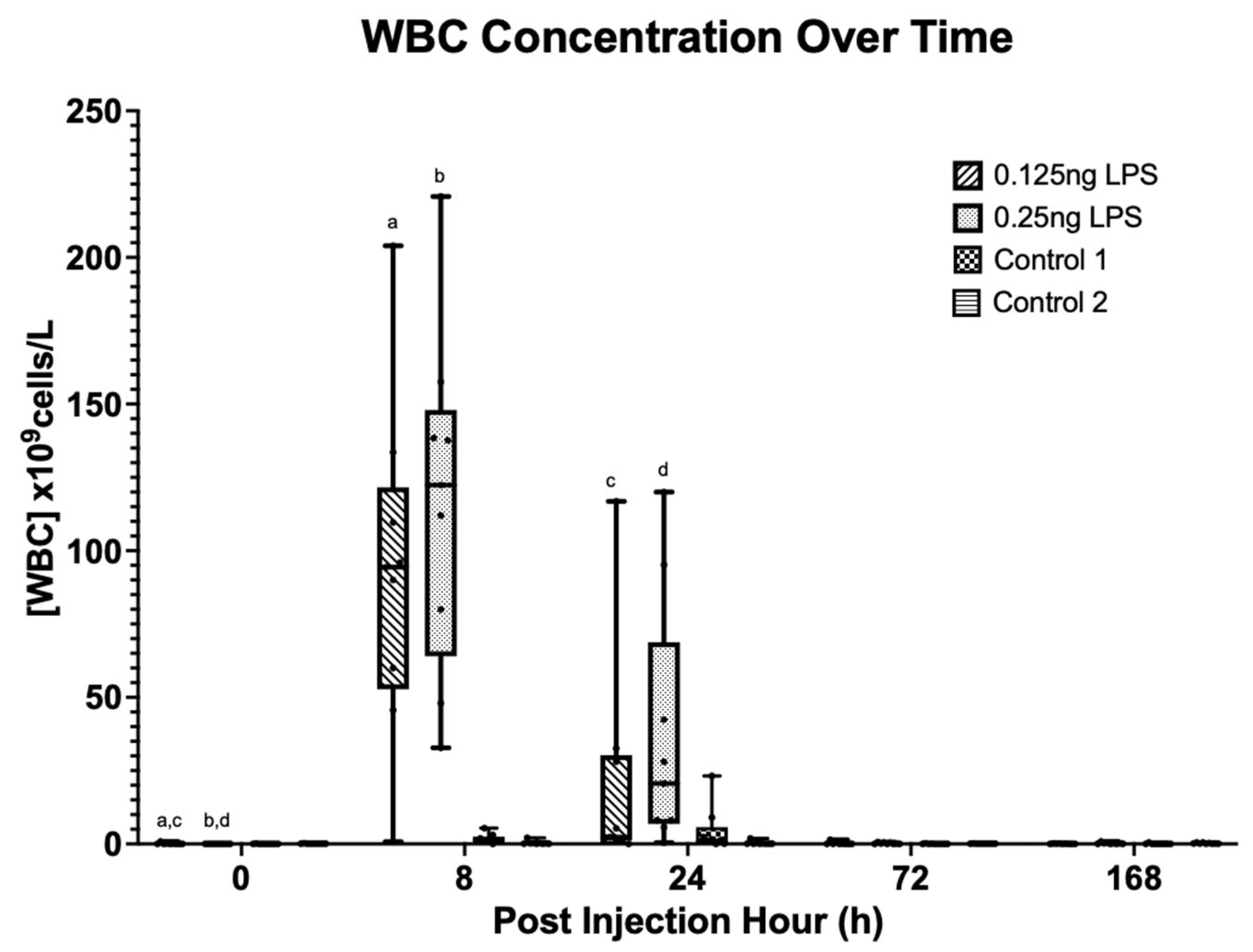
3.3. Synovial Cytology
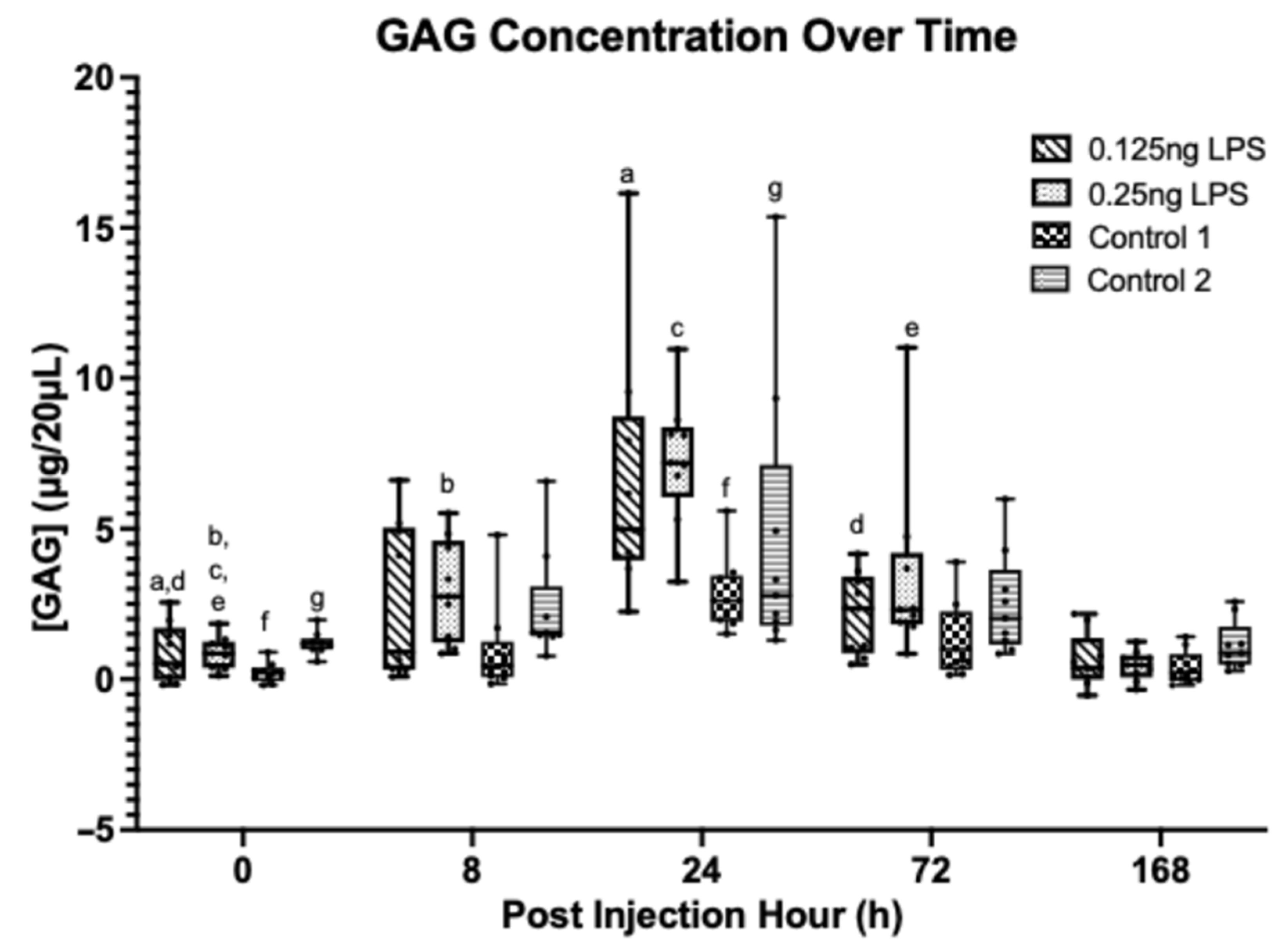
3.4. Synovial Biomarkers
3.5. Objective Gait Analysis
3.6. Thermographic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Firth, E.C.; Wensing, T.; Seuren, F. An Induced Synovitis Disease Model in Ponies. Cornell Vet. 1987, 77, 107–118. [Google Scholar]
- Palmer, J.L.; Bertone, A.L. Experimentally-Induced Synovitis as a Model for Acute Synovitis in the Horse. Equine Vet. J. 1994, 26, 492–495. [Google Scholar] [CrossRef]
- Kay, A.T.; Bolt, D.M.; Ishihara, A.; Rajala-Schultz, P.J.; Bertone, A.L. Anti-Inflammatory and Analgesic Effects of Intra-Articular Injection of Triamcinolone Acetonide, Mepivacaine Hydrochloride, or Both on Lipopolysaccharide-Induced Lameness in Horses. Am. J. Vet. Res. 2008, 69, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- de Grauw, J.C.; van de Lest, C.H.A.; Brama, P.A.J.; Rambags, B.P.B.; van Weeren, P.R. In Vivo Effects of Meloxicam on Inflammatory Mediators, MMP Activity and Cartilage Biomarkers in Equine Joints with Acute Synovitis. Equine Vet. J. 2009, 41, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Ross, T.N.; Kisiday, J.D.; Hess, T.; McIlwraith, C.W. Evaluation of the Inflammatory Response in Experimentally Induced Synovitis in the Horse: A Comparison of Recombinant Equine Interleukin 1 Beta and Lipopolysaccharide. Osteoarthr. Cartil. 2012, 20, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Sladek, S.; Kearney, C.; Crean, D.; Brama, P.A.J.; Tajber, L.; Fawcett, K.; Labberte, M.C.; Leggett, B.; Brayden, D.J. Intra-Articular Delivery of a Nanocomplex Comprising Salmon Calcitonin, Hyaluronic Acid, and Chitosan Using an Equine Model of Joint Inflammation. Drug Deliv. Transl. Res. 2018, 8, 1421–1435. [Google Scholar] [CrossRef]
- Knych, H.K.; Arthur, R.M.; McKemie, D.S.; Baden, R.W.; Seminoff, K.; Kass, P.H. Pharmacokinetics and Anti-Inflammatory Effects of Flunixin Meglumine as a Sole Agent and in Combination with Phenylbutazone in Exercised Thoroughbred Horses. Equine Vet. J. 2021, 53, 102–116. [Google Scholar] [CrossRef]
- Van de Water, E.; Oosterlinck, M.; Korthagen, N.M.; Duchateau, L.; Dumoulin, M.; van Weeren, P.R.; Olijve, J.; van Doorn, D.A.; Pille, F. The Lipopolysaccharide Model for the Experimental Induction of Transient Lameness and Synovitis in Standardbred Horses. Vet. J. 2021, 270, 105626. [Google Scholar] [CrossRef]
- Castro-Cuellar, G.; Cremer, J.; Queiroz-Williams, P.; Knych, H.K.; Leise, B.S. Pharmacokinetics of Intra-articular Buprenorphine in Horses with Lipopolysaccharide-induced Synovitis. J. Vet. Pharmacol. Ther. 2023, 46, jvp.13119. [Google Scholar] [CrossRef]
- McCoy, A.M. Animal Models of Osteoarthritis: Comparisons and Key Considerations. Vet. Pathol. 2015, 52, 803–818. [Google Scholar] [CrossRef]
- Bertoni, L.; Jacquet-Guibon, S.; Branly, T.; Legendre, F.; Desancé, M.; Mespoulhes, C.; Melin, M.; Hartmann, D.-J.; Schmutz, A.; Denoix, J.-M.; et al. An Experimentally Induced Osteoarthritis Model in Horses Performed on Both Metacarpophalangeal and Metatarsophalangeal Joints: Technical, Clinical, Imaging, Biochemical, Macroscopic and Microscopic Characterization. PLoS ONE 2020, 15, e0235251. [Google Scholar] [CrossRef]
- Manivong, S.; Cullier, A.; Audigié, F.; Banquy, X.; Moldovan, F.; Demoor, M.; Roullin, V.G. New Trends for Osteoarthritis: Biomaterials, Models and Modeling. Drug Discov. Today 2023, 28, 103488. [Google Scholar] [CrossRef] [PubMed]
- Poole, S.; Dawson, P.; Gaines Das, R.E. Second International Standard for Endotoxin: Calibration in an International Collaborative Study. J. Endotoxin Res. 1997, 4, 221–231. [Google Scholar] [CrossRef]
- Hawkins, D.L.; MacKay, R.J.; Gum, G.G.; Colahan, P.T.; Meyer, J.C. Effects of Intra-Articularly Administered Endotoxin on Clinical Signs of Disease and Synovial Fluid Tumor Necrosis Factor, Interleukin 6, and Prostaglandin E2 Values in Horses. Am. J. Vet. Res. 1993, 54, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Kearney, C.M.; Korthagen, N.M.; Plomp, S.G.M.; Labberté, M.C.; de Grauw, J.C.; van Weeren, P.R.; Brama, P.A.J. Treatment Effects of Intra-Articular Triamcinolone Acetonide in an Equine Model of Recurrent Joint Inflammation. Equine Vet. J. 2021, 53, 1277–1286. [Google Scholar] [CrossRef]
- Kearney, C.M.; Khatab, S.; van Buul, G.M.; Plomp, S.G.M.; Korthagen, N.M.; Labberté, M.C.; Goodrich, L.R.; Kisiday, J.D.; Van Weeren, P.R.; van Osch, G.J.V.M.; et al. Treatment Effects of Intra-Articular Allogenic Mesenchymal Stem Cell Secretome in an Equine Model of Joint Inflammation. Front. Vet. Sci. 2022, 9, 907616. [Google Scholar] [CrossRef]
- Owens, J.G.; Kamerling, S.G.; Stanton, S.R.; Keowen, M.L.; Prescott-Mathews, J.S. Effects of Pretreatment with Ketoprofen and Phenylbutazone on Experimentally Induced Synovitis in Horses. Am. J. Vet. Res. 1996, 57, 866–874. [Google Scholar] [CrossRef]
- Pfau, T. EquiGait Wireless: Instruction Manual; EquiGait Ltd.: Hertford, UK, 2018. [Google Scholar]
- McCracken, M.J.; Kramer, J.; Keegan, K.G.; Lopes, M.; Wilson, D.A.; Reed, S.K.; LaCarrubba, A.; Rasch, M. Comparison of an Inertial Sensor System of Lameness Quantification with Subjective Lameness Evaluation. Equine Vet. J. 2012, 44, 652–656. [Google Scholar] [CrossRef]
- Neumann, U.; Kubota, H.; Frei, K.; Ganu, V.; Leppert, D. Characterization of Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2, a Fluorogenic Substrate with Increased Specificity Constants for Collagenases and Tumor Necrosis Factor Converting Enzyme. Anal. Biochem. 2004, 328, 166–173. [Google Scholar] [CrossRef]
- Levenston, M. Fact versus Artifact: Avoiding Erroneous Estimates of Sulfated Glycosaminoglycan Content Using the Dimethylmethylene Blue Colorimetric Assay for Tissue-Engineered Constructs. Eur. Cell. Mater. 2015, 29, 224–236. [Google Scholar] [CrossRef]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved Quantitation and Discrimination of Sulphated Glycosaminoglycans by Use of Dimethylmethylene Blue. Biochim. Biophys. Acta 1986, 883, 173–177. [Google Scholar] [CrossRef]
- de Grauw, J.C.; van Loon, J.P.A.M.; van de Lest, C.H.A.; Brunott, A.; van Weeren, P.R. In Vivo Effects of Phenylbutazone on Inflammation and Cartilage-Derived Biomarkers in Equine Joints with Acute Synovitis. Vet. J. 2014, 201, 51–56. [Google Scholar] [CrossRef]
- Kearney, C.M.; Korthagen, N.M.; Plomp, S.G.M.; Labberté, M.C.; de Grauw, J.C.; van Weeren, P.R.; Brama, P.A.J. A Translational Model for Repeated Episodes of Joint Inflammation: Welfare, Clinical and Synovial Fluid Biomarker Assessment. Animals 2023, 13, 3190. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Khumsap, S.; Lanovaz, J.L.; Rosenstein, D.S.; Byron, C.; Clayton, H.M. Effect of Induced Unilateral Synovitis of Distal Intertarsal and Tarsometatarsal Joints on Sagittal Plane Kinematics and Kinetics of Trotting Horses. Am. J. Vet. Res. 2003, 64, 1491–1495. [Google Scholar] [CrossRef]
- van Loon, J.P.A.M.; De Grauw, J.C.; van Dierendonck, M.; L’ami, J.J.; Back, W.; Van Weeren, P.R. Intra-Articular Opioid Analgesia Is Effective in Reducing Pain and Inflammation in an Equine LPS Induced Synovitis Model. Equine Vet. J. 2010, 42, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, J.P.A.M.; Menke, E.S.; L’Ami, J.J.; Jonckheer-Sheehy, V.S.M.; Back, W.; René Van Weeren, P. Analgesic and Anti-Hyperalgesic Effects of Epidural Morphine in an Equine LPS-Induced Acute Synovitis Model. Vet. J. 2012, 193, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, S.M.; Vinther, A.M.L.; Nielsen, S.S.; Andersen, P.H.; Tnibar, A.; Kristensen, A.T.; Jacobsen, S. Changes in Concentrations of Haemostatic and Inflammatory Biomarkers in Synovial Fluid after Intra-Articular Injection of Lipopolysaccharide in Horses. BMC Vet. Res. 2017, 13, 182. [Google Scholar] [CrossRef] [PubMed]
- De Grauw, J.C.; Van De Lest, C.H.A.; Van Weeren, R.; Brommer, H.; Brama, P.A.J. Arthrogenic Lameness of the Fetlock: Synovial Fluid Markers of Inflammation and Cartilage Turnover in Relation to Clinical Joint Pain. Equine Vet. J. 2006, 38, 305–311. [Google Scholar] [CrossRef]
- Bertone, A.L.; Palmer, J.L.; Jones, J. Synovial Fluid Cytokines and Eicosanoids as Markers of Joint Disease in Horses. Vet. Surg. 2001, 30, 528–538. [Google Scholar] [CrossRef]
- Baccarin, R.Y.A.; Seidel, S.R.T.; Michelacci, Y.M.; Tokawa, P.K.A.; Oliveira, T.M. Osteoarthritis: A Common Disease That Should Be Avoided in the Athletic Horse’s Life. Anim. Front. Rev. Mag. Anim. Agric. 2022, 12, 25–36. [Google Scholar] [CrossRef]
- Cokelaere, S.M.; Plomp, S.G.M.; de Boef, E.; de Leeuw, M.; Bool, S.; van de Lest, C.H.A.; van Weeren, P.R.; Korthagen, N.M. Sustained Intra-Articular Release of Celecoxib in an Equine Repeated LPS Synovitis Model. Eur. J. Pharm. Biopharm. 2018, 128, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Lucia, J.L.; Coverdale, J.A.; Arnold, C.E.; Winsco, K.N. Influence of an Intra-Articular Lipopolysaccharide Challenge on Markers of Inflammation and Cartilage Metabolism in Young Horses. Am. Soc. Anim. Sci. 2013, 91, 2693–2699. [Google Scholar] [CrossRef]
- Hunt, C.L.; Leatherwood, J.L.; Coverdale, J.A.; Sigler, D.L.; Vogelsang, M.M.; Arnold, C.E. Effects of Repeated Arthrocentesis on Systemic Cytokine Expression and Leukocyte Population in Young Horses Challenged with Intra-Articular Lipopolysaccharide. J. Anim. Sci. 2019, 97, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.A. Diagnostic Thermography. Vet. Clin. North Am. Equine Pract. 2001, 17, 95–114. [Google Scholar] [CrossRef]
- Figueiredo, T.; Dzyekanski, B.; Pimpão, C.T.; Silveira, A.B.; Capriglione, L.G.; Michelotto, P.V. Use of Infrared Thermography to Detect Intrasynovial Injections in Horses. J. Equine Vet. Sci. 2013, 33, 257–260. [Google Scholar] [CrossRef]
- Soroko, M.; Howell, K. Infrared Thermography: Current Applications in Equine Medicine. J. Equine Vet. Sci. 2018, 60, 90–96.e2. [Google Scholar] [CrossRef]
- Hewetson, M.; Christley, R.M.; Hunt, I.D.; Voute, L.C. Investigations of the Reliability of Observational Gait Analysis for the Assessment of Lameness in Horses. Vet. Rec. 2006, 158, 852–858. [Google Scholar] [CrossRef]
- de Grauw, J.C.; van de Lest, C.H.; van Weeren, P.R. Inflammatory Mediators and Cartilage Biomarkers in Synovial Fluid after a Single Inflammatory Insult: A Longitudinal Experimental Study. Arthritis Res. Ther. 2009, 11, R35. [Google Scholar] [CrossRef]
- Partridge, E.; Adam, E.; Wood, C.; Parker, J.; Johnson, M.; Horohov, D.; Page, A. Residual Effects of Intra-Articular Betamethasone and Triamcinolone Acetonide in an Equine Acute Synovitis Model. Equine Vet. J. 2023, 55, 905–915. [Google Scholar] [CrossRef]
- Knych, H.K.; Weiner, D.; Harrison, L.; McKemie, D.S. Pharmacokinetics and Pharmacodynamics of Intra-Articular Isoflupredone Following Administration to Horses with Lipopolysaccharide-Induced Synovitis. BMC Vet. Res. 2022, 18, 436. [Google Scholar] [CrossRef]
- Urayama, S.; Tanaka, A.; Kusano, K.; Sato, H.; Muranaka, M.; Mita, H.; Nagashima, T.; Matsuda, H. Oral Administration of Meloxicam and Flunixin Meglumine Have Similar Analgesic Effects After Lipopolysaccharide-Induced Inflammatory Response in Thoroughbred Horses. J. Equine Vet. Sci. 2023, 121, 104205. [Google Scholar] [CrossRef]
- Ekstrand, C.; Bondesson, U.; Giving, E.; Hedeland, M.; Ingvast-Larsson, C.; Jacobsen, S.; Löfgren, M.; Moen, L.; Rhodin, M.; Saetra, T.; et al. Disposition and Effect of Intra-Articularly Administered Dexamethasone on Lipopolysaccharide Induced Equine Synovitis. Acta Vet. Scand. 2019, 61, 28. [Google Scholar] [CrossRef]
- Malyala, P.; Singh, M. Endotoxin Limits in Formulations for Preclinical Research. J. Pharm. Sci. 2008, 97, 2041–2044. [Google Scholar] [CrossRef]
- van de Water, E.; Oosterlinck, M.; Dumoulin, M.; Korthagen, N.M.; van Weeren, P.R.; van den Broek, J.; Everts, H.; Pille, F.; van Doorn, D.A. The Preventive Effects of Two Nutraceuticals on Experimentally Induced Acute Synovitis. Equine Vet. J. 2017, 49, 532–538. [Google Scholar] [CrossRef]
- Bar-Or, D.; Rael, L.T.; Brody, E.N. Use of Saline as a Placebo in Intra-Articular Injections in Osteoarthritis: Potential Contributions to Nociceptive Pain Relief. Open Rheumatol. J. 2017, 11, 16–22. [Google Scholar] [CrossRef]
- Sugimura, N.; Ikeuchi, M.; Izumi, M.; Kawano, T.; Aso, K.; Kato, T.; Ushida, T.; Yokoyama, M.; Tani, T. Repeated Intra-Articular Injections of Acidic Saline Produce Long-Lasting Joint Pain and Widespread Hyperalgesia. Eur. J. Pain 2015, 19, 629–638. [Google Scholar] [CrossRef]



| Peak Values (PIH 8) | Breed | WBC (×109 cells/L) | TP (g/L) |
|---|---|---|---|
| This Study | TB | 116.1 ± 57.6 | 52.2 ± 11.3 |
| (Van de Water et al., 2021) [8] | SB | 126.6 ± 21.8 | 51.5 ± 6.7 |
| (Kearney et al., 2023) [24] | Mixed | 102.8 ± 39.6 | 56.3 ± 16.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duggan, M.J.S.; Kearney, C.; Baltrimaite, M.; Labberté, M.C.; Gibney, R.; Brama, P.A.J. Refinement of the Lipopolysaccharide-Induced Synovitis Model in Equine Middle Carpal Joints. Animals 2025, 15, 2474. https://doi.org/10.3390/ani15172474
Duggan MJS, Kearney C, Baltrimaite M, Labberté MC, Gibney R, Brama PAJ. Refinement of the Lipopolysaccharide-Induced Synovitis Model in Equine Middle Carpal Joints. Animals. 2025; 15(17):2474. https://doi.org/10.3390/ani15172474
Chicago/Turabian StyleDuggan, Michael J. S., Clodagh Kearney, Milda Baltrimaite, Margot C. Labberté, Rory Gibney, and Pieter A. J. Brama. 2025. "Refinement of the Lipopolysaccharide-Induced Synovitis Model in Equine Middle Carpal Joints" Animals 15, no. 17: 2474. https://doi.org/10.3390/ani15172474
APA StyleDuggan, M. J. S., Kearney, C., Baltrimaite, M., Labberté, M. C., Gibney, R., & Brama, P. A. J. (2025). Refinement of the Lipopolysaccharide-Induced Synovitis Model in Equine Middle Carpal Joints. Animals, 15(17), 2474. https://doi.org/10.3390/ani15172474







