Mitochondrial Quality Control in Bovine Oocyte Maturation: Mechanisms, Challenges, and Prospects for Enhancing Reproductive Efficiency
Simple Summary
Abstract
1. Introduction
2. Oocyte Maturation
3. Mitochondrial Quality Control
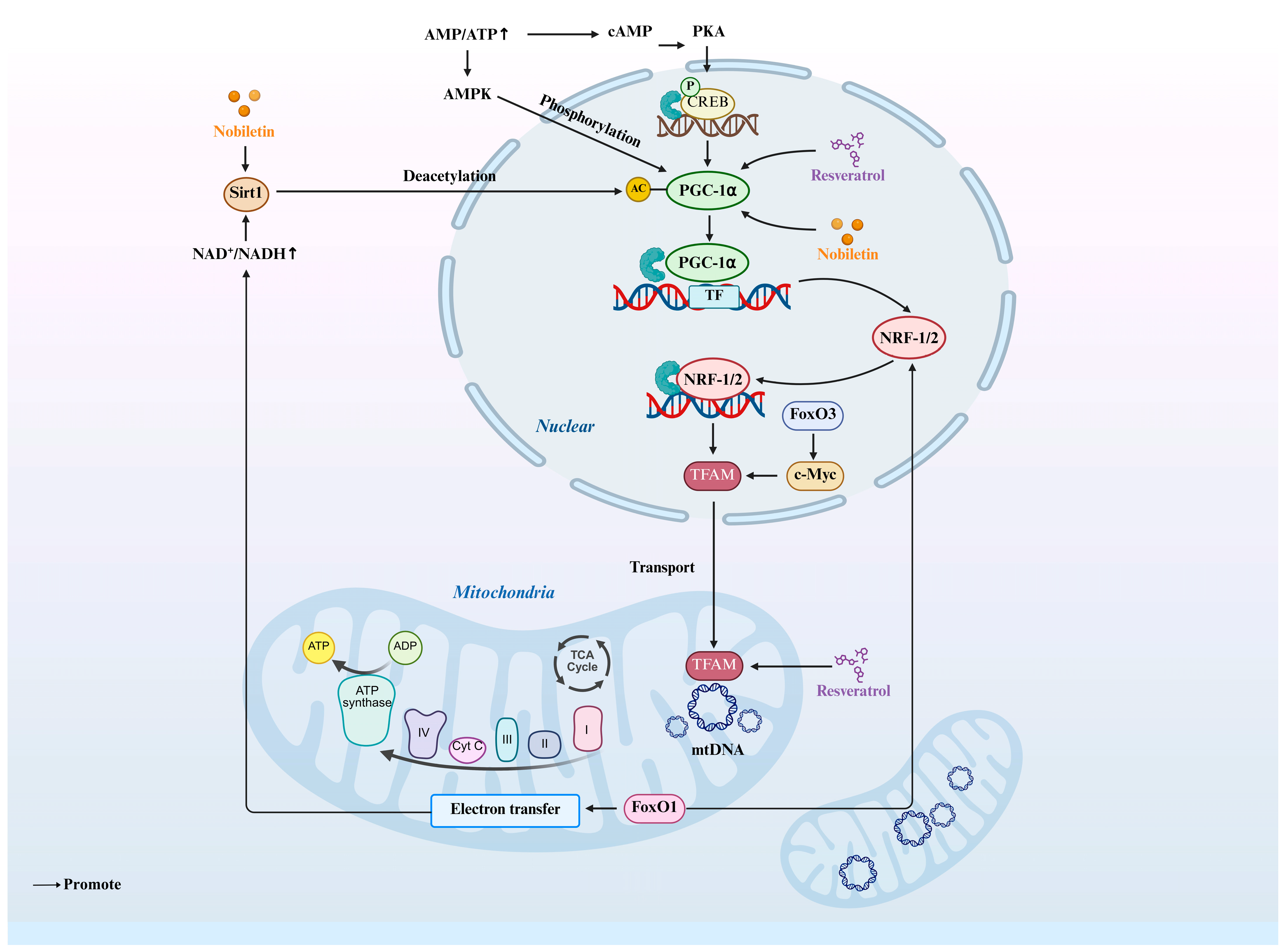
4. Mitochondrial Biogenesis and Its Molecular Role in Oocyte Maturation
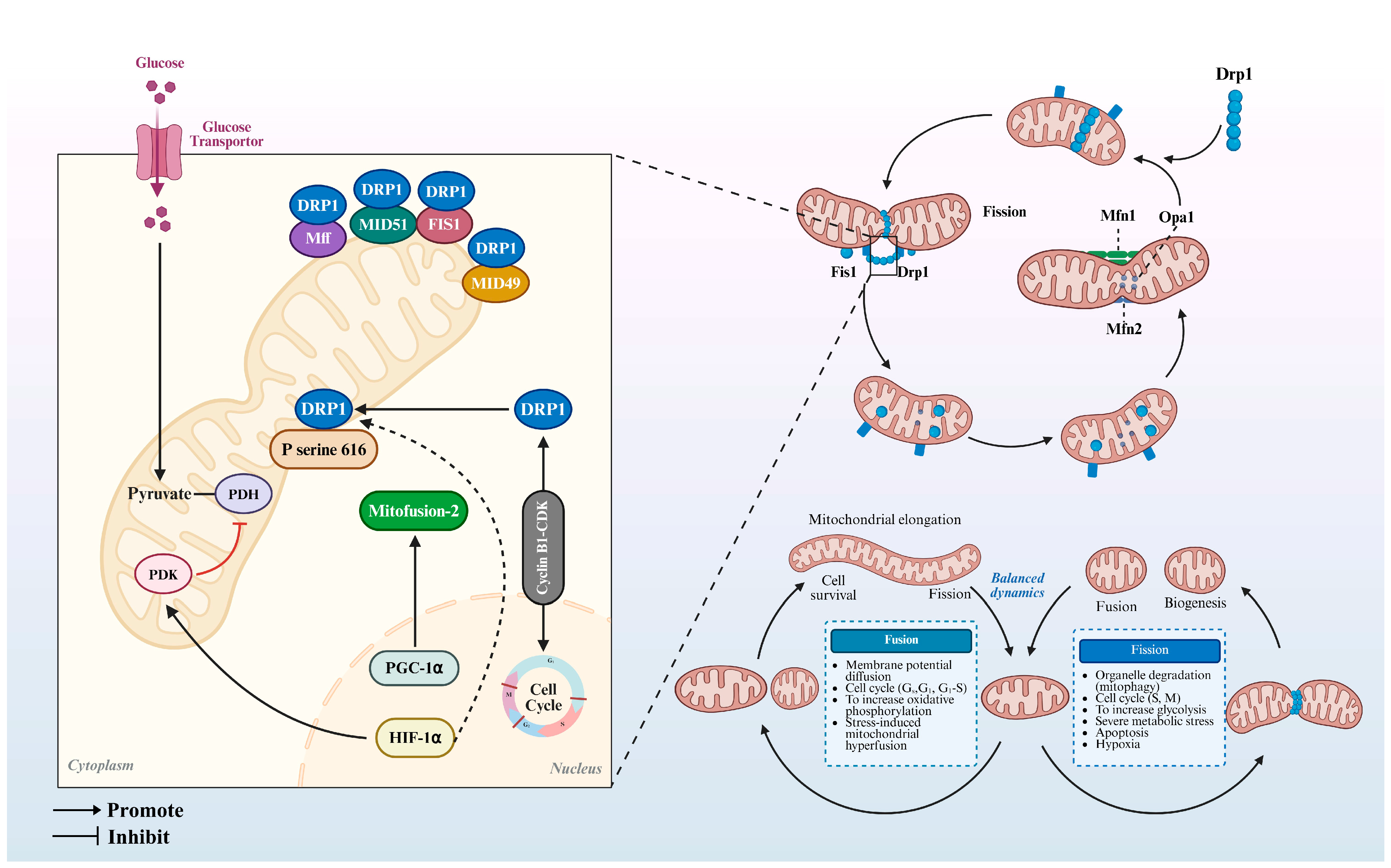
5. Mitochondrial Dynamics and Their Regulatory Mechanisms in Oocyte Maturation
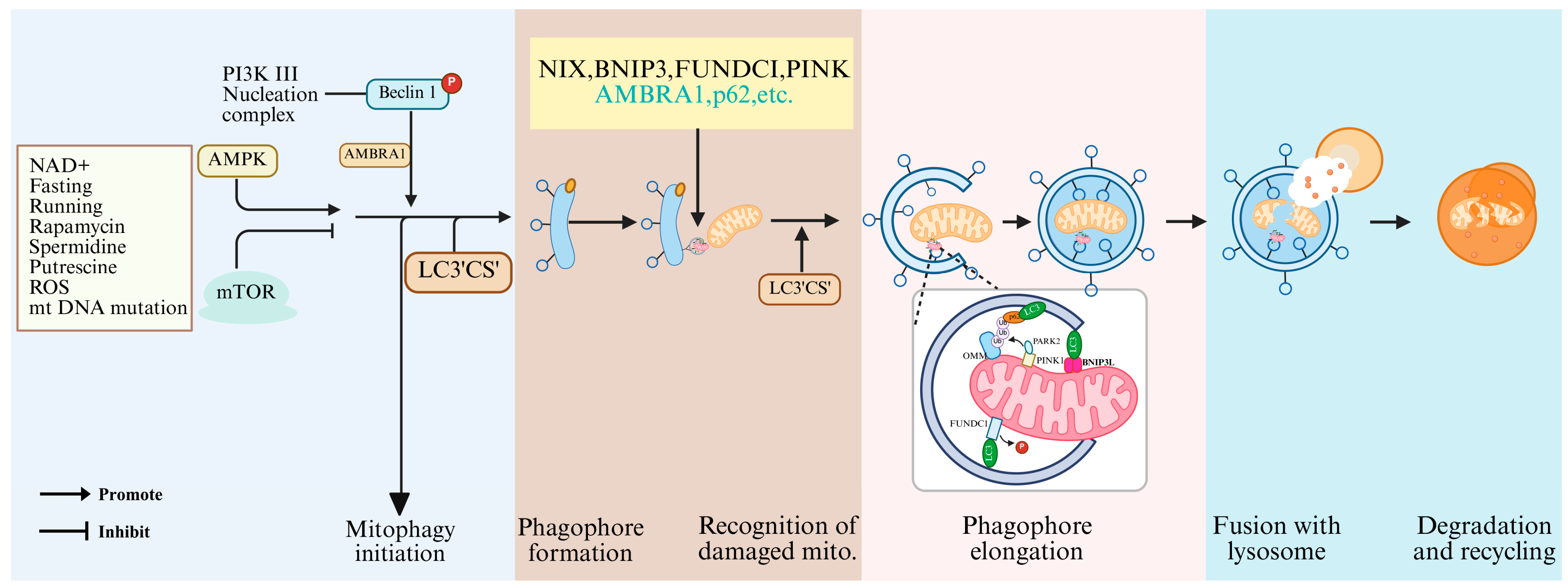
6. Molecular Mechanisms by Which Mitophagy Regulates Oocyte Maturation
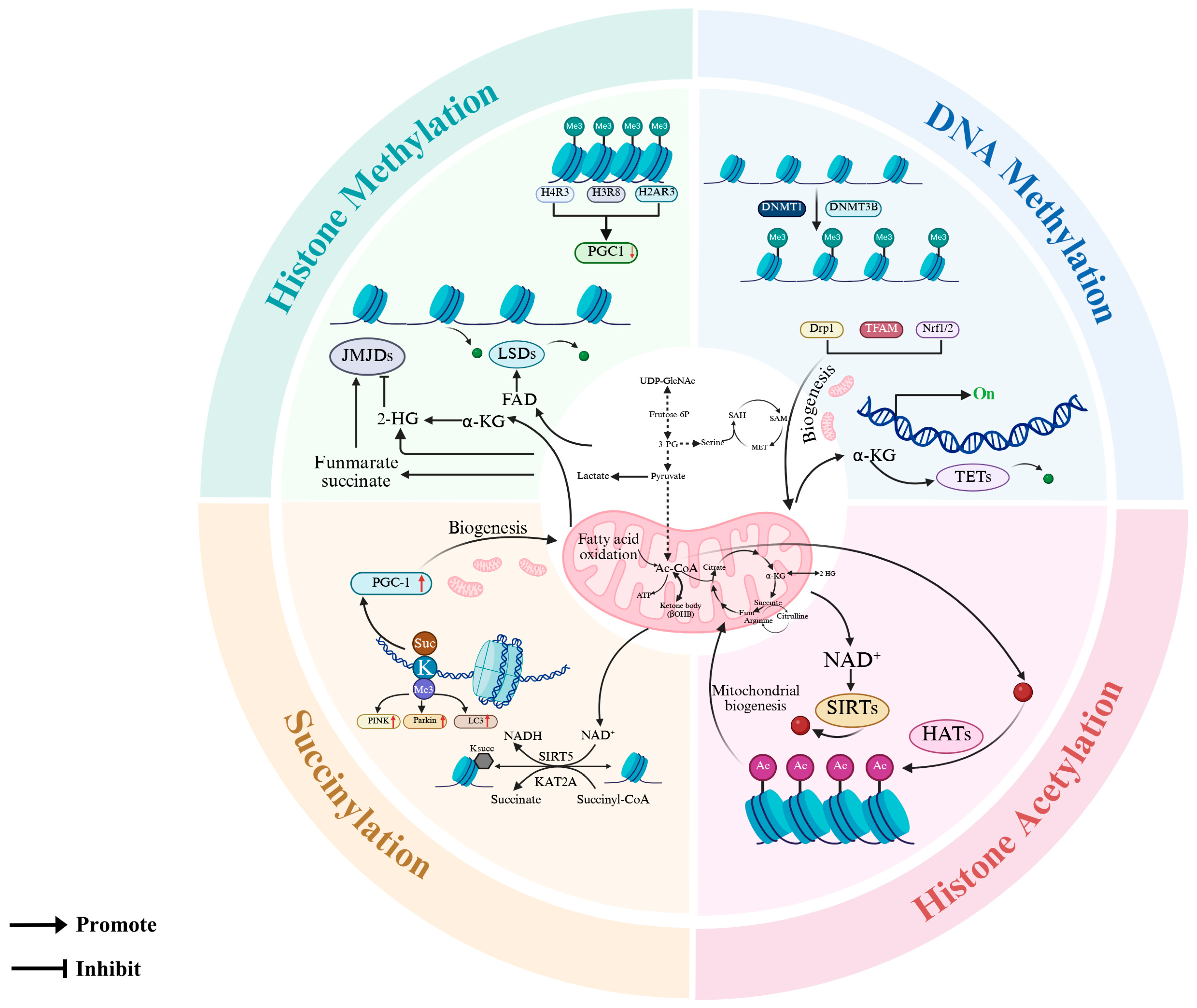
7. Epigenetic Modifications in Mitochondrial Quality Control: Molecular Mechanisms and Functional Consequences
8. Methodological Advances in Mitochondrial Quality Control Research of Oocytes
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.Y.; Li, Y.C.; Tian, X.Y.; Zhou, Y.; Guo, K.P.; Fan, H.Y.; Liang, X.W.; Ou, X.H.; Sha, Q.Q. A long-acting recombinant FSH supports high-quality mouse follicle development and oocyte maturation in vitro by coordinating somatic and germ cell transcriptomes. Mol. Hum. Reprod. 2023, 29, gaad013. [Google Scholar] [CrossRef] [PubMed]
- Dieci, C.; Lodde, V.; Labreque, R.; Dufort, I.; Tessaro, I.; Sirard, M.A.; Luciano, A.M. Differences in cumulus cell gene expression indicate the benefit of a pre-maturation step to improve in-vitro bovine embryo production. Mol. Hum. Reprod. 2016, 22, 882–897. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zaffagnini, G.; Cheng, S.; Salzer, M.C.; Pernaute, B.; Duran, J.M.; Irimia, M.; Schuh, M.; Böke, E. Mouse oocytes sequester aggregated proteins in degradative super-organelles. Cell 2024, 187, 1109–1126.e1121. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Wang, Y.Y.; Shi, Z.Y.; Ding, Y.Y.; Wen, H.Q.; Wu, M.P.; Sun, S.C.; Cai, Y.F.; Zhang, Y. SIRT5 modulates mitochondria function via mitophagy and antioxidant mechanisms to facilitate oocyte maturation in mice. Int. J. Biol. Macromol. 2025, 306, 141488. [Google Scholar] [CrossRef]
- Cai, J.; Li, Y.; Zhao, B.; Bao, Z.; Li, J.; Sun, S.; Chen, Y.; Wu, X. N-Acetylcysteine Alleviates D-Galactose-Induced Injury of Ovarian Granulosa Cells in Female Rabbits by Regulating the PI3K/Akt/mTOR Signaling Pathway. Antioxidants 2024, 13, 384. [Google Scholar] [CrossRef]
- Li, C.W.; Ge, W. Spatiotemporal expression of bone morphogenetic protein family ligands and receptors in the zebrafish ovary: A potential paracrine-signaling mechanism for oocyte-follicle cell communication. Biol. Reprod. 2011, 85, 977–986. [Google Scholar] [CrossRef]
- Marei, W.F.A.; Van den Bosch, L.; Pintelon, I.; Mohey-Elsaeed, O.; Bols, P.E.J.; Leroy, J. Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: A bovine in vitro model. Hum. Reprod. 2019, 34, 1984–1998. [Google Scholar] [CrossRef]
- Kumar, K.; Venturas, M.; Needleman, D.J.; Racowsky, C.; Wells, D. Extensive analysis of mitochondrial DNA quantity and sequence variation in human cumulus cells and assisted reproduction outcomes. Hum. Reprod. 2021, 37, 66–79. [Google Scholar] [CrossRef]
- Yang, Q.; Xi, Q.; Wang, M.; Long, R.; Hu, J.; Li, Z.; Ren, X.; Zhu, L.; Jin, L. Rapamycin improves the quality and developmental competence of mice oocytes by promoting DNA damage repair during in vitro maturation. Reprod. Biol. Endocrinol. 2022, 20, 67. [Google Scholar] [CrossRef]
- Bilodeau-Goeseels, S.; Panich, P.L.; Kastelic, J.P. Activation of AMP-activated protein kinase may not be involved in AICAR- and metformin-mediated meiotic arrest in bovine denuded and cumulus-enclosed oocytes in vitro. Zygote 2011, 19, 97–106. [Google Scholar] [CrossRef]
- Santiquet, N.; Sasseville, M.; Laforest, M.; Guillemette, C.; Gilchrist, R.B.; Richard, F.J. Activation of 5’adenosine monophosphate-activated protein kinase blocks cumulus cell expansion through inhibition of protein synthesis during in vitro maturation in Swine. Biol. Reprod. 2014, 91, 51. [Google Scholar] [CrossRef] [PubMed]
- Ran, Z.; Liu, R.; Shi, H.; Wang, X.; Wu, Z.; Zhou, S.; Liao, J.; Hu, L.; Hu, Y.; Zhou, J.; et al. mTOR signaling mediates energy metabolic equilibrium in bovine and mouse oocytes during the ovulatory phase†. Biol. Reprod. 2025, 112, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ying, H.; Dai, Y.; Rong, Y.; Chen, J.; Zhou, F.; Wang, S.; Xu, S.; Tong, X.; Zhang, S. Upregulated let-7 expression in the follicular fluid of patients with endometriomas leads to dysfunction of granulosa cells through targeting of IGF1R. Hum. Reprod. 2025, 40, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.T.; Rodgers, J.T.; Arlow, D.H.; Vazquez, F.; Mootha, V.K.; Puigserver, P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 2007, 450, 736–740. [Google Scholar] [CrossRef]
- Ramanathan, A.; Schreiber, S.L. Direct control of mitochondrial function by mTOR. Proc. Natl. Acad. Sci. USA 2009, 106, 22229–22232. [Google Scholar] [CrossRef]
- Morita, M.; Prudent, J.; Basu, K.; Goyon, V.; Katsumura, S.; Hulea, L.; Pearl, D.; Siddiqui, N.; Strack, S.; McGuirk, S.; et al. mTOR Controls Mitochondrial Dynamics and Cell Survival via MTFP1. Mol. Cell 2017, 67, 922–935.e925. [Google Scholar] [CrossRef]
- El-Sheikh, M.; Mesalam, A.A.; Kang, S.M.; Joo, M.D.; Soliman, S.S.; Khalil, A.A.K.; Ahn, M.J.; Kong, I.K. Modulation of Apoptosis and Autophagy by Melatonin in Juglone-Exposed Bovine Oocytes. Animals 2023, 13, 1475. [Google Scholar] [CrossRef]
- El-Sheikh, M.; Mesalam, A.; Khalil, A.A.K.; Idrees, M.; Ahn, M.J.; Mesalam, A.A.; Kong, I.K. Downregulation of PI3K/AKT/mTOR Pathway in Juglone-Treated Bovine Oocytes. Antioxidants 2023, 12, 114. [Google Scholar] [CrossRef]
- Ellederová, Z.; Cais, O.; Susor, A.; Uhlírová, K.; Kovárová, H.; Jelínková, L.; Tomek, W.; Kubelka, M. ERK1/2 map kinase metabolic pathway is responsible for phosphorylation of translation initiation factor eIF4E during in vitro maturation of pig oocytes. Mol. Reprod. Dev. 2008, 75, 309–317. [Google Scholar] [CrossRef]
- Tomek, W.; Melo Sterza, F.A.; Kubelka, M.; Wollenhaupt, K.; Torner, H.; Anger, M.; Kanitz, W. Regulation of translation during in vitro maturation of bovine oocytes: The role of MAP kinase, eIF4E (cap binding protein) phosphorylation, and eIF4E-BP1. Biol. Reprod. 2002, 66, 1274–1282. [Google Scholar] [CrossRef]
- Jansova, D.; Koncicka, M.; Tetkova, A.; Cerna, R.; Malik, R.; Del Llano, E.; Kubelka, M.; Susor, A. Regulation of 4E-BP1 activity in the mammalian oocyte. Cell Cycle 2017, 16, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.; Wrenzycki, C.; Tomek, W. Inactivation of mTor arrests bovine oocytes in the metaphase-I stage, despite reversible inhibition of 4E-BP1 phosphorylation. Mol. Reprod. Dev. 2014, 81, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, M.; Harris, S.E.; Collado Fernandez, E.; Lu, J.; Huntriss, J.D.; Campbell, B.K.; Picton, H.M. The activity and copy number of mitochondrial DNA in ovine oocytes throughout oogenesis in vivo and during oocyte maturation in vitro. Mol. Hum. Reprod. 2013, 19, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Tollner, T.L.; Hu, Z.; Dai, M.; Li, X.; Guan, H.; Shan, D.; Zhang, X.; Lv, J.; Huang, C.; et al. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol. Reprod. Dev. 2012, 79, 392–401. [Google Scholar] [CrossRef]
- Jeseta, M.; Ctvrtlikova Knitlova, D.; Hanzalova, K.; Hulinska, P.; Hanulakova, S.; Milakovic, I.; Nemcova, L.; Kanka, J.; Machatkova, M. Mitochondrial patterns in bovine oocytes with different meiotic competence related to their in vitro maturation. Reprod. Domest. Anim. 2014, 49, 469–475. [Google Scholar] [CrossRef]
- Němcová, L.; Hulínská, P.; Ješeta, M.; Kempisty, B.; Kaňka, J.; Machatková, M. Expression of selected mitochondrial genes during in vitro maturation of bovine oocytes related to their meiotic competence. Theriogenology 2019, 133, 104–112. [Google Scholar] [CrossRef]
- Wang, X.H.; Yin, S.; Ou, X.H.; Luo, S.M. Increase of mitochondria surrounding spindle causes mouse oocytes arrested at metaphase I stage. Biochem. Biophys. Res. Commun. 2020, 527, 1043–1049. [Google Scholar] [CrossRef]
- Pan, Z.N.; Zhang, H.L.; Zhang, K.H.; Ju, J.Q.; Liu, J.C.; Sun, S.C. Insufficient MIRO1 contributes to declined oocyte quality during reproductive aging. Sci. China-Life Sci. 2025, 68, 764–776. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Romagnoli, A.; Refolo, G.; Vescovo, T.; Ciccosanti, F.; Zuchegna, C.; Lozzi, F.; Occhigrossi, L.; Piacentini, M.; Fimia, G.M. Role of AMBRA1 in mitophagy regulation: Emerging evidence in aging-related diseases. Autophagy 2024, 20, 2602–2615. [Google Scholar] [CrossRef]
- Liu, Y.P.; He, B.; Wang, W.X.; Pan, W.L.; Jiao, L.; Yan, J.J.; Sun, S.C.; Zhang, Y. PKD regulates mitophagy to prevent oxidative stress and mitochondrial dysfunction during mouse oocyte maturation. Mitochondrion 2024, 78, 101946. [Google Scholar] [CrossRef]
- Dudkina, N.V.; Eubel, H.; Keegstra, W.; Boekema, E.J.; Braun, H.P. Structure of a mitochondrial supercomplex formed by respiratory-chain complexes I and III. Proc. Natl. Acad. Sci. USA 2005, 102, 3225–3229. [Google Scholar] [CrossRef]
- Magalhaes-Novais, S.; Blecha, J.; Naraine, R.; Mikesova, J.; Abaffy, P.; Pecinova, A.; Milosevic, M.; Bohuslavova, R.; Prochazka, J.; Khan, S.; et al. Mitochondrial respiration supports autophagy to provide stress resistance during quiescence. Autophagy 2022, 18, 2409–2426. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.H.; Rathore, M.G.; Allende-Vega, N.; Vo, D.N.; Belkhala, S.; Orecchioni, S.; Talarico, G.; Bertolini, F.; Cartron, G.; Lecellier, C.H.; et al. Human Leukemic Cells performing Oxidative Phosphorylation (OXPHOS) Generate an Antioxidant Response Independently of Reactive Oxygen species (ROS) Production. EBioMedicine 2016, 3, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Coticchio, G.; Dal Canto, M.; Mignini Renzini, M.; Guglielmo, M.C.; Brambillasca, F.; Turchi, D.; Novara, P.V.; Fadini, R. Oocyte maturation: Gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum. Reprod. Update 2015, 21, 427–454. [Google Scholar] [CrossRef]
- Richani, D.; Gilchrist, R.B. The epidermal growth factor network: Role in oocyte growth, maturation and developmental competence. Hum. Reprod. Update 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Gu, L.; Wang, Q.; Sun, Q.Y. Histone modifications during mammalian oocyte maturation: Dynamics, regulation and functions. Cell Cycle 2010, 9, 1942–1950. [Google Scholar] [CrossRef]
- Adhikari, D.; Lee, I.W.; Yuen, W.S.; Carroll, J. Oocyte mitochondria-key regulators of oocyte function and potential therapeutic targets for improving fertility. Biol. Reprod. 2022, 106, 366–377. [Google Scholar] [CrossRef]
- Kirillova, A.; Smitz, J.E.J.; Sukhikh, G.T.; Mazunin, I. The Role of Mitochondria in Oocyte Maturation. Cells 2021, 10, 2484. [Google Scholar] [CrossRef]
- Giotto, A.B.; Brum, D.D.; Santos, F.W.; Guimaraes, A.C.G.; Gonçalves, C.G.M.; Pavin, C.U.M.; Folchini, N.P.; Moyses, A.B.; Missio, D.; Leivas, F.G. Oxygen tension and oocyte density during in vitro maturation affect the in vitro fertilization of bovine oocytes. Semin.-Cienc. Agrar. 2015, 36, 4277–4287. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Q.; Li, Y.; Tang, Q.; Wu, T.; Chen, L.; Pu, S.; Zhao, Y.; Zhang, G.; Huang, C.; et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte 2020, 9, 484–494. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Nuclear control of respiratory gene expression in mammalian cells. J. Cell. Biochem. 2006, 97, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Quirós, P.M.; Langer, T.; López-Otín, C. New roles for mitochondrial proteases in health, ageing and disease. Nat. Rev. Mol. Cell Biol. 2015, 16, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.L. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 2013, 369, 2236–2251. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Jinks, R.N.; Puffenberger, E.G.; Venkatesh, S.; Singh, K.; Cheng, I.; Mikita, N.; Thilagavathi, J.; Lee, J.; Sarafianos, S.; et al. CODAS syndrome is associated with mutations of LONP1, encoding mitochondrial AAA+ Lon protease. Am. J. Hum. Genet. 2015, 96, 121–135. [Google Scholar] [CrossRef]
- Bao, F.; Zhou, L.; Zhou, R.; Huang, Q.; Chen, J.; Zeng, S.; Wu, Y.; Yang, L.; Qian, S.; Wang, M.; et al. Mitolysosome exocytosis, a mitophagy-independent mitochondrial quality control in flunarizine-induced parkinsonism-like symptoms. Sci. Adv. 2022, 8, eabk2376. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Zhang, S.; Zhang, T.; Liu, Y.; Zhang, L. Cellular mitophagy: Mechanism, roles in diseases and small molecule pharmacological regulation. Theranostics 2023, 13, 736–766. [Google Scholar] [CrossRef]
- Qi, X.Y.; Yuan, J.D.; Liu, Z.Y.; Jiang, X.Q.; Zhang, Q.; Zhang, S.L.; Zhao, L.; Ke, L.Y.; Zhang, C.Y.; Li, Y.; et al. Sirtuin 3-mediated deacetylation of superoxide dismutase 2 ameliorates sodium fluoride-induced mitochondrial dysfunction in porcine oocytes. Sci. Total Environ. 2024, 908, 168306. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Q.; Shi, J.; Zhou, S. Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson’s disease. Biomed. Pharmacother. 2020, 132, 110928. [Google Scholar] [CrossRef]
- Li, X.; Duan, J.; Wang, S.; Cheng, J.; Chen, H.; Zhang, Z.; Yang, L.; Hua, R.; Li, Q. Isorhamnetin protects porcine oocytes from zearalenone-induced reproductive toxicity through the PI3K/Akt signaling pathway. J. Anim. Sci. Biotechnol. 2023, 14, 22. [Google Scholar] [CrossRef]
- Alcantara da Silva, J.V.; Ispada, J.; Nociti, R.P.; da Fonseca Junior, A.M.; de Lima, C.B.; Dos Santos, E.C.; Chiaratti, M.R.; Milazzotto, M.P. The central role of pyruvate metabolism on the epigenetic maturation and transcriptional profile of bovine oocytes. Reproduction 2024, 167, e230181. [Google Scholar] [CrossRef]
- Richani, D.; Dunning, K.R.; Thompson, J.G.; Gilchrist, R.B. Metabolic co-dependence of the oocyte and cumulus cells: Essential role in determining oocyte developmental competence. Hum. Reprod. Update 2021, 27, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Robker, R.L. Promoting lipid utilization with l-carnitine to improve oocyte quality. Anim. Reprod. Sci. 2012, 134, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Akison, L.K.; Russell, D.L.; Norman, R.J.; Robker, R.L. Increased beta-oxidation and improved oocyte developmental competence in response to l-carnitine during ovarian in vitro follicle development in mice. Biol. Reprod. 2011, 85, 548–555. [Google Scholar] [CrossRef]
- Leitão, A.M.F.; Silva, B.R.; Barbalho, E.C.; Paulino, L.R.M.; Costa, F.D.C.; Martins, F.S.; Silva, J.R.V. The role of L-carnitine in the control of oxidative stress and lipid β-oxidation during in vitro follicle growth, oocyte maturation, embryonic development and cryopreservation: A review. Zygote 2024, 32, 335–340. [Google Scholar] [CrossRef]
- Xu, D.; Li, C.; Huang, Y.; Hu, K.; Wang, C.; Zhou, P.; Shen, H.; Liu, C.; Xu, J.; He, J.; et al. Ferric ammonium citrate regulates iron death in mature porcine oocytes and their embryonic development in vitro through the NRF2 signaling pathway. Theriogenology 2025, 232, 1–8. [Google Scholar] [CrossRef]
- Colpman, P.; Dasgupta, A.; Archer, S.L. The Role of Mitochondrial Dynamics and Mitotic Fission in Regulating the Cell Cycle in Cancer and Pulmonary Arterial Hypertension: Implications for Dynamin-Related Protein 1 and Mitofusin2 in Hyperproliferative Diseases. Cells 2023, 12, 1897. [Google Scholar] [CrossRef]
- Ago, T.; Yeh, I.; Yamamoto, M.; Schinke-Braun, M.; Brown, J.A.; Tian, B.; Sadoshima, J. Thioredoxin1 upregulates mitochondrial proteins related to oxidative phosphorylation and TCA cycle in the heart. Antioxid. Redox Signal. 2006, 8, 1635–1650. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Li, Z.; Zhao, M.; Wang, D.; Sun, Z.; Wen, P.; Dai, Y.; Gou, F.; Ji, Y.; et al. PINK1/Parkin-mediated mitophagy in neurodegenerative diseases. Ageing Res. Rev. 2023, 84, 101817. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Padman, B.S.; Lazarou, M. Deciphering the Molecular Signals of PINK1/Parkin Mitophagy. Trends Cell Biol. 2016, 26, 733–744. [Google Scholar] [CrossRef]
- Tang, J.; Peng, W.; Ji, J.; Peng, C.; Wang, T.; Yang, P.; Gu, J.; Feng, Y.; Jin, K.; Wang, X.; et al. GPR176 Promotes Cancer Progression by Interacting with G Protein GNAS to Restrain Cell Mitophagy in Colorectal Cancer. Adv. Sci. 2023, 10, e2205627. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial dysfunction: Roles in skeletal muscle atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Cheng, X.; Ma, Z.Q.; Wang, H.P.; Zhou, C.; Li, J.; Zhang, D.L.; Hu, L.L.; Cui, Y.F.; Huang, J.; et al. Polystyrene nanoplastics induce apoptosis, autophagy, and steroidogenesis disruption in granulosa cells to reduce oocyte quality and fertility by inhibiting the PI3K/AKT pathway in female mice. J. Nanobiotechnol. 2024, 22, 460. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.J.; Wang, X.Y.; Dong, Y.H.; Wang, D.H.; Han, Z.; Zhang, X.J.; Sun, Q.Y.; Carroll, J.; Liang, C.G. CENP-F-dependent DRP1 function regulates APC/C activity during oocyte meiosis I. Nat. Commun. 2022, 13, 7732. [Google Scholar] [CrossRef] [PubMed]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Fan, S.; Zhao, X.; Xie, W.; Yang, X.; Yu, W.; Tang, Z.; Chen, Y.; Yuan, Z.; Han, Y.; Sheng, X.; et al. The effect of 3-Methyl-4-Nitrophenol on the early ovarian follicle development in mice by disrupting the clock genes expression. Chem. Biol. Interact. 2022, 363, 110001. [Google Scholar] [CrossRef]
- Ma, C.; Xu, Y.; Zhang, X.; Shi, X.; Zhang, Y.; Luo, M.; Wu, C.; Ding, Z.; Xiang, H.; Cao, Y. Melatonin mitigates PNMC-induced disruption of spindle assembly and mitochondrial function in mouse Oocytes. Ecotoxicol. Environ. Saf. 2024, 282, 116703. [Google Scholar] [CrossRef]
- Zhang, Z.; Jia, Z. Pre-IVM with C-type natriuretic peptide promotes mitochondrial biogenesis of bovine oocytes via activation of CREB. Sci. Rep. 2024, 14, 16260. [Google Scholar] [CrossRef]
- Jia, Z.; Yang, X.; Liu, K. Treatment of cattle oocytes with C-type natriuretic peptide before in vitro maturation enhances oocyte mitochondrial function. Anim. Reprod. Sci. 2021, 225, 106685. [Google Scholar] [CrossRef]
- Ardehjani, N.A.; Agha-Hosseini, M.; Nashtaei, M.S.; Khodarahmian, M.; Shabani, M.; Jabarpour, M.; Fereidouni, F.; Rastegar, T.; Amidi, F. Resveratrol ameliorates mitochondrial biogenesis and reproductive outcomes in women with polycystic ovary syndrome undergoing assisted reproduction: A randomized, triple-blind, placebo-controlled clinical trial. J. Ovarian Res. 2024, 17, 143. [Google Scholar] [CrossRef]
- Gherardi, G.; Corbioli, G.; Ruzza, F.; Rizzuto, R. CoQ(10) and Resveratrol Effects to Ameliorate Aged-Related Mitochondrial Dysfunctions. Nutrients 2022, 14, 4326. [Google Scholar] [CrossRef]
- Itami, N.; Shirasuna, K.; Kuwayama, T.; Iwata, H. Short-term heat stress induces mitochondrial degradation and biogenesis and enhances mitochondrial quality in porcine oocytes. J. Therm. Biol. 2018, 74, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Roth, Z. Symposium review: Reduction in oocyte developmental competence by stress is associated with alterations in mitochondrial function. J. Dairy Sci. 2018, 101, 3642–3654. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.J.; Zhou, W.; Nie, Z.W.; Shin, K.T.; Cui, X.S. Melatonin enhances mitochondrial biogenesis and protects against rotenone-induced mitochondrial deficiency in early porcine embryos. J. Pineal Res. 2020, 68, e12627. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.; Romero, S.; De Vos, M.; Verheyen, G.; Smitz, J. Human cumulus-enclosed germinal vesicle oocytes from early antral follicles reveal heterogeneous cellular and molecular features associated with in vitro maturation capacity. Hum. Reprod. 2015, 30, 1396–1409. [Google Scholar] [CrossRef]
- Xu, D.; Wu, L.; Jiang, X.; Yang, L.; Cheng, J.; Chen, H.; Hua, R.; Geng, G.; Yang, L.; Li, Q. SIRT2 Inhibition Results in Meiotic Arrest, Mitochondrial Dysfunction, and Disturbance of Redox Homeostasis during Bovine Oocyte Maturation. Int. J. Mol. Sci. 2019, 20, 1365. [Google Scholar] [CrossRef]
- Zhang, L.; Hou, X.; Ma, R.; Moley, K.; Schedl, T.; Wang, Q. Sirt2 functions in spindle organization and chromosome alignment in mouse oocyte meiosis. FASEB J. 2014, 28, 1435–1445. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Soares, M.; Almeida-Santos, T.; Ramalho-Santos, J.; Sousa, A.P. Aging and oocyte competence: A molecular cell perspective. WIREs Mech. Dis. 2023, 15, e1613. [Google Scholar] [CrossRef]
- Mikwar, M.; MacFarlane, A.J.; Marchetti, F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutat. Res. Rev. Mutat. Res. 2020, 785, 108320. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Gómora-García, J.C.; Montiel, T.; Hüttenrauch, M.; Salcido-Gómez, A.; García-Velázquez, L.; Ramiro-Cortés, Y.; Gomora, J.C.; Castro-Obregón, S.; Massieu, L. Effect of the Ketone Body, D-β-Hydroxybutyrate, on Sirtuin2-Mediated Regulation of Mitochondrial Quality Control and the Autophagy-Lysosomal Pathway. Cells 2023, 12, 486. [Google Scholar] [CrossRef]
- Cheng, Z. FoxO transcription factors in mitochondrial homeostasis. Biochem. J. 2022, 479, 525–536. [Google Scholar] [CrossRef]
- Chao, X.; Wang, S.; Zhao, K.; Li, Y.; Williams, J.A.; Li, T.; Chavan, H.; Krishnamurthy, P.; He, X.C.; Li, L.; et al. Impaired TFEB-Mediated Lysosome Biogenesis and Autophagy Promote Chronic Ethanol-Induced Liver Injury and Steatosis in Mice. Gastroenterology 2018, 155, 865–879.e812. [Google Scholar] [CrossRef]
- Perales, J.A.; Lawan, A.; Bajpeyi, S.; Han, S.M.; Bennett, A.M.; Min, K. MAP Kinase Phosphatase-5 Deficiency Improves Endurance Exercise Capacity. Cells 2025, 14, 410. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, Y.; Yang, H.; Tan, J.; Zhang, J.; Zi, D. Pyrroloquinoline quinone promotes human mesenchymal stem cell-derived mitochondria to improve premature ovarian insufficiency in mice through the SIRT1/ATM/p53 pathway. Stem Cell Res. Ther. 2024, 15, 97. [Google Scholar] [CrossRef]
- Hao, X.; Zhao, J.; Rodriguez-Wallberg, K.A. Comprehensive atlas of mitochondrial distribution and dynamics during oocyte maturation in mouse models. Biomark. Res. 2024, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Zhang, S.; Gong, F.; Lu, C.; Lin, G.; Hu, L. The mitochondrial DNA copy number of cumulus granulosa cells may be related to the maturity of oocyte cytoplasm. Hum. Reprod. 2020, 35, 1120–1129. [Google Scholar] [CrossRef]
- Sandoval, H.; Yao, C.K.; Chen, K.; Jaiswal, M.; Donti, T.; Lin, Y.Q.; Bayat, V.; Xiong, B.; Zhang, K.; David, G.; et al. Mitochondrial fusion but not fission regulates larval growth and synaptic development through steroid hormone production. Elife 2014, 3, e03558. [Google Scholar] [CrossRef]
- Piao, L.; Fang, Y.H.; Fisher, M.; Hamanaka, R.B.; Ousta, A.; Wu, R.X.; Mutlu, G.M.; Garcia, A.J.; Archer, S.L.; Sharp, W.W. Dynamin-related protein 1 is a critical regulator of mitochondrial calcium homeostasis during myocardial ischemia/reperfusion injury. FASEB J. 2024, 38, e23379. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, T.; Gao, L.; Fu, X.; Zhu, S.; Hou, Y. Enriched endoplasmic reticulum-mitochondria interactions result in mitochondrial dysfunction and apoptosis in oocytes from obese mice. J. Anim. Sci. Biotechnol. 2017, 8, 62. [Google Scholar] [CrossRef]
- Wu, S.; Lu, Q.; Ding, Y.; Wu, Y.; Qiu, Y.; Wang, P.; Mao, X.; Huang, K.; Xie, Z.; Zou, M.H. Hyperglycemia-Driven Inhibition of AMP-Activated Protein Kinase α2 Induces Diabetic Cardiomyopathy by Promoting Mitochondria-Associated Endoplasmic Reticulum Membranes In Vivo. Circulation 2019, 139, 1913–1936. [Google Scholar] [CrossRef]
- Wang, F.; Meng, T.G.; Li, J.; Hou, Y.; Luo, S.M.; Schatten, H.; Sun, Q.Y.; Ou, X.H. Mitochondrial Ca(2 +) Is Related to Mitochondrial Activity and Dynamic Events in Mouse Oocytes. Front. Cell Dev. Biol. 2020, 8, 585932. [Google Scholar] [CrossRef]
- Boucret, L.; Chao de la Barca, J.M.; Morinière, C.; Desquiret, V.; Ferré-L’Hôtellier, V.; Descamps, P.; Marcaillou, C.; Reynier, P.; Procaccio, V.; May-Panloup, P. Relationship between diminished ovarian reserve and mitochondrial biogenesis in cumulus cells. Hum. Reprod. 2015, 30, 1653–1664. [Google Scholar] [CrossRef] [PubMed]
- Machiela, E.; Liontis, T.; Dues, D.J.; Rudich, P.D.; Traa, A.; Wyman, L.; Kaufman, C.; Cooper, J.F.; Lew, L.; Nadarajan, S.; et al. Disruption of mitochondrial dynamics increases stress resistance through activation of multiple stress response pathways. FASEB J. 2020, 34, 8475–8492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, Q.; Li, H.; Wang, Y.; Jiang, Y.; Wang, H.; Cong, L.; Xu, J.; Shen, Z.; Chen, W.; et al. Sirt3 deficiency accelerates ovarian senescence without affecting spermatogenesis in aging mice. Free Radic. Biol. Med. 2022, 193, 511–525. [Google Scholar] [CrossRef]
- Han, T.; Zhao, Y.; Jiao, A.; Sun, Z.; Zhang, H.; Zhao, D.; Wang, H.; Gao, Q. OPA1 deficiency induces mitophagy through PINK1/Parkin pathway during bovine oocytes maturation. Theriogenology 2025, 234, 51–63. [Google Scholar] [CrossRef]
- Martinez, A.; Sanchez-Martinez, A.; Pickering, J.T.; Twyning, M.J.; Terriente-Felix, A.; Chen, P.L.; Chen, C.H.; Whitworth, A.J. Mitochondrial CISD1/Cisd accumulation blocks mitophagy and genetic or pharmacological inhibition rescues neurodegenerative phenotypes in Pink1/parkin models. Mol. Neurodegener. 2024, 19, 12. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, R.; Yan, J.; Chen, H.; Sang, L.; Yao, X.; Fan, D.; Wang, X.; Zuo, X.; Zhang, X.; et al. Mitochondrial deoxyguanosine kinase is required for female fertility in mice. Acta Biochim. Biophys. Sin. 2024, 56, 427–439. [Google Scholar] [CrossRef]
- Van Der Kelen, A.; Li Piani, L.; Mertens, J.; Regin, M.; Couvreu de Deckersberg, E.; Van de Velde, H.; Sermon, K.; Tournaye, H.; Verpoest, W.; Hes, F.J.; et al. The interplay between mitochondrial DNA genotypes, female infertility, ovarian response, and mutagenesis in oocytes. Hum. Reprod. Open 2025, 2025, hoae074. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, Y.L.; Ji, S.Y.; Zhao, L.W.; Shang, W.N.; Li, D.; Chen, Z.; Tong, C.; Fan, H.Y. Mitochondrial Function Regulated by Mitoguardin-1/2 Is Crucial for Ovarian Endocrine Functions and Ovulation. Endocrinology 2017, 158, 3988–3999. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Bai, J.; Tian, X.; Zhao, X.; Liu, W.; Duan, X.; Shang, W.; Fan, H.Y.; Tong, C. Mitoguardin Regulates Mitochondrial Fusion through MitoPLD and Is Required for Neuronal Homeostasis. Mol. Cell 2016, 61, 111–124. [Google Scholar] [CrossRef]
- Lee, S.H.; Sun, M.H.; Zhou, D.; Jiang, W.J.; Li, X.H.; Heo, G.; Cui, X.S. High Temperature Disrupts Organelle Distribution and Functions Affecting Meiotic Maturation in Porcine Oocytes. Front. Cell Dev. Biol. 2022, 10, 826801. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Burgess, J.D.; Faroqi, A.H.; DeMeo, N.N.; Fiesel, F.C.; Springer, W.; Delenclos, M.; McLean, P.J. Alpha-synuclein-induced mitochondrial dysfunction is mediated via a sirtuin 3-dependent pathway. Mol. Neurodegener. 2020, 15, 5. [Google Scholar] [CrossRef]
- Garcia, B.M.; Machado, T.S.; Carvalho, K.F.; Nolasco, P.; Nociti, R.P.; Del Collado, M.; Capo Bianco, M.J.D.; Grejo, M.P.; Augusto Neto, J.D.; Sugiyama, F.H.C.; et al. Mice born to females with oocyte-specific deletion of mitofusin 2 have increased weight gain and impaired glucose homeostasis. Mol. Hum. Reprod. 2020, 26, 938–952. [Google Scholar] [CrossRef] [PubMed]
- Naón, D.; Hernández-Alvarez, M.I.; Shinjo, S.; Wieczor, M.; Ivanova, S.; Martins de Brito, O.; Quintana, A.; Hidalgo, J.; Palacín, M.; Aparicio, P.; et al. Splice variants of mitofusin 2 shape the endoplasmic reticulum and tether it to mitochondria. Science 2023, 380, eadh9351. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, A.; Bai, J.; Liu, H.; Zhu, Y.; Luo, Y.; Zheng, L.; Hou, Y.; Li, J.; Fu, X. Decreased ATF5 level contributes to improved mitochondrial function in oocytes exposed to vitrification stress. Front. Cell Dev. Biol. 2024, 12, 1431683. [Google Scholar] [CrossRef]
- Zhang, D.; Fang, X.; Xia, W.; Sun, Q.; Zhang, X.; Qi, Y.; Yu, Y.; Zhou, Z.; Du, D.; Tao, C.; et al. Rutin enhances mitochondrial function and improves the developmental potential of vitrified ovine GV-stage oocyte. Theriogenology 2024, 229, 214–224. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, Y.; Li, H.; Zhang, L. Effect of mitophagy in oocytes and granulosa cells on oocyte quality†. Biol. Reprod. 2021, 104, 294–304. [Google Scholar] [CrossRef]
- May-Panloup, P.; Boucret, L.; Chao de la Barca, J.M.; Desquiret-Dumas, V.; Ferré-L’Hotellier, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef]
- Li, A.; Gao, M.; Liu, B.; Qin, Y.; Chen, L.; Liu, H.; Wu, H.; Gong, G. Mitochondrial autophagy: Molecular mechanisms and implications for cardiovascular disease. Cell Death Dis. 2022, 13, 444. [Google Scholar] [CrossRef]
- Uoselis, L.; Nguyen, T.N.; Lazarou, M. Mitochondrial degradation: Mitophagy and beyond. Mol. Cell 2023, 83, 3404–3420. [Google Scholar] [CrossRef]
- Imberechts, D.; Kinnart, I.; Wauters, F.; Terbeek, J.; Manders, L.; Wierda, K.; Eggermont, K.; Madeiro, R.F.; Sue, C.; Verfaillie, C.; et al. DJ-1 is an essential downstream mediator in PINK1/parkin-dependent mitophagy. Brain 2022, 145, 4368–4384. [Google Scholar] [CrossRef] [PubMed]
- Ouni, E.; Bouzin, C.; Dolmans, M.M.; Marbaix, E.; Pyr Dit Ruys, S.; Vertommen, D.; Amorim, C.A. Spatiotemporal changes in mechanical matrisome components of the human ovary from prepuberty to menopause. Hum. Reprod. 2020, 35, 1391–1410. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, L.; Wu, C.; Zhang, S.; Ju, S.; Rui, R.; Zhang, D.; Dai, J. Involvement of PINK1/Parkin-mediated mitophagy in mitochondrial functional disruption under oxidative stress in vitrified porcine oocytes. Theriogenology 2021, 174, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, C.; Liu, Q.; Li, J.; Wu, H.; Xu, R.; Sun, Y.; Cheng, M.; Zhao, X.; Pan, M.; et al. C-type natriuretic peptide improves maternally aged oocytes quality by inhibiting excessive PINK1/Parkin-mediated mitophagy. Elife 2023, 12, RP88523. [Google Scholar] [CrossRef]
- Showell, M.G.; Mackenzie-Proctor, R.; Jordan, V.; Hart, R.J. Antioxidants for female subfertility. Cochrane Database Syst. Rev. 2020, 8, Cd007807. [Google Scholar] [CrossRef]
- Fang, X.; Xia, W.; Qi, Y.; Yu, Y.; Sun, Q.; Zhang, D.; Zhou, Z.; Qin, T.; Tao, C.; Li, J. SIRT2 regulates apoptosis by inducing mitophagy in sheep cumulus cells. Theriogenology 2024, 218, 163–173. [Google Scholar] [CrossRef]
- Sun, Y.L.; Tang, S.B.; Shen, W.; Yin, S.; Sun, Q.Y. Roles of Resveratrol in Improving the Quality of Postovulatory Aging Oocytes In Vitro. Cells 2019, 8, 1132. [Google Scholar] [CrossRef]
- Fang, X.; Xia, W.; Li, S.; Qi, Y.; Liu, M.; Yu, Y.; Li, H.; Li, M.; Tao, C.; Wang, Z.; et al. SIRT2 Is Critical for Sheep Oocyte Maturation through Regulating Function of Surrounding Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 5013. [Google Scholar] [CrossRef]
- Shin, J.-H.; Yang, S.-G.; Hyo-Jin, P.; Koo, D.-B. Oocyte quality is closely linked to DRP1 derived-mitochondrial fission and mitophagy by the NAD+ biosynthesis in a postovulatory-aging model of pigs. J. Anim. Reprod. Biotechnol. 2024, 39, 67–80. [Google Scholar] [CrossRef]
- Ng, A.Q.E.; Chan, S.N.; Pek, J.W. Nutrient-dependent regulation of a stable intron modulates germline mitochondrial quality control. Nat. Commun. 2024, 15, 1252. [Google Scholar] [CrossRef]
- Wong, J.T.; Akhbar, F.; Ng, A.Y.E.; Tay, M.L.; Loi, G.J.E.; Pek, J.W. DIP1 modulates stem cell homeostasis in Drosophila through regulation of sisR-1. Nat. Commun. 2017, 8, 759. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, K.; Wang, L.; Liu, W.; Zhang, C.; Qiu, Y.; Liu, W.; Zhang, H.; Zhang, D.; Yang, Z.; et al. RAB7 activity is required for the regulation of mitophagy in oocyte meiosis and oocyte quality control during ovarian aging. Autophagy 2022, 18, 643–660. [Google Scholar] [CrossRef]
- Yan, B.R.; Li, T.; Coyaud, E.; Laurent, E.M.N.; St-Germain, J.; Zhou, Y.; Kim, P.K.; Raught, B.; Brumell, J.H. C5orf51 is a component of the MON1-CCZ1 complex and controls RAB7A localization and stability during mitophagy. Autophagy 2022, 18, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Shen, M.; Wu, J.; Ye, C.; Tan, Y. Mechanism Research of DHEA Treatment Improving Diminished Ovarian Reserve by Attenuating the AMPK-SIRT1 Signaling and Mitophagy. Reprod. Sci. 2024, 31, 2059–2072. [Google Scholar] [CrossRef]
- Furat Rencber, S.; Kurnaz Ozbek, S.; Eraldemır, C.; Sezer, Z.; Kum, T.; Ceylan, S.; Guzel, E. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: An experimental study. J. Ovarian Res. 2018, 11, 55. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, J.; Cui, Z.; Li, Y.; Gao, Q.; Miao, Y.; Xiong, B. Polyamine metabolite spermidine rejuvenates oocyte quality by enhancing mitophagy during female reproductive aging. Nat. Aging 2023, 3, 1372–1386. [Google Scholar] [CrossRef]
- Field, J.T.; Gordon, J.W. BNIP3 and Nix: Atypical regulators of cell fate. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119325. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, W.; Lu, Y.; Zheng, Y.; Pan, L.; Wu, X.; Yuan, Y.; Shen, Z.; Ma, S.; Zhang, X.; et al. BNIP3L/NIX-mediated mitophagy: Molecular mechanisms and implications for human disease. Cell Death Dis. 2021, 13, 14. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Liu, L.; Feng, D.; Chen, G.; Chen, M.; Zheng, Q.; Song, P.; Ma, Q.; Zhu, C.; Wang, R.; Qi, W.; et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 2012, 14, 177–185. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Z.; Wang, Y.; Tan, Z.; Zhu, C.; Li, Y.; Han, Z.; Chen, L.; Gao, R.; Liu, L.; et al. Mitophagy receptor FUNDC1 regulates mitochondrial dynamics and mitophagy. Autophagy 2016, 12, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, P.; Li, R.; Ren, J.; Zhou, H. Fundc1-dependent mitophagy is obligatory to ischemic preconditioning-conferred renoprotection in ischemic AKI via suppression of Drp1-mediated mitochondrial fission. Redox Biol. 2020, 30, 101415. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, W.; Chen, H.; Jiang, L.; Zhu, R.; Feng, D. FUNDC1 is a novel mitochondrial-associated-membrane (MAM) protein required for hypoxia-induced mitochondrial fission and mitophagy. Autophagy 2016, 12, 1675–1676. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Yang, Y.; Sun, F.; Tao, H.; Lu, C.; Yang, J.J. Mitochondrial quality control in liver fibrosis: Epigenetic hallmarks and therapeutic strategies. Cell. Signal. 2024, 115, 111035. [Google Scholar] [CrossRef]
- Lin, L.C.; Tu, B.; Song, K.; Liu, Z.Y.; Sun, H.; Zhou, Y.; Sha, J.M.; Yang, J.J.; Zhang, Y.; Zhao, J.Y.; et al. Mitochondrial quality control in cardiac fibrosis: Epigenetic mechanisms and therapeutic strategies. Metabolism 2023, 145, 155626. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, Y. The nuclear-mitochondrial crosstalk in aging: From mechanisms to therapeutics. Free Radic. Biol. Med. 2025, 232, 391–397. [Google Scholar] [CrossRef]
- Naderi, N.; Tavalaee, M.; Nasr-Esfahani, M.H. The epigenetic approach of varicocele: A focus on sperm DNA and m6A-RNA methylation. Hum. Reprod. Update 2025, 31, 81–101. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Jadhav, G.; Sakharkar, A.J. Repeated mild traumatic brain injuries perturb the mitochondrial biogenesis via DNA methylation in the hippocampus of rat. Mitochondrion 2021, 61, 11–24. [Google Scholar] [CrossRef]
- Balasubramanian, N.; Sagarkar, S.; Choudhary, A.G.; Kokare, D.M.; Sakharkar, A.J. Epigenetic Blockade of Hippocampal SOD2 Via DNMT3b-Mediated DNA Methylation: Implications in Mild Traumatic Brain Injury-Induced Persistent Oxidative Damage. Mol. Neurobiol. 2021, 58, 1162–1184. [Google Scholar] [CrossRef]
- Janssen, B.G.; Byun, H.M.; Gyselaers, W.; Lefebvre, W.; Baccarelli, A.A.; Nawrot, T.S. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIRONAGE birth cohort study. Epigenetics 2015, 10, 536–544. [Google Scholar] [CrossRef]
- Janssen, B.G.; Byun, H.M.; Roels, H.A.; Gyselaers, W.; Penders, J.; Baccarelli, A.A.; Nawrot, T.S. Regulating role of fetal thyroid hormones on placental mitochondrial DNA methylation: Epidemiological evidence from the ENVIRONAGE birth cohort study. Clin. Epigenet. 2017, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Qu, Q.; Chen, X.; Zhao, L.; Jiao, Z.; Wan, Z.; Kwok, H.F.; Qu, S. Downregulation of Ambra1 by altered DNA methylation exacerbates dopaminergic neuron damage in a fenpropathrin-induced Parkinson-like mouse model. Ecotoxicol. Environ. Saf. 2024, 271, 115995. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhao, Y.; Li, Z.; Zhu, M.; Wang, Z.; Li, Y.; Xu, T.; Feng, D.; Zhang, S.; Tang, F.; et al. miR-103a-3p regulates mitophagy in Parkinson’s disease through Parkin/Ambra1 signaling. Pharmacol. Res. 2020, 160, 105197. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yao, J.; Xiao, N.; Han, Y.; Wu, X.; Ci, C.; Chen, K.; Geng, X. DNA methyltransferase 1 (DNMT1) suppresses mitophagy and aggravates heart failure via the microRNA-152-3p/ETS1/RhoH axis. Lab. Investig. 2022, 102, 782–793. [Google Scholar] [CrossRef]
- Jin, F.; Li, M.; Li, X.; Zheng, Y.; Zhang, K.; Liu, X.; Cai, B.; Yin, G. DNMT1-mediated methylation inhibits microRNA-214-3p and promotes hair follicle stem cell differentiate into adipogenic lineages. Stem Cell Res. Ther. 2020, 11, 444. [Google Scholar] [CrossRef]
- Jiang, S.X.; Zhou, Z.Y.; Tu, B.; Song, K.; Lin, L.C.; Liu, Z.Y.; Cao, W.; Zhao, J.Y.; Tao, H. Epigenetic regulation of mitochondrial fission and cardiac fibrosis via sFRP3 promoter methylation. Cell. Mol. Life Sci. 2024, 81, 483. [Google Scholar] [CrossRef]
- Jia, Y.; Xie, H.; Wu, S.; Dong, J.; Ying, H. Induction of FAM46C expression mediated by DNMT3A downregulation is involved in early-onset preeclampsia through gene body methylation. Cell. Signal. 2025, 125, 111506. [Google Scholar] [CrossRef]
- Li, X.C.; Jin, F.; Wang, B.Y.; Yin, X.J.; Hong, W.; Tian, F.J. The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA. Theranostics 2019, 9, 3853–3865. [Google Scholar] [CrossRef]
- Cotney, J.; McKay, S.E.; Shadel, G.S. Elucidation of separate, but collaborative functions of the rRNA methyltransferase-related human mitochondrial transcription factors B1 and B2 in mitochondrial biogenesis reveals new insight into maternally inherited deafness. Hum. Mol. Genet. 2009, 18, 2670–2682. [Google Scholar] [CrossRef]
- Raimundo, N.; Song, L.; Shutt, T.E.; McKay, S.E.; Cotney, J.; Guan, M.X.; Gilliland, T.C.; Hohuan, D.; Santos-Sacchi, J.; Shadel, G.S. Mitochondrial stress engages E2F1 apoptotic signaling to cause deafness. Cell 2012, 148, 716–726. [Google Scholar] [CrossRef]
- Kahl, M.; Xu, Z.; Arumugam, S.; Edens, B.; Fischietti, M.; Zhu, A.C.; Platanias, L.C.; He, C.; Zhuang, X.; Ma, Y.C. m6A RNA methylation regulates mitochondrial function. Hum. Mol. Genet. 2024, 33, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yang, B.; Chen, S.; Chen, G.; Zeng, X.; Min, H.; Xu, L. M6A RNA Methylation-Mediated TUG1 Stability Maintains Mitochondrial Homeostasis during Kidney Aging by Epigenetically Regulating PGC1-α Expression. Antioxid. Redox Signal. 2024, 41, 993–1013. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Song, K.; Zhou, Y.; Sun, H.; Liu, Z.Y.; Lin, L.C.; Ding, J.F.; Sha, J.M.; Shi, Y.; Yang, J.J.; et al. METTL3 boosts mitochondrial fission and induces cardiac fibrosis by enhancing LncRNA GAS5 methylation. Pharmacol. Res. 2023, 194, 106840. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, K.; Tu, B.; Sun, H.; Ding, J.F.; Luo, Y.; Sha, J.M.; Li, R.; Zhang, Y.; Zhao, J.Y.; et al. METTL3 boosts glycolysis and cardiac fibroblast proliferation by increasing AR methylation. Int. J. Biol. Macromol. 2022, 223, 899–915. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Zhong, K.; Tao, L.; Lin, Y.; Xie, G.; Tan, Y.; Wu, Y.; Lu, Y.; Chen, Z.; et al. N6-methyladenosine facilitates mitochondrial fusion of colorectal cancer cells via induction of GSH synthesis and stabilization of OPA1 mRNA. Natl. Sci. Rev. 2024, 11, nwae039. [Google Scholar] [CrossRef]
- Shen, C.; Xuan, B.; Yan, T.; Ma, Y.; Xu, P.; Tian, X.; Zhang, X.; Cao, Y.; Ma, D.; Zhu, X.; et al. m(6)A-dependent glycolysis enhances colorectal cancer progression. Mol. Cancer 2020, 19, 72. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Li, C.; Zhang, X.; Shan, Y.; Zhang, Z.; Bo, H.; Zhang, Y. Endurance exercise-induced histone methylation modification involved in skeletal muscle fiber type transition and mitochondrial biogenesis. Sci. Rep. 2024, 14, 21154. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Bo, H.; Zhang, Y. Exercise couples mitochondrial function with skeletal muscle fiber type via ROS-mediated epigenetic modification. Free Radic. Biol. Med. 2024, 213, 409–425. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Mu, Q.; Hou, X.; Yu, W.; Guo, J. Histone H3 Acetylation Is Involved in Retinoid Acid-Induced Neural Differentiation through Increasing Mitochondrial Function. Biomedicines 2023, 11, 3251. [Google Scholar] [CrossRef]
- Wiesel-Motiuk, N.; Assaraf, Y.G. The key roles of the lysine acetyltransferases KAT6A and KAT6B in physiology and pathology. Drug Resist. Updates 2020, 53, 100729. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Zhao, L.; Tian, Q.; deAvila, J.M.; Zhu, M.J.; Du, M. Dietary succinate supplementation to maternal mice improves fetal brown adipose tissue development and thermogenesis of female offspring. J. Nutr. Biochem. 2022, 100, 108908. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jia, H.; Chen, S.; Ma, X.; Zhou, S.; Qiu, L.; Wu, X.; Li, P.; Chu, H.; Zhang, G. Inhibition of OGG1 ameliorates pulmonary fibrosis via preventing M2 macrophage polarization and activating PINK1-mediated mitophagy. Mol. Med. 2024, 30, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Wang, C.; Xue, L.; Liu, L.; Yang, X.; Liu, Z.; Zhang, S.; Luo, D. The SMYD3-MTHFD1L-formate metabolic regulatory axis mediates mitophagy to inhibit M1 polarization in macrophages. Int. Immunopharmacol. 2022, 113, 109352. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Xiang, W. The impact of mitochondrial dysfunction on ovarian aging. J. Transl. Med. 2025, 23, 211. [Google Scholar] [CrossRef]
- Huang, F.; Luo, X.; Ou, Y.; Gao, Z.; Tang, Q.; Chu, Z.; Zhu, X.; He, Y. Control of histone demethylation by nuclear-localized α-ketoglutarate dehydrogenase. Science 2023, 381, eadf8822. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef]
- Mizuarai, S.; Miki, S.; Araki, H.; Takahashi, K.; Kotani, H. Identification of dicarboxylate carrier Slc25a10 as malate transporter in de novo fatty acid synthesis. J. Biol. Chem. 2005, 280, 32434–32441. [Google Scholar] [CrossRef]
- Yuan, H.; Wu, X.; Wu, Q.; Chatoff, A.; Megill, E.; Gao, J.; Huang, T.; Duan, T.; Yang, K.; Jin, C.; et al. Lysine catabolism reprograms tumour immunity through histone crotonylation. Nature 2023, 617, 818–826. [Google Scholar] [CrossRef]
- Zhu, D.; Li, X.; Tian, Y. Mitochondrial-to-nuclear communication in aging: An epigenetic perspective. Trends Biochem. Sci. 2022, 47, 645–659. [Google Scholar] [CrossRef]
- Trefely, S.; Lovell, C.D.; Snyder, N.W.; Wellen, K.E. Compartmentalised acyl-CoA metabolism and roles in chromatin regulation. Mol. Metab. 2020, 38, 100941. [Google Scholar] [CrossRef]
- Hao, X.; Kuang, C.F.; Gu, Z.T.; Wang, Y.F.; Li, S.A.; Ku, Y.L.; Li, Y.H.; Ge, J.H.; Liu, X. From microscopy to nanoscopy via visible light. Light-Sci. Appl. 2013, 2, e108. [Google Scholar] [CrossRef]
- Bialas, N.; Sokolova, V.; van der Meer, S.B.; Knuschke, T.; Ruks, T.; Klein, K.; Westendorf, A.M.; Epple, M. Bacteria (E. coli) take up ultrasmall gold nanoparticles (2 nm) as shown by different optical microscopic techniques (CLSM, SIM, STORM). Nano Select 2022, 3, 1407–1420. [Google Scholar] [CrossRef]
- Frolikova, M.; Blazikova, M.; Capek, M.; Chmelova, H.; Valecka, J.; Kolackova, V.; Valaskova, E.; Gregor, M.; Komrskova, K.; Horvath, O.; et al. Innovative sample preparation using alcohol dehydration and high refractive index medium enables acquisition of two-channel super-resolution 3D STED image of an entire oocyte. J. Microsc. 2025, 297, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; He, X.; Fang, J.B.; Ju, M.Z. Spatiotemporal dynamic monitoring of protein conformation changes by super-resolution imaging. Dye. Pigment. 2025, 236, 112689. [Google Scholar] [CrossRef]
- Yang, X.Z.; Fang, H.B.; Li, S.M.; Chu, C.Y.; Zhang, Y.H.; Yang, Y.; He, W.J.; Chen, Y.C.; Guo, Z.J. Aggregation-based dual-target probe for dual-colour super-resolution monitoring mitophagy and evaluating drugs regulating mitochondria. Aggregate 2025, 6, e641. [Google Scholar] [CrossRef]
- Gao, S.; Sun, J.; Hou, Y.; Ge, X.; Shi, M.; Zheng, H.; Zhang, Y.; Li, M.; Gao, B.; Xi, P. HBimmCue: A Versatile Fluorescent Probe for Multi-Scale Imaging of Lipid Polarity and Membrane Order in Inner Mitochondrial Membrane. Adv. Sci. 2025, 12, e2414343. [Google Scholar] [CrossRef]
- Vargas-Ordaz, E.; Newman, H.; Austin, C.; Catt, S.; Nosrati, R.; Cadarso, V.J.; Neild, A.; Horta, F. Novel application of metabolic imaging of early embryos using a light-sheet on-a-chip device: A proof-of-concept study. Hum. Reprod. 2025, 40, 41–55. [Google Scholar] [CrossRef]
- Chakraborty, C.; Bhattacharya, M.; Agoramoorthy, G. Single-cell sequencing of miRNAs: A modified technology. Cell Biol. Int. 2020, 44, 1773–1780. [Google Scholar] [CrossRef]
- Song, K.; Yang, X.; An, G.; Xia, X.; Zhao, J.; Xu, X.; Wan, C.; Liu, T.; Zheng, Y.; Ren, S.; et al. Targeting APLN/APJ restores blood-testis barrier and improves spermatogenesis in murine and human diabetic models. Nat. Commun. 2022, 13, 7335. [Google Scholar] [CrossRef]
- Liu, C.; Zuo, W.; Yan, G.; Wang, S.; Sun, S.; Li, S.; Tang, X.; Li, Y.; Cai, C.; Wang, H.; et al. Granulosa cell mevalonate pathway abnormalities contribute to oocyte meiotic defects and aneuploidy. Nat. Aging 2023, 3, 670–687. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, C.; Zheng, Y.; Wu, D.; Chen, X.; Lan, H.; Zheng, X.; Wu, H.; Li, S. Glycine regulates lipid peroxidation promoting porcine oocyte maturation and early embryonic development. J. Anim. Sci. 2023, 101, skac425. [Google Scholar] [CrossRef]
- Hashimshony, T.; Senderovich, N.; Avital, G.; Klochendler, A.; de Leeuw, Y.; Anavy, L.; Gennert, D.; Li, S.; Livak, K.J.; Rozenblatt-Rosen, O.; et al. CEL-Seq2: Sensitive highly-multiplexed single-cell RNA-Seq. Genome Biol. 2016, 17, 77. [Google Scholar] [CrossRef]
- Hashimshony, T.; Wagner, F.; Sher, N.; Yanai, I. CEL-Seq: Single-cell RNA-Seq by multiplexed linear amplification. Cell Rep. 2012, 2, 666–673. [Google Scholar] [CrossRef]
- Sasagawa, Y.; Nikaido, I.; Hayashi, T.; Danno, H.; Uno, K.D.; Imai, T.; Ueda, H.R. Quartz-Seq: A highly reproducible and sensitive single-cell RNA sequencing method, reveals non-genetic gene-expression heterogeneity. Genome Biol. 2013, 14, R31. [Google Scholar] [CrossRef]
- Homberger, C.; Imdahl, F.; Hayward, R.J.; Barquist, L.; Saliba, A.E.; Vogel, J. Transcriptomic profiling of individual bacteria by MATQ-seq. Nat. Protoc. 2025, 1–30. [Google Scholar] [CrossRef]
- Sheng, K.; Cao, W.; Niu, Y.; Deng, Q.; Zong, C. Effective detection of variation in single-cell transcriptomes using MATQ-seq. Nat. Methods 2017, 14, 267–270. [Google Scholar] [CrossRef]
- Sun, F.; Li, H.; Sun, D.; Fu, S.; Gu, L.; Shao, X.; Wang, Q.; Dong, X.; Duan, B.; Xing, F.; et al. Single-cell omics: Experimental workflow, data analyses and applications. Sci. China Life Sci. 2025, 68, 5–102. [Google Scholar] [CrossRef]
- Love, A.M.A.; Edwards, C.; Cai, R.Y.; Gibbs, V. Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults. Autism Adulthood 2023, 5, 389–400. [Google Scholar] [CrossRef]
- Huang, J.; Chen, P.; Jia, L.; Li, T.; Yang, X.; Liang, Q.; Zeng, Y.; Liu, J.; Wu, T.; Hu, W.; et al. Multi-Omics Analysis Reveals Translational Landscapes and Regulations in Mouse and Human Oocyte Aging. Adv. Sci. 2023, 10, e2301538. [Google Scholar] [CrossRef]
- Wu, Y.W.; Li, S.; Zheng, W.; Li, Y.C.; Chen, L.; Zhou, Y.; Deng, Z.Q.; Lin, G.; Fan, H.Y.; Sha, Q.Q. Dynamic mRNA degradome analyses indicate a role of histone H3K4 trimethylation in association with meiosis-coupled mRNA decay in oocyte aging. Nat. Commun. 2022, 13, 3191. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, Y. Exploring the Cancer Immune Epigenome by ATAC-Seq. In Cancer Immunosurveillance. Methods in Molecular Biology; Humana: New York, NY, USA, 2025; Volume 2930, pp. 219–227. [Google Scholar] [CrossRef]
- Ming, H.; Sun, J.; Pasquariello, R.; Gatenby, L.; Herrick, J.R.; Yuan, Y.; Pinto, C.R.; Bondioli, K.R.; Krisher, R.L.; Jiang, Z. The landscape of accessible chromatin in bovine oocytes and early embryos. Epigenetics 2021, 16, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.T.; Lim, D.H.; Ma, W.; Aubol, B.E.; Hao, Y.; Wang, X.; Zhao, J.; Liang, Z.; Shao, C.; Zhang, X.; et al. Initiation of Parental Genome Reprogramming in Fertilized Oocyte by Splicing Kinase SRPK1-Catalyzed Protamine Phosphorylation. Cell 2020, 180, 1212–1227.e1214. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Dean, J. Reduced female fertility due to sequestration of RNA Pol II by pervasive transcription in exosome RNase-depleted oocytes. Cell Rep. 2023, 42, 113247. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Halstead, M.M.; Bonnet-Garnier, A.; Schultz, R.M.; Ross, P.J. Histone remodeling reflects conserved mechanisms of bovine and human preimplantation development. EMBO Rep. 2023, 24, e55726. [Google Scholar] [CrossRef]
- Zhang, B.; Qi, T.; Lin, J.; Zhai, S.; Wang, X.; Zhou, L.; Deng, X. KLF6-mediated recruitment of the p300 complex enhances H3K23su and cooperatively upregulates SEMA3C with FOSL2 to drive 5-FU resistance in colon cancer cells. Exp. Mol. Med. 2025, 57, 667–685. [Google Scholar] [CrossRef]
- Zhao, X.M.; Wang, N.; Hao, H.S.; Li, C.Y.; Zhao, Y.H.; Yan, C.L.; Wang, H.Y.; Du, W.H.; Wang, D.; Liu, Y.; et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res. 2018, 64, e12445. [Google Scholar] [CrossRef]
- Gu, J.; Hua, R.; Wu, H.; Guo, C.; Hai, Z.; Xiao, Y.; Yeung, W.S.B.; Liu, K.; Babayev, E.; Wang, T. Salidroside Improves Oocyte Competence of Reproductively Old Mice by Enhancing Mitophagy. Aging Cell 2025, e14475. [Google Scholar] [CrossRef]
- Leathersich, S.J.; Roche, C.S.; Walls, M.; Nathan, E.; Hart, R.J. Particulate air pollution at the time of oocyte retrieval is independently associated with reduced odds of live birth in subsequent frozen embryo transfers. Hum. Reprod. 2025, 40, 110–118. [Google Scholar] [CrossRef]
- Erdenetogtokh, P.; Kanno, C.; Sakaguchi, K.; Yanagawa, Y.; Katagiri, S.; Nagano, M. Effect of astaxanthin addition to an individual culture system for in vitro maturation of bovine oocytes on accumulation of reactive oxygen species and mitochondrial activity. Jpn. J. Vet. Res. 2018, 66, 325–329. [Google Scholar] [CrossRef]
- Khan, A.M.; Idrees, M.; Perera, C.D.; Haider, Z.; Joo, M.D.; Kang, J.S.; Lee, S.H.; Kong, I.K. The effects of cycloastragenol on bovine embryo development, implantation potential and telomerase activity. Reprod. Fertil. Dev. 2023, 35, 527–538. [Google Scholar] [CrossRef]
- Gutierrez-Castillo, E.; Diaz, F.A.; Talbot, S.A.; Bondioli, K.R. Effect of bovine oocyte vitrification with EGTA and post-warming recovery with resveratrol on meiotic spindle, mitochondrial function, reactive oxygen species, and developmental competence. Theriogenology 2023, 196, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Castillo, E.; Diaz, F.A.; Talbot, S.A.; Bondioli, K.R. Recovery of spindle morphology and mitochondrial function through extended culture after vitrification-warming of bovine oocytes. Theriogenology 2022, 189, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Chinen, S.; Yamanaka, T.; Hirabayashi, M.; Hochi, S. Rescue of vitrified-warmed bovine mature oocytes by short-term recovery culture with resveratrol. Cryobiology 2020, 97, 185–190. [Google Scholar] [CrossRef]
- Sprícigo, J.F.; Morató, R.; Arcarons, N.; Yeste, M.; Dode, M.A.; López-Bejar, M.; Mogas, T. Assessment of the effect of adding L-carnitine and/or resveratrol to maturation medium before vitrification on in vitro-matured calf oocytes. Theriogenology 2017, 89, 47–57. [Google Scholar] [CrossRef]
- Ito, J.; Iwata, H. Age-related advanced glycation end-product accumulation impairs mitochondrial regulation after vitrification†. Biol. Reprod. 2023, 109, 271–281. [Google Scholar] [CrossRef]
- Yang, C.X.; Liu, S.; Miao, J.K.; Mou, Q.; Liu, X.M.; Wang, P.C.; Huo, L.J.; Du, Z.Q. CoQ10 improves meiotic maturation of pig oocytes through enhancing mitochondrial function and suppressing oxidative stress. Theriogenology 2021, 159, 77–86. [Google Scholar] [CrossRef]
- He, X.; Chen, H.; Liao, M.; Zhao, X.; Zhang, D.; Jiang, M.; Jiang, Z. The role of CoQ10 in embryonic development. J. Assist. Reprod. Genet. 2024, 41, 767–779. [Google Scholar] [CrossRef]
- Ratchamak, R.; Authaida, S.; Koedkanmark, T.; Boonkum, W.; Chankitisakul, V. Coenzyme Q10 Supplementation Effects on In Vitro Oocyte Maturation, Lipid Peroxidation, and Embryonic Development in Prepubertal and Aging Thai-Holstein Cows. Animals 2024, 15, 18. [Google Scholar] [CrossRef]
- Bisogno, S.; Depciuch, J.; Gulzar, H.; Heber, M.F.; Kobiałka, M.; Gąsior, Ł.; Bereta, A.; Pieczara, A.; Fic, K.; Musson, R.; et al. Female-age-dependent changes in the lipid fingerprint of the mammalian oocytes. Hum. Reprod. 2024, 39, 2754–2767. [Google Scholar] [CrossRef]
- Laplacette, A.L.; Rial, C.; Sitko, E.; Perez, M.M.; Tompkins, S.; Stangaferro, M.L.; Thomas, M.J.; Giordano, J.O. Delaying induction of ovulation and timed artificial insemination in a Double-Ovsynch protocol increased expression of estrus and altered first-service reproductive outcomes of lactating dairy cows. J. Dairy Sci. 2025, 108, 1103–1124. [Google Scholar] [CrossRef]
- Ramírez-Martín, N.; Buigues, A.; Rodríguez-Varela, C.; Martínez, J.; Blázquez-Simón, P.; Rodríguez-Hernández, C.; Pellicer, N.; Pellicer, A.; Escribá, M.J.; Herraiz, S. Nicotinamide mononucleotide supplementation improves oocyte developmental competence in different ovarian damage conditions. Am. J. Obstet. Gynecol. 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qin, X.; Bojan, N.; Cao, C.; Chai, J.; Pang, W. Nicotinamide mononucleotide enhances porcine sperm quality by activating the SIRT3-SOD2/ROS pathway and promoting oxidative phosphorylation. Anim. Reprod. Sci. 2025, 275, 107797. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Sutton-McDowall, M.L.; Wang, X.; Sugimura, S.; Thompson, J.G.; Gilchrist, R.B. Extending prematuration with cAMP modulators enhances the cumulus contribution to oocyte antioxidant defence and oocyte quality via gap junctions. Hum. Reprod. 2016, 31, 810–821. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Peng, W.; Cheng, Y.; Lu, Z.; Zhou, C.; Zhang, Y.; Su, J. Melatonin supplementation during in vitro maturation of oocyte enhances subsequent development of bovine cloned embryos. J. Cell. Physiol. 2019, 234, 17370–17381. [Google Scholar] [CrossRef]
- El Sheikh, M.; Mesalam, A.; Mesalam, A.A.; Idrees, M.; Lee, K.L.; Kong, I.K. Melatonin Abrogates the Anti-Developmental Effect of the AKT Inhibitor SH6 in Bovine Oocytes and Embryos. Int. J. Mol. Sci. 2019, 20, 2956. [Google Scholar] [CrossRef]
- Santiquet, N.W.; Greene, A.F.; Becker, J.; Barfield, J.P.; Schoolcraft, W.B.; Krisher, R.L. A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence. Mol. Hum. Reprod. 2017, 23, 594–606. [Google Scholar] [CrossRef]
- Truong, T.T.; Soh, Y.M.; Gardner, D.K. Antioxidants improve mouse preimplantation embryo development and viability. Hum. Reprod. 2016, 31, 1445–1454. [Google Scholar] [CrossRef]
- Novielli, C.; Anelli, G.M.; Lisso, F.; Marzorati, A.; Parrilla, B.; Oneta, M.; Savasi, V.M.; Cetin, I.; Mandò, C. Effects of α-lipoic acid and myo-inositol supplementation on the oocyte environment of infertile obese women: A preliminary study. Reprod. Biol. 2020, 20, 541–546. [Google Scholar] [CrossRef]
- Silva, B.R.; Silva, J.R.V. Mechanisms of action of non-enzymatic antioxidants to control oxidative stress during in vitro follicle growth, oocyte maturation, and embryo development. Anim. Reprod. Sci. 2023, 249, 107186. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Y.; Zhao, Y.; Gao, Q.; Jin, Q.; Yan, C.; Xu, Y. l-carnitine supplementation during in vitro culture regulates oxidative stress in embryos from bovine aged oocytes. Theriogenology 2020, 143, 64–73. [Google Scholar] [CrossRef]


| Substances | Function | References |
|---|---|---|
| Resveratrol | Meiotic spindle stabilization; intracellular ROS reduction; mitochondrial biogenesis activation; cytoplasmic maturation enhancement; blastocyst formation promotion | [107,197,202,203,204,205,206] |
| Coenzyme Q10 | Suppresses ROS generation; mitigates oxidative stress-induced apoptosis; facilitates nuclear maturation; enhances oocyte quality; improves embryonic developmental competence; alleviates oxidative stress; reinforces mitochondrial function; accelerates developmental progression; promotes blastocyst formation rates | [207,208,209,210,211] |
| Nicotinamide Mononucleotide | ROS accumulation attenuation; meiotic chromosomal misalignment correction; mitochondrial membrane potential restoration; ATP synthesis augmentation; mitochondrial autophagy activation; oocyte maturation rate elevation; spindle assembly fidelity preservation; NAD+ pool replenishment in cumulus–oocyte complexes | [212,213] |
| Melatonin | ROS scavenging system activation (glutathione/antioxidant genes); mitochondrial architecture–function coordination; epigenetic regulation maintenance (methylation/hydroxymethylation) | [197,214,215,216,217] |
| α Lipoic Acid | Mitochondrial functional boost (activity/mtDNA); transcriptional fine-tuning; oxidative damage neutralization | [218,219] |
| L-carnitine | Metabolic flux optimization (fatty acid/glucose/respiratory chain); oxidative stress–apoptosis axis suppression; oocyte rejuvenation triad (glutathione/membrane potential/cytoplasmic maturation) | [220,221] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-R.; Xu, D.-J. Mitochondrial Quality Control in Bovine Oocyte Maturation: Mechanisms, Challenges, and Prospects for Enhancing Reproductive Efficiency. Animals 2025, 15, 2000. https://doi.org/10.3390/ani15132000
Zhang Y-R, Xu D-J. Mitochondrial Quality Control in Bovine Oocyte Maturation: Mechanisms, Challenges, and Prospects for Enhancing Reproductive Efficiency. Animals. 2025; 15(13):2000. https://doi.org/10.3390/ani15132000
Chicago/Turabian StyleZhang, Yi-Ran, and De-Jun Xu. 2025. "Mitochondrial Quality Control in Bovine Oocyte Maturation: Mechanisms, Challenges, and Prospects for Enhancing Reproductive Efficiency" Animals 15, no. 13: 2000. https://doi.org/10.3390/ani15132000
APA StyleZhang, Y.-R., & Xu, D.-J. (2025). Mitochondrial Quality Control in Bovine Oocyte Maturation: Mechanisms, Challenges, and Prospects for Enhancing Reproductive Efficiency. Animals, 15(13), 2000. https://doi.org/10.3390/ani15132000





