Multi-Detector Helical Computed Tomography, Transrectal Ultrasonography, and Histology of the Sacroiliac Joint: A Comparative Study in Adult Warmblood Horse Cadavers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diagnostic Imaging
2.2. Histological Examination
2.3. Statistical Analysis
3. Results
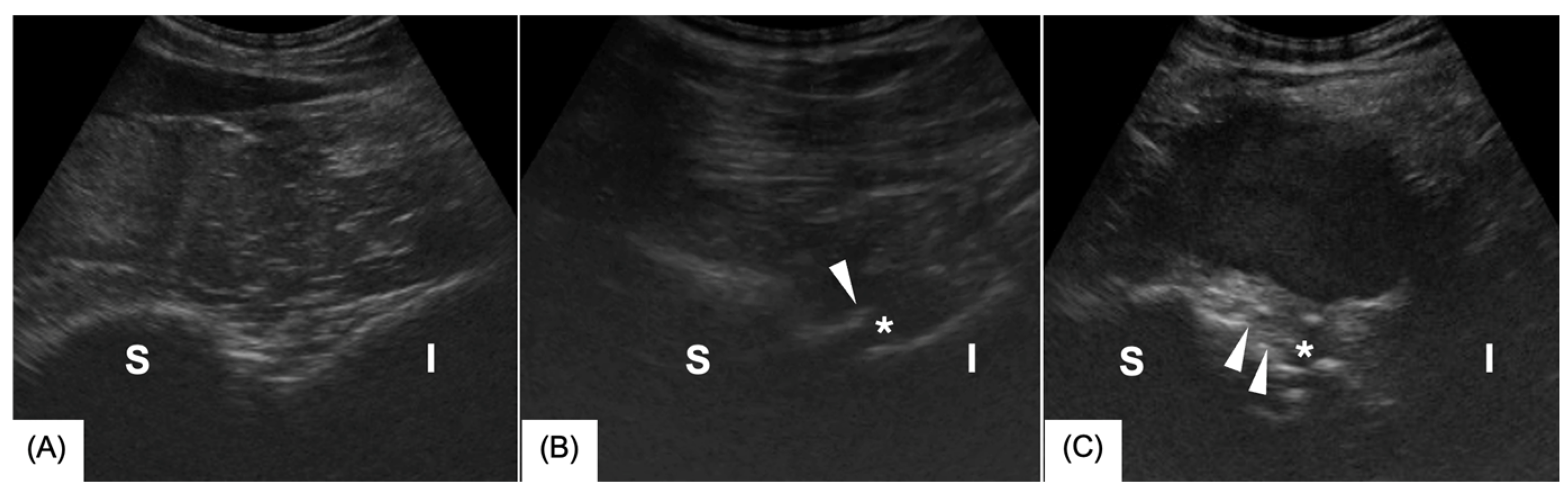
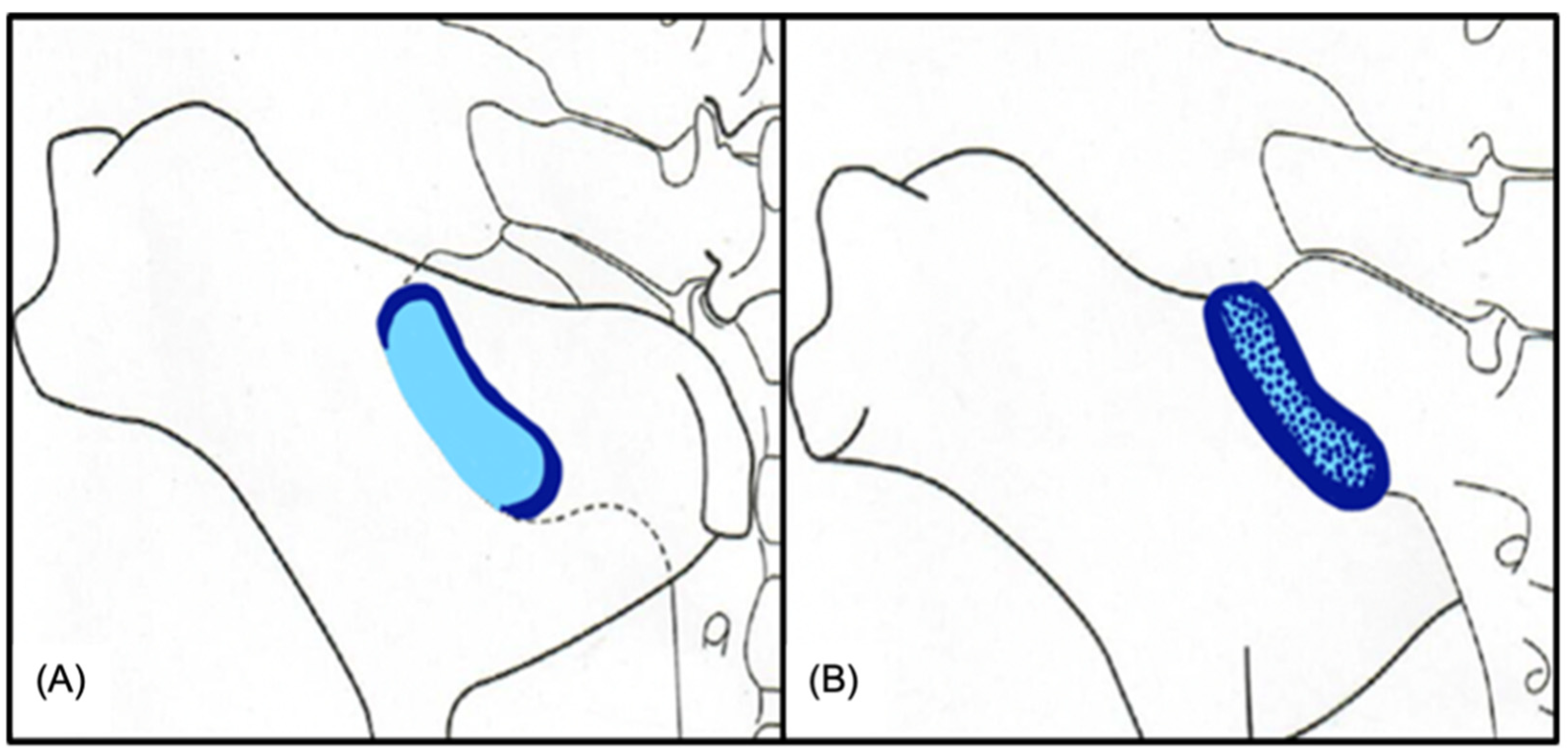
3.1. Ultrasonography
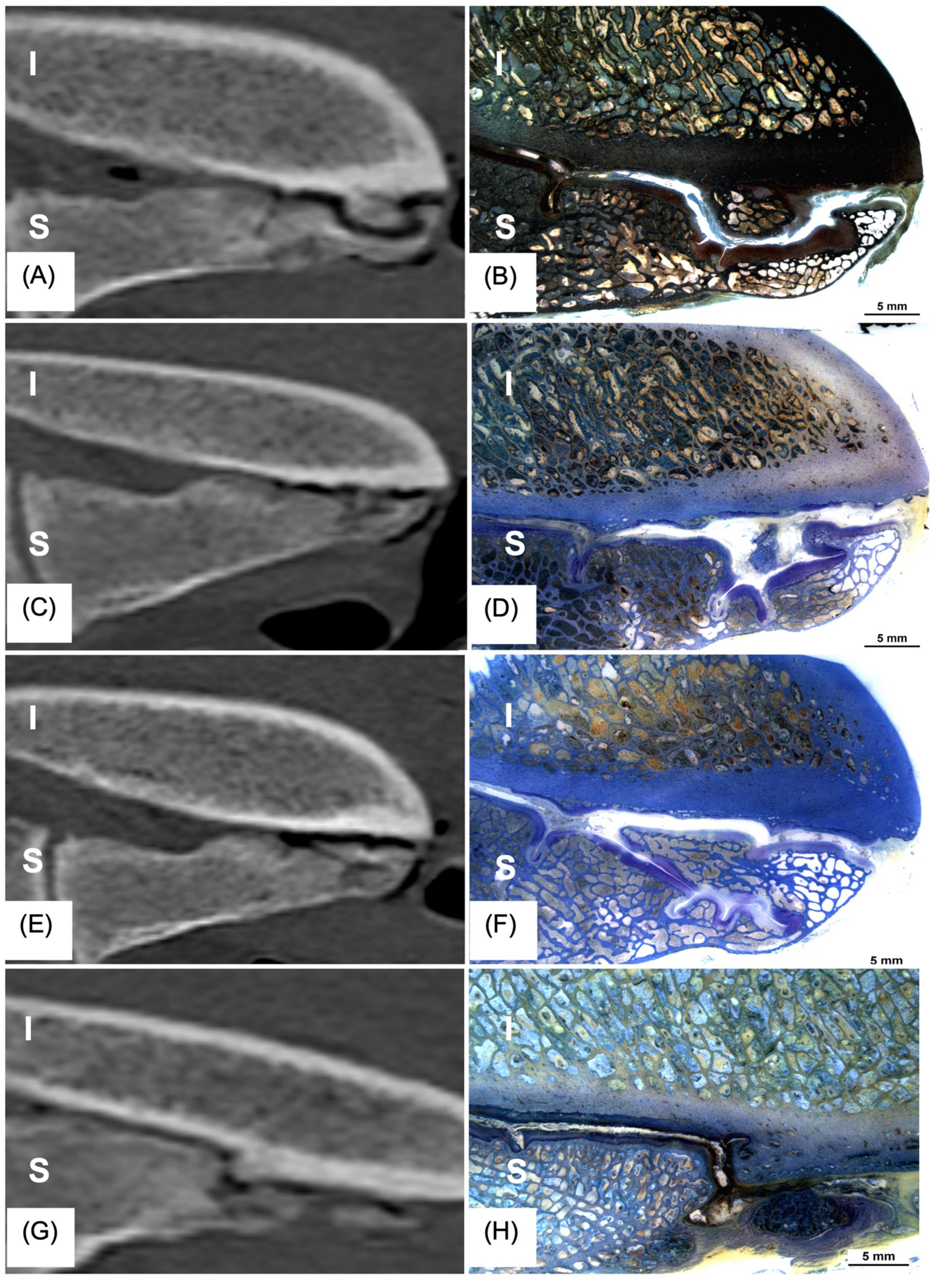
3.2. CT
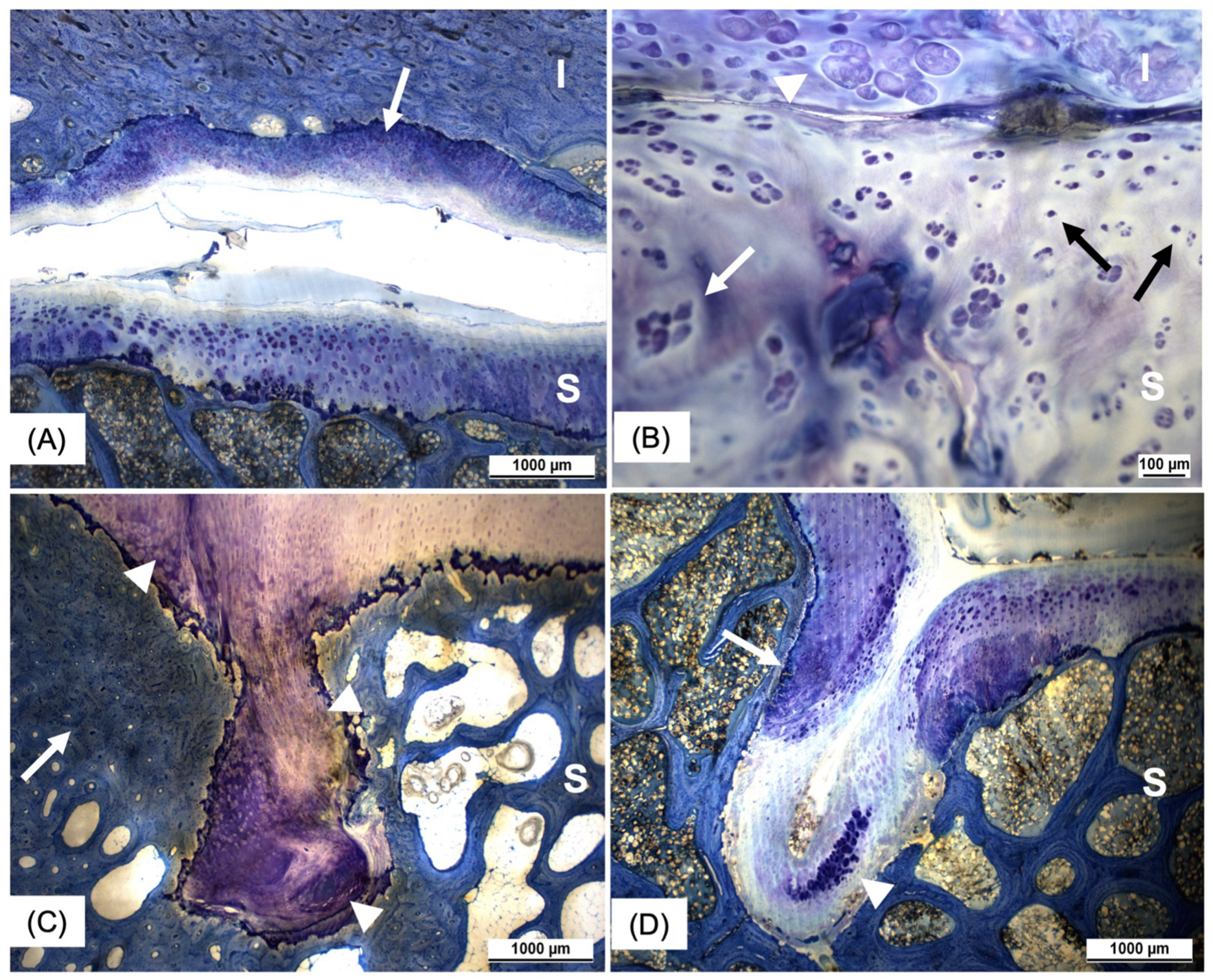
3.3. Histology
3.4. Correlation Between Imaging Modalities (CT, US) and Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed Tomography |
| ECVDI | European College of Veterinary Diagnostic Imaging |
| ECVS | European College of Veterinary Surgery |
| SIJ | Sacroiliac Joint |
| US | Ultrasonography |
References
- Dyson, S.; Murray, R. Pain associated with the sacroiliac joint region: A clinical study of 74 horses. Equine Vet. J. 2003, 35, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Barstow, A.; Dyson, S. Clinical features and diagnosis of sacroiliac joint region pain in 296 horses: 2004-2014. Equine Vet. Educ. 2015, 27, 637–647. [Google Scholar] [CrossRef]
- Jeffcott, L.B.; Dalin, G.; Ekman, S.; Olsson, S.E. Sacroiliac lesions as a cause of chronic poor performance in competitive horses. Equine Vet. J. 1985, 17, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Haussler, K.K. Functional Anatomy and Pathophysiology of Sacroiliac Joint Disease. Proc. Annu. Conv. Am. Assoc. Equine Pract. 2004, 50, 361–366. [Google Scholar]
- Gorgas, D.; Kircher, P.; Doherr, M.G.; Ueltschi, G.; Lang, J. Radiographic technique and anatomy of the equine sacroiliac region. Vet. Radiol. Ultrasound 2007, 48, 501–506. [Google Scholar] [CrossRef]
- Gorgas, D.; Luder, P.; Lang, J.; Doherr, M.G.; Ueltschi, G.; Kircher, P. Scintigraphic and radiographic appearance of the sacroiliac region in horses with gait abnormalities or poor performance. Vet. Radiol. Ultrasound 2009, 50, 208–214. [Google Scholar] [CrossRef]
- Erichsen, C.; Berger, M.; Eksell, P. The scintigraphic anatomy of the equine sacroiliac joint. Vet. Radiol. Ultrasound 2002, 43, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, C.; Eksell, P.; Widstrom, C.; Berger, M.; Holm, K.R.; Johnston, C. Scintigraphy of the sacroiliac joint region in asymptomatic riding horses: Scintigraphic appearance and evaluation of method. Vet. Radiol. Ultrasound 2003, 44, 699–706. [Google Scholar] [CrossRef]
- Dyson, S.; Murray, R.; Branch, M.; Harding, E. The sacroiliac joints: Evaluation using nuclear scintigraphy. Part 2: Lame horses. Equine Vet. J. 2003, 35, 233–239. [Google Scholar] [CrossRef]
- Tallaj, A.; Coudry, V.; Denoix, J.M. Transrectal ultrasonographic examination of the sacroiliac joints of the horse: Abnormal findings and lesions. Equine Vet. Educ. 2017, 32, 33–38. [Google Scholar] [CrossRef]
- Quiney, L.E.; Ireland, J.L.; Dyson, S.J. Evaluation of the diagnostic accuracy of skeletal scintigraphy in lame and poorly performing sports horses. Vet. Radiol. Ultrasound 2018, 59, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Tallaj, A.; Coudry, V.; Denoix, J.M. Transrectal ultrasonographic examination of the sacroiliac joints of the horse: Technique and normal images. Equine Vet. Educ. 2017, 31, 666–671. [Google Scholar] [CrossRef]
- Tomlinson, J.E.; Sage, A.M.; Turner, T.A. Ultrasonographic abnormalities detected in the sacroiliac area in twenty cases of upper hindlimb lameness. Equine Vet. J. 2003, 35, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, J.E.; Sage, A.M.; Turner, T.A.; Feeney, D.A. Detailed ultrasonographic mapping of the pelvis in clinically normal horses and ponies. Am. J. Vet. Res. 2001, 62, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Bergman, E.H.J.; Puchalski, S.M.; Denoix, J.M. How to perform a transrectal ultrasound examination of the lumbosacral and sacroiliac joints. Proc. Am. Assoc. Equine Pract. 2013, 59, 229–230. [Google Scholar]
- Engeli, E.; Yeager, A.E.; Erb, H.N.; Haussler, K.K. Ultrasonographic technique and normal anatomic features of the sacroiliac region in horses. Vet. Radiol. Ultrasound 2006, 47, 391–403. [Google Scholar] [CrossRef]
- Backlund, J.; Clewett Dahl, E.; Skorpil, M. Is CT indicated in diagnosing sacroiliac joint degeneration? Clin. Radiol. 2017, 72, 693.E9–693.E13. [Google Scholar] [CrossRef]
- Carnevale, M.; Jones, J.; Holaskova, I.; Sponenberg, D.P. CT and gross pathology are comparable methods for detecting some degenerative sacroiliac joint lesions in dogs. Vet. Radiol. Ultrasound 2019, 60, 378–389. [Google Scholar] [CrossRef]
- Carnevale, M.; Jones, J.; Li, G.; Sharp, J.; Olson, K.; Bridges, W. Computed Tomographic Evaluation of the Sacroiliac Joints of Young Working Labrador Retrievers of Various Work Status Groups: Detected Lesions Vary Among the Different Groups and Finite Element Analyses of the Static Pelvis Yields Repeatable Measures of Sacroiliac Ligament Joint Strain. Front. Vet. Sci. 2020, 7, 528. [Google Scholar] [CrossRef]
- Wise, R.; Jones, J.; Werre, S.; Aguirre, M. The prevalence of sacroiliac joint CT and MRI findings is high in large breed dogs. Vet. Radiol. Ultrasound 2022, 63, 739–748. [Google Scholar] [CrossRef]
- Scilimati, N.; Angeli, G.; Di Meo, A.; Dall’Aglio, C.; Pepe, M.; Beccati, F. Post-Mortem Computed Tomographic Features of the Most Caudal Lumbar Vertebrae, Anatomical Variations and Acquired Osseous Pathological Changes, in a Mixed Population of Horses. Animals 2023, 13, 743. [Google Scholar] [CrossRef]
- Ogden, N.K.E.; Winderickx, K.; Bennell, A.; Stack, J.D. Computed tomography of the equine caudal spine and pelvis: Technique, image quality and anatomical variation in 56 clinical cases (2018-2023). Equine Vet. J. 2024, 1–14. [Google Scholar] [CrossRef]
- Dalin, G.; Jeffcott, L.B. Sacroiliac joint of the horse. 1. Gross morphology. Anat. Histol. Embryol. 1986, 15, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Rümens, D.; Patan, B.; Probst, A.; Polsterer, E.; Macher, R.; Stanek, C.; König, H.E. The iliosacral connection: A problem associated area of the equine back. Pferdeheilkunde Equine Med. 2007, 23, 21–26. [Google Scholar] [CrossRef]
- Cassidy, J.; Townsend, H. Sacroiliac joint strain as a cause of back pain in man—Implications for the horse. Proc. Annu. Conv. Am. Assoc. Equine Pract. 1986, 317–333. [Google Scholar]
- Dalin, G.; Jeffcott, L.B. Sacroiliac joint of the horse. 2. Morphometric features. Anat. Histol. Embryol. 1986, 15, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Denoix, J.M. Ligament injuries of the axial skeleton in the horse: Supraspinal and sacroiliac desmopathies. In Proceedings of the Dubai International Equine Symposium, Dubai, United Arab Emirates, 27–30 March 1996; pp. 273–286. [Google Scholar]
- Quiney, L.; Stewart, J.; Routh, J.; Dyson, S. Gross post-mortem and histological features in 27 horses with confirmed lumbosacral region pain and five control horses: A descriptive cadaveric study. Equine Vet. J. 2022, 54, 726–739. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E.; Fuller, C.J.; Hurtig, M.; Cruz, A. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the horse. Osteoarthr. Cartil. 2010, 18 (Suppl. S3), S93–S105. [Google Scholar] [CrossRef]
- Faflia, C.P.; Prassopoulos, P.K.; Daskalogiannaki, M.E.; Gourtsoyiannis, N.C. Variation in the appearance of the normal sacroiliac joint on pelvic CT. Clin. Radiol. 1998, 53, 742–746. [Google Scholar] [CrossRef]
- Haussler, K.K.; Stover, S.M.; Willits, N.H. Pathologic changes in the lumbosacral vertebrae and pelvis in Thoroughbred racehorses. Am. J. Vet. Res. 1999, 60, 143–153. [Google Scholar] [CrossRef]
- Vogler, J.B., 3rd; Brown, W.H.; Helms, C.A.; Genant, H.K. The normal sacroiliac joint: A CT study of asymptomatic patients. Radiology 1984, 151, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Shirai, Y.; Miyamoto, M. The aging process in the sacroiliac joint: Helical computed tomography analysis. J. Orthop. Sci. 2002, 7, 12–18. [Google Scholar] [CrossRef]
- Ogden, N.K.E.; Winderickx, K.; Stack, J.D. Computed tomography of the equine caudal spine and pelvis. Pathological findings in 56 clinical cases (2018–2023). Equine Vet. J. 2024, 1–11. [Google Scholar] [CrossRef]
- Engeli, E.; Haussler, K. Review of injection techniques targeting the sacroiliac region in horses. Equine Vet. Educ. 2012, 24, 529–541. [Google Scholar] [CrossRef]
- Ekman, S.; Dalin, G.; Olsson, S.E.; Jeffcott, L.B. Sacroiliac joint of the horse. 3. Histological appearance. Anat. Histol. Embryol. 1986, 15, 108–121. [Google Scholar] [CrossRef]
- Dreier, R. Hypertrophic differentiation of chondrocytes in osteoarthritis: The developmental aspect of degenerative joint disorders. Arthritis Res. Ther. 2010, 12, 216. [Google Scholar] [CrossRef]
- Jaswal, A.P.; Bandyopadhyay, A. Re-examining osteoarthritis therapy from a developmental biologist’s perspective. Biochem. Pharmacol. 2019, 165, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Scilimati, N.; Beccati, F.; Dall’Aglio, C.; Di Meo, A.; Pepe, M. Age and sex correlate with bony changes and anatomic variations of the lumbosacroiliac region of the vertebral column in a mixed population of horses. J. Am. Vet. Med. Assoc. 2022, 261, 258–265. [Google Scholar] [CrossRef]
- Haussler, K.K. Anatomy of the thoracolumbar vertebral region. Vet. Clin. N. Am. Equine Pract. 1999, 15, 13–26. [Google Scholar] [CrossRef]
- Haussler, K.K.; McGilvray, K.C.; Ayturk, U.M.; Puttlitz, C.M.; Hills, A.E.; McIlwraith, C.W. Deformation of the equine pelvis in response to in vitro 3D sacroiliac joint loading. Equine Vet. J. 2009, 41, 207–212. [Google Scholar] [CrossRef]
- Müller-Quirin, J.; Dittmann, M.T.; Roepstorff, C.; Arpagaus, S.; Latif, S.N.; Weishaupt, M.A. Riding Soundness-Comparison of Subjective with Objective Lameness Assessments of Owner-Sound Horses at Trot on a Treadmill. J. Equine Vet. Sci. 2020, 95, 103314. [Google Scholar] [CrossRef] [PubMed]
| Grade | Articular Margins | Modeling | Enthesophyte Formation | Ventral Sacroiliac Ligament | Joint Effusion |
|---|---|---|---|---|---|
| 0 | Normal articular margins | No change of shape | No enthesophytes present | Normal echogenicity of ventral sacroiliac ligament | No joint effusion |
| 1 | Mild irregular articular joint margins | Mild modeling | Mild enthesophyte formation | Heterogenous echogenicity of ventral sacroiliac ligament | Suspected or mild effusion |
| 2 | Moderate irregular articular joint margins | Moderate modeling | Moderate enthesophyte formation | Fibrosis and/or atrophy of the ventral sacroiliac ligament | Moderate joint effusion |
| 3 | Severely irregular articular joint margins | Severe modeling | Severe enthesophyte formation | Severe fibrosis and/or atrophy of the ventral sacroiliac ligament | Severe joint effusion |
| Grade | Osteophyte Formation | Periarticular Modeling (Enthesophyte Formation) | Subchondral Bone Lesions | Subchondral Bone Sclerosis | Sacrum/Ilium Bone Modeling |
|---|---|---|---|---|---|
| 0 | Normal | Normal | Normal | Normal | Normal |
| 1 | Periarticular osteophytes | Mild enthesophyte formation | Mild erosion and/or one small cyst | Mild/focal sclerosis | Mild modeling |
| 2 | Intra-articular bone spurs | Moderate enthesophyte formation | Moderate erosion and/or multiple cysts | Moderate/multifocal sclerosis | Moderate modeling |
| 3 | Intra-articular ankylosis | Severe enthesophyte formation and/or ankylosis | Severe erosion and/or >3 cysts | Severe/diffuse sclerosis | Severe modeling |
| Histological Feature | Grade | Description |
|---|---|---|
| Articular cartilage | ||
| Hypertrophic chondrocytes without group formation | 0 | No hypertrophic chondrocyte |
| 1 | Isolated hypertrophic chondrocytes | |
| Chondrocyte group formation without hypertrophy | 0 | No groups (>2 cells) without hypertrophy |
| 1 | Groups (>2 cells) without hypertrophy | |
| Chondrocyte group formation with hypertrophy | 0 | No groups (>2 cells) with hypertrophy |
| 1 | Groups (>2 cells) with hypertrophy | |
| Hypertrophic chondrocytes adjacent to fissures | 0 | No hypertrophic chondrocytes adjacent to fissures of the articular cartilage |
| 1 | Hypertrophic chondrocytes adjacent to fissures of the articular cartilage | |
| Pyknotic chondrocytes | 0 | ≤1 pyknotic chondrocyte near the articular surface (20× magnification) |
| 1 | >1 pyknotic chondrocyte near the articular surface or in the basal layer | |
| Indentations in the cartilage without disturbance of the cartilage alignment | 0 | No indentation; cartilage alignment intact |
| 1 | Indentation present; cartilage alignment intact | |
| Indentations in the cartilage with disturbance of the cartilage alignment | 0 | No indentation |
| 1 | Indentation present with disturbed cartilage alignment | |
| Tidemark | 0 | Tidemark intact |
| 1 | Tidemark penetrated by vessels | |
| Subchondral bone | ||
| Subchondral bone regularity | 0 | Regular subchondral bone |
| 1 | Irregular subchondral bone | |
| Subchondral bone thinning | 0 | Subjectively normal subchondral bone thickness |
| 1 | Subjectively reduced subchondral bone thickness | |
| Subchondral bone thickening/sclerosis | 0 | Subjectively normal subchondral bone thickness |
| 1 | Subjectively increased subchondral bone thickness | |
| Subchondral bone lesions | 0 | No lesion in the area of the subchondral bone |
| 1 | Presence of subchondral bone cysts, fissures, clefts or several of these within the subchondral bone | |
| Joint capsule | ||
| Cellularity of the joint capsule | 0 | Normal cellularity |
| 1 | Increased cellularity | |
| Histological Finding | Iliac Joint Surface (% of Occurrence in 104 Histological Slides) | Sacral Joint Surface (% of Occurrence in 104 Histological Slides) |
|---|---|---|
| Group formation (>2 chondrocytes) without hypertrophy | 44.3% | * 61.3% (p = 0.015) |
| Group formation (>2 chondrocytes) with hypertrophy | 17% | * 50% (p < 0.001) |
| Pyknotic chondrocytes | 26.4% | * 42.5% (p = 0.016) |
| Vascular disruption of tidemark | 17.9% | * 30.2% (p = 0.25) |
| Hypertrophy without group formation | 7.5% | * 21.7% (p = 0.004) |
| Cartilage indentations with disruption of cell alignment | 21.7% | 26.4% |
| Cartilage indentation without disruption of cell alignment | 10.4% | 11.3% |
| Sclerosis of subchondral bone | 15.1% | * 40.6% (p < 0.001) |
| Irregular subchondral bone | 21.7% | 33% |
| Subchondral bone lesions (cysts, clefts…) | 26.4% | 31.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathys, R.A.; Schmitz, T.R.; Geyer, H.; Borel, N.; Hilbe, M.; Ohlerth, S.; Bischofberger, A.S. Multi-Detector Helical Computed Tomography, Transrectal Ultrasonography, and Histology of the Sacroiliac Joint: A Comparative Study in Adult Warmblood Horse Cadavers. Animals 2025, 15, 1995. https://doi.org/10.3390/ani15131995
Mathys RA, Schmitz TR, Geyer H, Borel N, Hilbe M, Ohlerth S, Bischofberger AS. Multi-Detector Helical Computed Tomography, Transrectal Ultrasonography, and Histology of the Sacroiliac Joint: A Comparative Study in Adult Warmblood Horse Cadavers. Animals. 2025; 15(13):1995. https://doi.org/10.3390/ani15131995
Chicago/Turabian StyleMathys, Rebecca A., Thomas R. Schmitz, Hans Geyer, Nicole Borel, Monika Hilbe, Stefanie Ohlerth, and Andrea S. Bischofberger. 2025. "Multi-Detector Helical Computed Tomography, Transrectal Ultrasonography, and Histology of the Sacroiliac Joint: A Comparative Study in Adult Warmblood Horse Cadavers" Animals 15, no. 13: 1995. https://doi.org/10.3390/ani15131995
APA StyleMathys, R. A., Schmitz, T. R., Geyer, H., Borel, N., Hilbe, M., Ohlerth, S., & Bischofberger, A. S. (2025). Multi-Detector Helical Computed Tomography, Transrectal Ultrasonography, and Histology of the Sacroiliac Joint: A Comparative Study in Adult Warmblood Horse Cadavers. Animals, 15(13), 1995. https://doi.org/10.3390/ani15131995






