Dietary Tea Polyphenols Improve Growth Performance and Intestinal Microbiota Under Chronic Crowding Stress in Hybrid Crucian Carp
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. TPs and Experimental Diets
2.2. Fish and Experimental Conditions
2.3. Harvesting and Sample Collection
2.4. Growth Performance
2.5. Serum Biochemical Indices
2.6. Antioxidant Capacity
2.7. mRNA Expression
2.8. Intestinal Microbiota Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of TPs Supplementation on Growth Performance
3.2. Effects of TPs Supplementation on Serum Biochemical Indices
3.3. Effects of TPs Supplementation on Antioxidant Capacity
3.4. Effects of TPs Supplementation on mRNA Expression
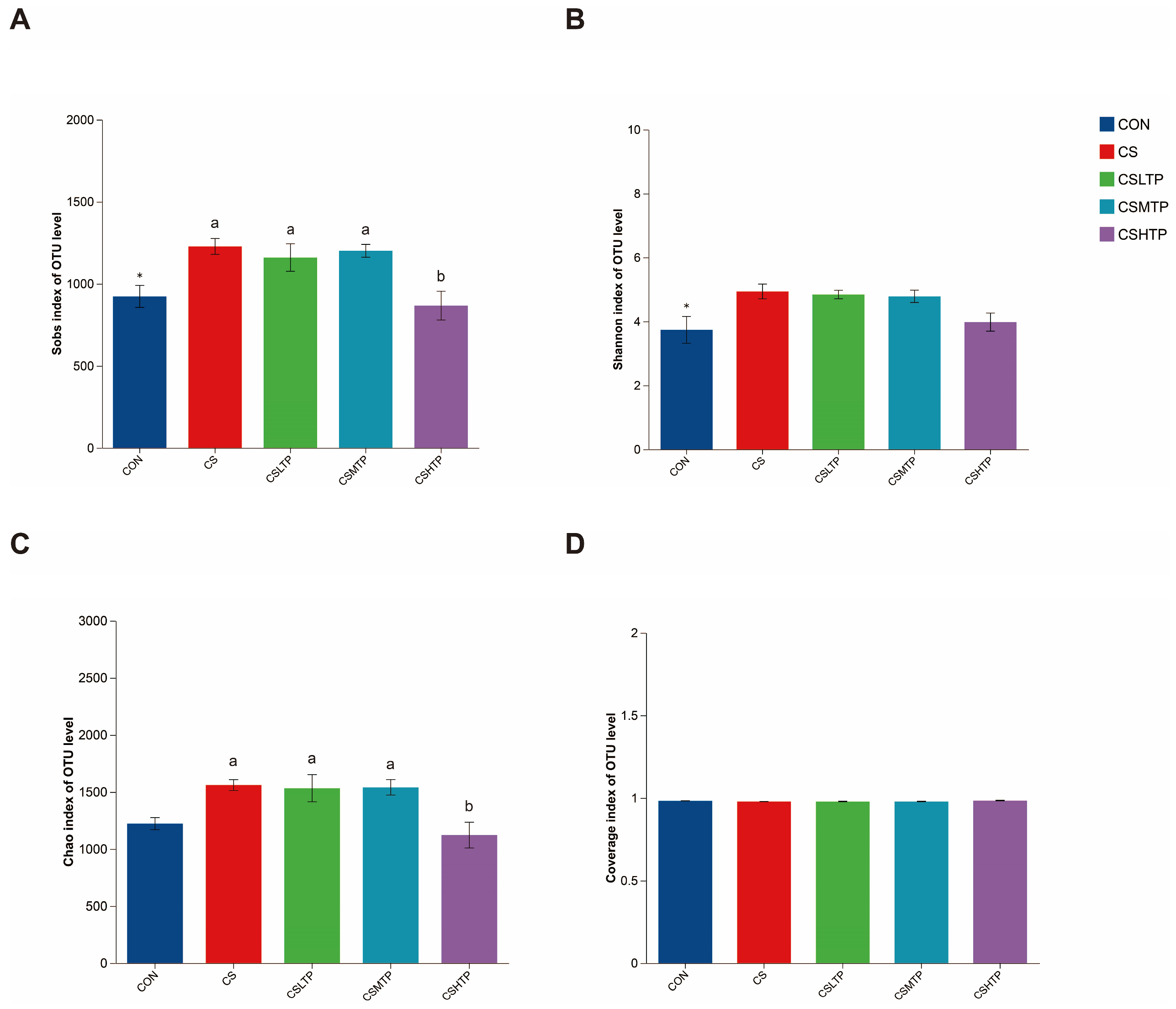
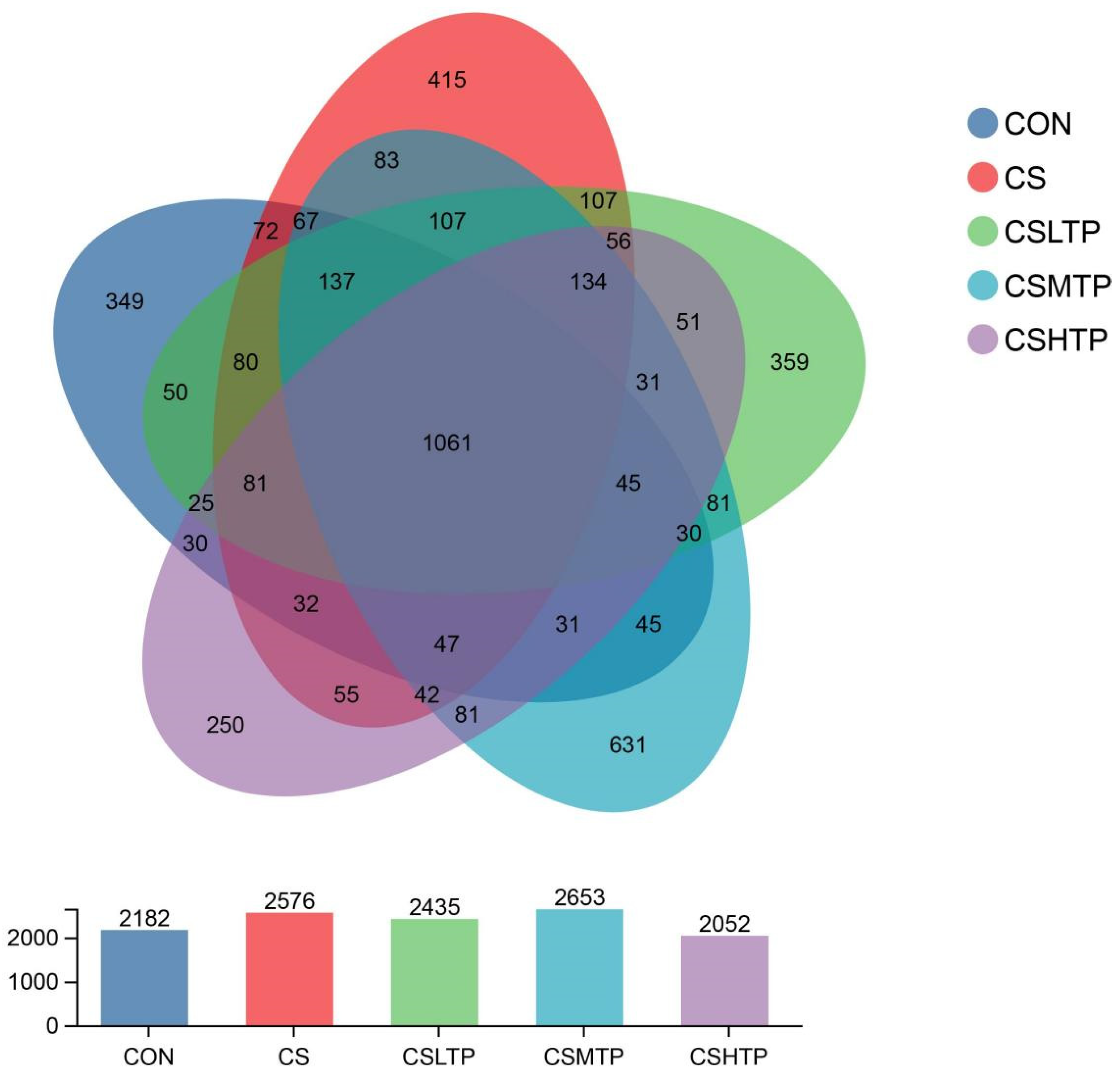
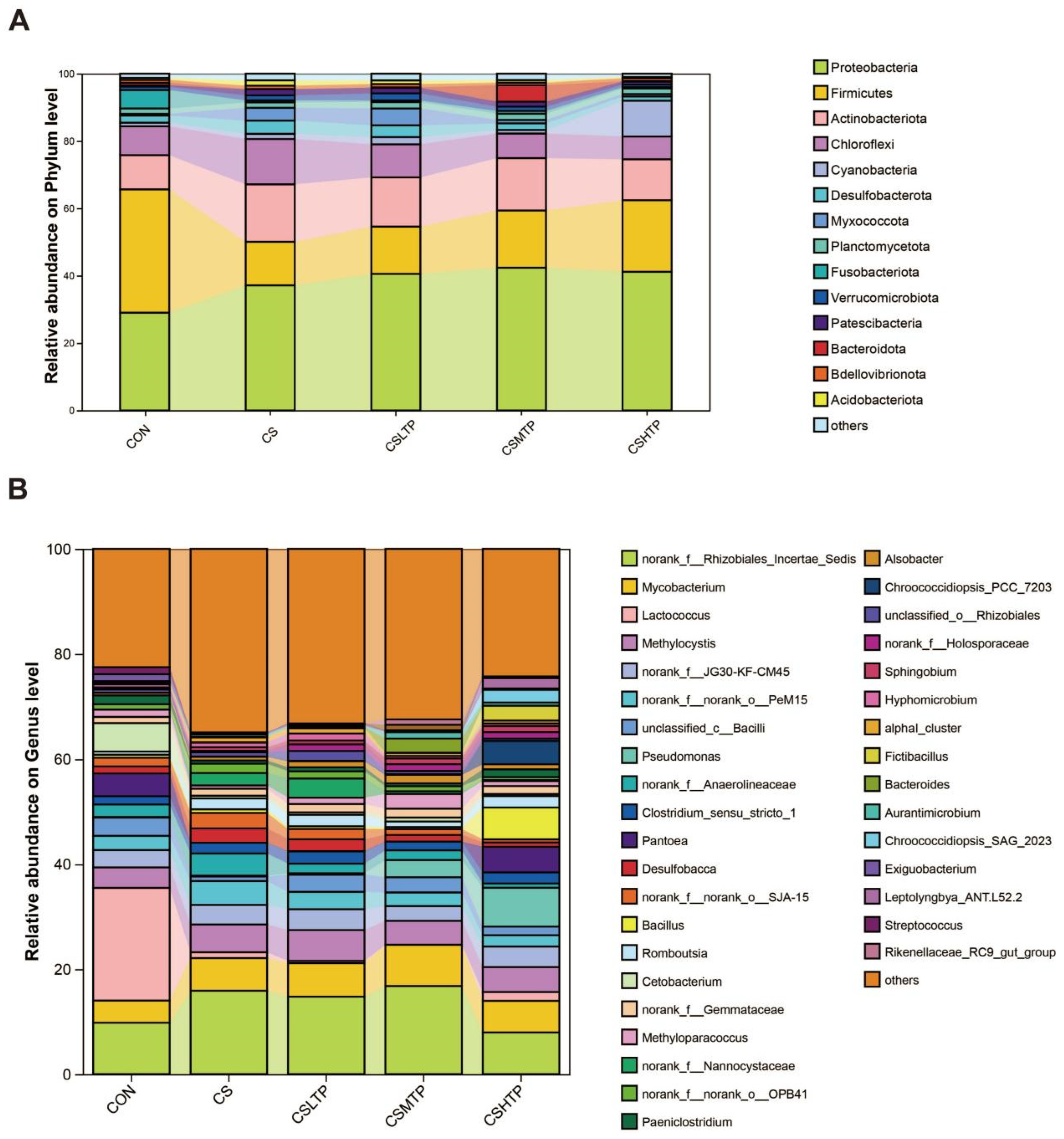
3.5. Microbial Sample Information and Composition Analysis
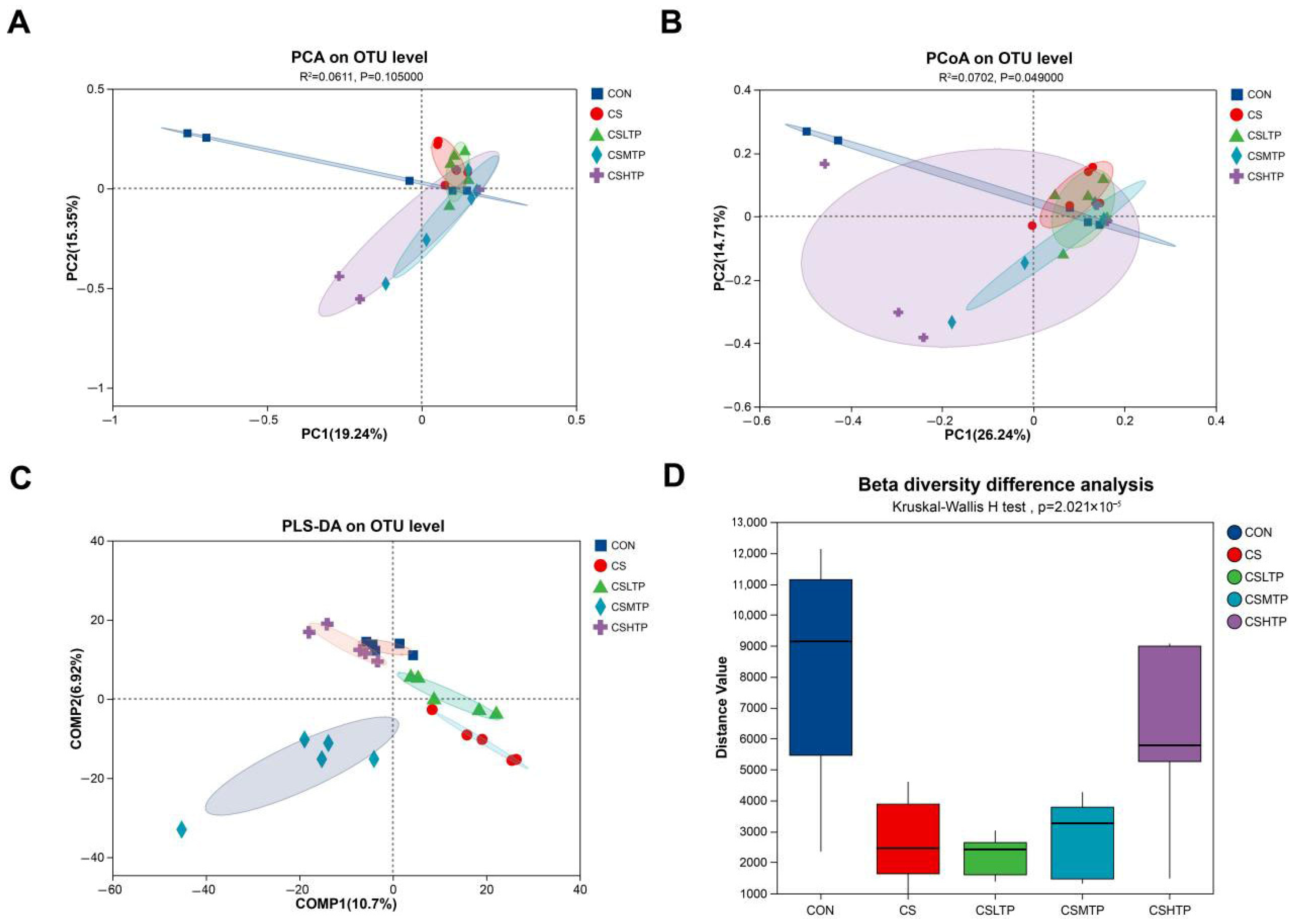
3.6. Comparative Analysis of Microbiota
4. Discussion
4.1. Effects on Growth Performance
4.2. Effects on Serum Biochemical Indices
4.3. Effects on Antioxidant Capacity
4.4. Effects on Gut Microbiota
4.4.1. Microbial Richness and Diversity Changes
4.4.2. Community Structure and Inter-Group Variation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garlock, T.; Asche, F.; Anderson, J.; Bjorndal, T.; Kumar, G.; Lorenzen, K.; Ropicki, A.; Smith, M.D.; Tyeterås, R. A global blue revolution: Aquaculture growth across regions, species, and countries. Rev. Fish. Sci. Aquac. 2020, 28, 107–116. [Google Scholar] [CrossRef]
- Liu, Q.Z.; Wang, S.; Tang, C.C.; Tao, M.; Zhang, C.; Zhou, Y.; Qin, Q.B.; Luo, K.K.; Wu, C.; Hu, F.Z.; et al. The research advances in distant hybridization and gynogenesis in fish. Rev. Aquac. 2025, 17, e12972. [Google Scholar] [CrossRef]
- Liu, Q.F.; Liu, J.M.; Liang, Q.L.; Qi, Y.H.; Tao, M.; Zhang, C.; Qin, Q.B.; Zhao, R.R.; Chen, B.; Liu, S.J. A hybrid lineage derived from hybridization of Carassius cuvieri and Carassius auratus red var. and a new type of improved fish obtained by backcrossing. Aquaculture 2019, 505, 173–182. [Google Scholar] [CrossRef]
- Saraiva, J.L.; Rachinas-Lopes, P.; Arechavala-Lopez, P. Finding the “golden stocking density”: A balance between fish welfare and farmers’ perspectives. Front. Vet. Sci. 2022, 9, 930221. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef]
- Lin, W.; Li, L.; Chen, J.; Li, D.P.; Hou, J.; Guo, H.H.; Shen, J.Z. Long-term crowding stress causes compromised nonspecific immunity and increases apoptosis of spleen in grass carp Ctenopharyngodon idella. Fish Shellfish Immunol. 2018, 80, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Best, C.; Faught, E.; Vijayan, M.M.; Gilmour, K.M. Negative feedback regulation in the hypothalamic-pituitary-interrenal axis of rainbow trout subjected to chronic social stress. Gen. Comp. Endocrinol. 2023, 341, 114332. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xie, Y.M.; Luo, K.; Wang, M.Y.; Liu, L.Z.; Li, C.L.; Tian, X.L. Physiological and intestinal microbiota responses of sea cucumber Apostichopus japonicus to various stress and signatures of intestinal microbiota dysbiosis. Front. Microbiol. 2024, 15, 1528275. [Google Scholar] [CrossRef]
- Ghafarifarsani, H.; Hoseinifar, S.H.; Javahery, S.; Van Doan, H. Effects of dietary vitamin c, thyme essential oil, and quercetin on the immunological and antioxidant status of common carp (Cyprinus carpio). Aquaculture 2022, 553, 738053. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef]
- Yan, Z.M.; Zhong, Y.Z.; Duan, Y.H.; Chen, Q.H.; Li, F.N. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Wang, S.Z.; Li, Z.L.; Ma, Y.T.; Liu, Y.; Lin, C.C.; Li, S.M.; Zhan, J.F.; Ho, C.T. Immunomodulatory effects of green tea polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef]
- Bansal, S.; Choudhary, S.; Sharma, M.; Kumar, S.S.; Lohan, S.; Bhardwaj, V.; Syan, N.; Jyoti, S. Tea: A native source of antimicrobial agents. Food Res. Int. 2013, 53, 568–584. [Google Scholar] [CrossRef]
- Li, Y.Y.; Gao, X.; Lou, Y.J. Interactions of tea polyphenols with intestinal microbiota and their implication for cellular signal conditioning mechanism. J. Food Biochem. 2019, 43, e12953. [Google Scholar] [CrossRef]
- Li, M.Y.; Qiu, P.X.; Shen, J.Y.; Wang, H.X.; Shao, Y.; Song, H.L.; Shen, L.D.; Zhang, S. Tea polyphenols postpone the evolution of multidrug tolerance in Escherichia coli under the stress of antibiotic in water. J. Clean. Prod. 2024, 457, 142467. [Google Scholar] [CrossRef]
- Wang, W.F.; Han, S.; Zha, X.N.; Cheng, J.R.; Song, J.Y.; Jiao, Z. Response surface optimization of supercritical carbon dioxide extraction of tea polyphenols from green tea scraps. J. AOAC Int. 2019, 102, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, H.; Shimoi, K. Anti-stress effects of polyphenols: Animal models and human trials. Food Funct. 2020, 11, 5702–5717. [Google Scholar] [CrossRef]
- Ma, Y.B.; Zhou, X.Q.; Jiang, W.D.; Wu, P.; Liu, Y.; Li, S.W.; Tang, L.; Zhang, L.; Mi, H.F.; Feng, L. Tea polyphenols protect against flavobacterium columnare-induced gill injury via suppression of oxidative stress, inflammation, and apoptosis in grass carp. Int. J. Biol. Macromol. 2024, 254, 127050. [Google Scholar] [CrossRef]
- Guo, H.H.; Lin, W.; Wang, L.K.; Zhang, D.N.; Wu, X.Y.; Li, L.; Li, D.P.; Tang, R.; Yang, L.P.; Qiu, Y.M. The supplementation of dietary selenium yeast and green tea-derived polyphenols improves antioxidant capacity and immune response in juvenile wuchang bream under ammonia stress. Aquac. Res. 2020, 51, 3790–3803. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, S.S.; Li, X.Y.; Liu, Y.D.; Luo, W.T.; Zhao, Y.T.; Huang, Z.F.; Zhao, Y.B.; Li, Z.B. Effects of oxidized fish oil diet supplemented with tea polyphenols on intestinal health and liver metabolism of spotted sea bass (Lateolabrax maculatus). Aquac. Rep. 2024, 37, 102201. [Google Scholar] [CrossRef]
- Chai, Y.Q.; Sun, S.H.; Li, Y.D. Assessing the effects of dietary tea polyphenols on the gut microbiota of loaches (Paramisgurnus dabryanus) under chronic ammonia nitrogen stress. Fishes 2024, 9, 180. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Xu, G.; Zhao, Q.; Wei, Z.; Liu, S. Effects of dietary glutathione on growth performance, muscle quality and lipid metabolism of hybrid crucian carp (Carassius auratus cuvieri ♀ × Carassius auratus red var. ♂) fed a high-fat diet. Reprod. Breed. 2023, 3, 89–98. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Chacoff, L.; Calvo, Á.; Ruiz-Jarabo, I.; Villarroel, F.; Muñoz, J.L.; Tinoco, A.B.; Cárdenas, S.; Mancera, J.M. Growth performance, osmoregulatory and metabolic modifications in red porgy fry, Pagrus pagrus, under different environmental salinities and stocking densities. Aquac. Res. 2011, 42, 1269–1278. [Google Scholar] [CrossRef]
- Nardocci, G.; Navarro, C.; Cortés, P.P.; Imarai, M.; Montoya, M.; Valenzuela, B.; Jara, P.; Acuña-Castillo, C.; Fernández, R. Neuroendocrine mechanisms for immune system regulation during stress in fish. Fish Shellfish Immunol. 2014, 40, 531–538. [Google Scholar] [CrossRef]
- Son, Y.Q.; Liang, X.; Chen, J.; Tang, R.; Li, L.; Li, D.P. Change in ubiquitin proteasome system of grass carp Ctenopharyngodon idellus reared in the different stocking densities. Front. Physiol. 2018, 9, 837. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.O.; Rizwan, M.; Yue, D.D.; Khan, I.M.; Yao, F.; Zhong, J.; Huang, S.J. Compensatory growth in gibel carp (Carassius auratus gibelio) after the stress of stocking density. Aquac. Res. 2021, 52, 1697–1704. [Google Scholar] [CrossRef]
- Pan, S.M.; Yan, X.B.; Li, T.; Suo, X.X.; Liu, H.; Tan, B.P.; Huang, W.B.; Yang, Y.Z.; Zhang, H.T.; Dong, X.H. Impacts of tea polyphenols on growth, antioxidant capacity and immunity in juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ x e. Lanceolatus ♂) fed high-lipid diets. Fish Shellfish Immunol. 2022, 128, 348–359. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Zhao, F.; Cairang, Z.; Zhou, Z.; Du, Q.; Wang, J.L.; Zhao, F.; Wang, Q.F.; Li, Z.Y.; Zhang, X.P. Role of dietary tea polyphenols on growth performance and gut health benefits in juvenile hybrid sturgeon (Acipenser baerii ♀ x a. Schrenckii ♂). Fish Shellfish Immunol. 2023, 139, 108911. [Google Scholar] [CrossRef]
- Hu, M.; Zhou, X.H.; Wang, Y.C.; Li, J.; Wu, Q.H.; Bao, S.S.; Jiang, L.; Liu, B. Use of fermented tea residues as a feed additive and effects on growth performance, body composition, intestinal enzyme activities, and inflammatory biomarkers in juvenile largemouth bass (Micropterus salmoides). Aquac. Rep. 2023, 31, 101671. [Google Scholar] [CrossRef]
- Li, W.; Li, A.; Zhang, X.; Fei, F.; Gao, X.; Fang, Y.; Cao, S.; Yang, H.; Li, W.; Liu, B. Transcriptomics reveals crowding stress inhibit the immune defense of the head kidney of the pearl gentian grouper juvenile through nf-κb signal pathway. Dev. Comp. Immunol. 2025, 162, 105299. [Google Scholar] [CrossRef] [PubMed]
- Gabr, G.A.; Ibrahim, Y.S.; Al-Shawi, S.G.; Abosaooda, M.; Gupta, J.; Oudaha, K.H.; Mavlonov, K.; Jalil, A.T.; Thalij, K.M.; Mustafa, Y.F.; et al. Single or combined consumption of resveratrol and the probiotic, Lactobacillus acidophilus attenuate the effects of crowding stress on growth, immune characteristics, and antioxidant defense in the common carp, (Cyprinus carpio). Aquac. Rep. 2023, 29, 101471. [Google Scholar] [CrossRef]
- Qian, Y.C.; Wang, X.; Ren, J.; Wang, J.; Limbu, S.M.; Li, R.X.; Zhou, W.H.; Qiao, F.; Zhang, M.L.; Du, Z.Y. Different effects of two dietary levels of tea polyphenols on the lipid deposition, immunity and antioxidant capacity of juvenile gift tilapia (oreochromis niloticus) fed a high-fat diet. Aquaculture 2021, 542, 736896. [Google Scholar] [CrossRef]
- Zhang, N.; Tao, J.S.; Yu, Q.F.; Sun, G.G.; Liu, X.P.; Tang, W.R.; Zhang, L.N.; Yang, Z. Dietary tea polyphenols alleviate acute-heat-stress-induced death of hybrid crucian carp hcc2: Involvement of modified lipid metabolisms in liver. Metabolites 2025, 15, 229. [Google Scholar] [CrossRef]
- Hou, Z.S.; Wen, H.S.; Li, J.F.; He, F.; Li, Y.; Qi, X. Effects of long-term crowding stress on neuro-endocrine-immune network of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2019, 95, 180–189. [Google Scholar] [CrossRef]
- Mi, J.L.; Liu, D.; Qin, C.B.; Yan, X.; Pang, P.; Yun, Y.H.; Wang, L.M.; Nie, G.X. Dietary (-)-epicatechin supplementation regulates myofiber development, fillet quality, and antioxidant status of yellow river carp (Cyprinus carpio). Aquaculture 2023, 572, 739542. [Google Scholar] [CrossRef]
- Xiong, N.X.; Fang, Z.X.; Kuang, X.Y.; Ou, J.; Luo, S.W.; Liu, S.J. Integrated analysis of gene expressions and metabolite features unravel immunometabolic interplay in hybrid fish (Carassius cuvieri ♀ x Carassius auratus red var ♂) infected with aeromonas hydrophila. Aquaculture 2023, 563, 738981. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of polyphenols and polyphenol-rich dietary sources on gut microbiota composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Li, M.J.; Yang, L.S.; Liu, Y.; Ma, H. Long-term crowding stress disrupts intestinal homeostasis in largemouth bass (Micropterus salmoides). Aquaculture 2025, 599, 742171. [Google Scholar] [CrossRef]
- Li, T.T.; Qi, M.T.; Gatesoupe, F.J.; Tian, D.C.; Jin, W.H.; Li, J.; Lin, Q.; Wu, S.J.; Li, H. Adaptation to fasting in crucian carp (Carassius auratus): Gut microbiota and its correlative relationship with immune function. Microb. Ecol. 2019, 78, 6–19. [Google Scholar] [CrossRef]
- Dhanasiri, A.K.S.; Li, Y.; Krogdahl, A.; Forberg, T.; Kortner, T.M. Longitudinal study on the effects of a synbiotic supplement to atlantic salmon diets on performance, gut microbiota and immune responses during antibiotic treatment and subsequent recovery. Anim. Microbiome 2024, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.S.; Li, M.J.; Zhang, J.; Liu, Y. Role of brain-gut axis in modulating stress responses via neurotransmitters and cytokines in largemouth bass (Micropterus salmoides) under acute crowding stress. Aquaculture 2025, 604, 742502. [Google Scholar] [CrossRef]
- Hu, X.D.; Ma, W.J.; Zhang, D.S.; Tian, Z.K.; Yang, Y.Q.; Huang, Y.; Hong, Y.H. Application of natural antioxidants as feed additives in aquaculture: A review. Biology 2025, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.N.; Ho, C.T.; Zhang, X. Interaction between tea polyphenols and intestinal microbiota in host metabolic diseases from the perspective of the gut-brain axis. Mol. Nutr. Food Res. 2020, 64, e2000187. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.W.; Dicksved, J.; Lundh, T.; Vidakovic, A.; Norouzitallab, P.; Huyben, D. A meta-analysis revealing the technical, environmental, and host-associated factors that shape the gut microbiota of atlantic salmon and rainbow trout. Rev. Aquac. 2024, 16, 1603–1620. [Google Scholar] [CrossRef]
- Du, F.K.; Li, Y.; Tang, Y.K.; Su, S.Y.; Yu, J.H.; Yu, F.; Li, J.L.; Li, H.X.; Wang, M.Y.; Xu, P. Response of the gut microbiome of Megalobrama amblycephala to crowding stress. Aquaculture 2019, 500, 586–596. [Google Scholar] [CrossRef]
- Moschos, S.; Kormas, K.A.; Karayanni, H. Prokaryotic diversity in marine and freshwater recirculating aquaculture systems. Rev. Aquac. 2022, 14, 1861–1886. [Google Scholar] [CrossRef]
- Zheng, J.S.; Wang, Z.X.; Pu, D.C.; Li, P.Y.; Wei, X.L.; Li, M.; Li, D.S.; Gao, L.H.; Zhai, X.L. Effects of stocking density on intestinal health of juvenile Micropterus salmoides in industrial aquaponics. Fishes 2023, 8, 555. [Google Scholar] [CrossRef]
- Ma, Y.B.; Jiang, W.D.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhou, X.Q.; Feng, L. Tea polyphenol alleviate Aeromonas hydrophila-induced intestinal physical barrier damage in grass carp (Ctenopharyngodon idella). Aquaculture 2021, 544, 737067. [Google Scholar] [CrossRef]
- Pardesi, B.; Roberton, A.M.; Lee, K.C.; Angert, E.R.; Rosendale, D.; Boycheva, S.; White, W.L.; Clements, K.D. Distinct microbiota composition and fermentation products indicate functional compartmentalization in the hindgut of a marine herbivorous fish. Mol. Ecol. 2022, 31, 2494–2509. [Google Scholar] [CrossRef]
- Song, Z.Y.; Zhang, X.; Hong, M.Y.; Wu, Z.F.; Luo, S.M.; Cheng, K.J. Oolong tea polyphenols affect the inflammatory response to improve cognitive function by regulating gut microbiota. J. Funct. Foods 2023, 105, 105584. [Google Scholar] [CrossRef]

| Ingredients | Experimental Groups Diets | ||||
|---|---|---|---|---|---|
| CON | CS | CSLTP | CSMTP | CSHTP | |
| Wheat Flour | 29.7 | 29.7 | 29.69 | 29.68 | 29.66 |
| Soybean Meal | 27 | 27 | 27 | 27 | 27 |
| Rapeseed Meal | 15 | 15 | 15 | 15 | 15 |
| Peanut Hulls | 12 | 12 | 12 | 12 | 12 |
| Fish Meal | 10 | 10 | 10 | 10 | 10 |
| Fish Oil | 2 | 2 | 2 | 2 | 2 |
| Ca(H2PO4)2 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Soybean Oil | 1 | 1 | 1 | 1 | 1 |
| Additive Premixes b | 1 | 1 | 1 | 1 | 1 |
| Choline Chloride | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Antimicrobial Agents | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Antioxidants | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Tea Polyphenols c | 0 | 0 | 0.01 | 0.02 | 0.04 |
| Nutrient Composition d (%) | |||||
| Crude Protein | 34.56 | 34.56 | 34.73 | 34.38 | 34.65 |
| Crude Fat | 5.44 | 5.44 | 5.49 | 5.55 | 5.61 |
| Moisture | 5.65 | 5.65 | 5.99 | 6.10 | 5.88 |
| Gene | Functional Category | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| β-actin | Reference gene | AAACGACCAACCCAAACC | GACGCTTCTGGAACGACTAA |
| CAT | Antioxidant enzyme | GCAAAGCCAAAGTGTTCG | CGCATCCCTGATAAAGAAG |
| nrf2 | Antioxidant transcription factor | AATATGTCGCTATTAGTGTCG | AATCCAACGGAGGTGAAG |
| SOD | Antioxidant enzyme | CCGCACTACAACCCTCAT | CGGAGTATTCCCCAAACA |
| GPX | Antioxidant enzyme | CGACTCCGTGTCCTTGAT | GTTTATTTCGCCCTCTTC |
| acox1 | Fatty acid β-oxidation enzyme | TGAGGACGCCTGGAACAA | CCGAGTGGACAGCCGTAT |
| CPT1 | Fatty acid β-oxidation transporter | CGAGCAACAGATTCAGCG | AAGGGACTTGGCATAGCG |
| LPL1 | Lipoprotein metabolism enzyme | CAGATCGCAGCATTGGG | CTCCGTAGCCGACCTTG |
| dgat2 | Triglyceride synthesis enzyme | TCTCAGCCTTACACGACC | ACATCAGCAGCAAAGAGC |
| Items 1 | CON | CS | CSLTP | CSMTP | CSHTP | CON vs. CS | CS vs. CS+TPs |
|---|---|---|---|---|---|---|---|
| p-Value | p-Value | ||||||
| IBW (g) | 2.03 ± 0.01 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 2.00 ± 0.00 | 0.87 | 0.99 |
| FBW (g) | 14.37 ± 0.35 * | 9.00 ± 0.84 b | 12.54 ± 0.93 a | 10.80 ± 0.26 ab | 9.29 ± 1.30 b | <0.01 | 0.09 |
| WGR (%) | 609.32 ± 13.64 * | 350.77 ± 42.28 b | 526.49 ± 46.09 a | 439.25 ± 11.98 ab | 364.07 ± 64.77 b | <0.01 | 0.09 |
| SGR (%/d) | 3.26 ± 0.09 * | 2.51 ± 0.30 b | 3.06 ± 0.27 a | 2.81 ± 0.08 ab | 2.56 ± 0.46 b | <0.01 | 0.09 |
| FCR | 1.45 ± 0.01 * | 1.80 ± 0.1 | 1.52 ± 0.05 | 1.62 ± 0.02 | 1.79 ± 0.13 | 0.03 | 0.14 |
| SR (%) | 100.00 ± 0.00 | 99.80 ± 0.2 | 99.67 ± 0.24 | 99.87 ± 0.07 | 99.80 ± 0.12 | 0.37 | 0.86 |
| CF (g/cm3) | 3.56 ± 0.05 | 3.51 ± 0.12 a | 3.42 ± 0.05 a | 3.17 ± 0.04 b | 3.36 ± 0.11 ab | 0.75 | 0.03 |
| Items 1 | CON | CS | CSLTP | CSMTP | CSHTP | CON vs. CS | CS vs. CS+TPs |
|---|---|---|---|---|---|---|---|
| p-Value | p-Value | ||||||
| LDH (U/L) | 538.00 ± 6.08 ** | 721.00 ± 14.93 a | 625.67 ± 19.64 b | 456.33 ± 4.98 c | 330.67 ± 11.57 d | <0.01 | <0.01 |
| GLU (mmol/L) | 16.30 ± 0.26 * | 10.47 ± 0.60 a | 7.90 ± 0.29 b | 9.37 ± 0.23 a | 6.30 ± 0.35 c | <0.01 | <0.01 |
| LACT (mmol/L) | 9.96 ± 0.25 | 9.27 ± 0.27 a | 8.24 ± 0.32 b | 8.09 ± 0.16 b | 7.62 ± 0.20 b | 0.06 | 0.01 |
| TG (mmol/L) | 2.79 ± 0.08 ** | 4.37 ± 0.06 b | 3.81 ± 0.07 c | 5.39 ± 0.11 a | 4.28 ± 0.19 b | <0.01 | <0.01 |
| TC (mmol/L) | 7.32 ± 0.22 | 6.64 ± 0.19 | 7.46 ± 0.40 | 6.61 ± 0.31 | 7.33 ± 0.26 | 0.08 | 0.16 |
| NEFA (mmol/L) | 0.93 ± 0.02 | 1.31 ± 0.19 a | 1.60 ± 0.17 a | 1.55 ± 0.15 a | 0.67 ± 0.05 b | 0.12 | 0.01 |
| LDL-C (mmol/L) | 1.03 ± 0.10 * | 0.55 ± 0.05 | 0.67 ± 0.06 | 0.53 ± 0.03 | 0.56 ± 0.01 | 0.02 | 0.16 |
| HDL-C (mmol/L) | 0.58 ± 0.01 | 0.5 ± 0.03 ab | 0.57 ± 0.03 a | 0.4 ± 0.02 b | 0.46 ± 0.02 ab | 0.14 | 0.01 |
| ALB (g/L) | 9.17 ± 0.20 | 9.93 ± 0.15 | 13.30 ± 1.97 | 10.40 ± 0.81 | 11.50 ± 0.31 | 0.06 | 0.2 |
| ALT (U/L) | 22.50 ± 2.10 * | 14.93 ± 0.79 b | 10.87 ± 1.39 a | 16.83 ± 0.46 b | 21.80 ± 0.55 a | 0.03 | <0.01 |
| AST (U/L) | 460.33 ± 3.38 * | 378.67 ± 17.68 ab | 425.67 ± 29.04 a | 339.33 ± 3.18 b | 349.67 ± 12.60 b | 0.01 | 0.04 |
| ALP (U/L) | 18.90 ± 0.59 * | 13.67 ± 0.33 b | 23.33 ± 2.73 a | 14.57 ± 0.30 b | 20.00 ± 0.58 a | <0.01 | <0.01 |
| Items 1 | CON | CS | CSLTP | CSMTP | CSHTP | CON vs. CS | CS vs. CS+TPs |
|---|---|---|---|---|---|---|---|
| p-Value | p-Value | ||||||
| Serum | |||||||
| T-AOC, μmol/mL | 0.57 ± 0.06 * | 0.71 ± 0.09 | 0.74 ± 0.08 | 0.65 ± 0.07 | 0.66 ± 0.06 | 0.03 | 0.48 |
| SOD, U/mL | 5.28 ± 0.44 | 5.73 ± 0.63 c | 8.46 ± 0.52 a | 7.69 ± 0.81 b | 7.89 ± 0.72 ab | 0.58 | 0.02 |
| CAT, U/mL | 26.3 ± 2.23 | 28.2 ± 2.32 b | 42.0 ± 3.45 a | 39.5 ± 3.61 a | 38.0 ± 4.01 a | 0.44 | 0.04 |
| GSH, μg/mL | 9.92 ± 0.97 ** | 5.49 ± 0.58 c | 8.33 ± 0.74 b | 11.85 ± 1.31 a | 9.28 ± 0.95 ab | <0.01 | <0.01 |
| Liver | |||||||
| T-AOC, μmol/g prot | 4.65 ± 0.39 | 4.52 ± 0.42 a | 4.69 ± 0.36 a | 4.63 ± 0.38 a | 3.83 ± 0.45 b | 0.33 | 0.11 |
| SOD, U/mg prot | 111.8 ± 9.8 | 131.4 ± 10.3 ab | 105.9 ± 10.2 b | 141.5 ± 11.7 a | 128.3 ± 12.6 ab | 0.06 | 0.09 |
| CAT, U/mg prot | 252.02 ± 20.58 | 259.65 ± 21.53 b | 212.84 ± 22.14 c | 276.52 ± 22.88 ab | 305.10 ± 24.82 a | 0.24 | 0.08 |
| GSH, μg/mg prot | 1021.86 ± 66.20 * | 951.12 ± 48.23 b | 988.73 ± 65.22 ab | 1060.66 ± 69.69 a | 1011.09 ± 77.47 ab | 0.04 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Sun, G.; Tao, J.; Tang, W.; Li, W.; Wei, Z.; Yu, Q. Dietary Tea Polyphenols Improve Growth Performance and Intestinal Microbiota Under Chronic Crowding Stress in Hybrid Crucian Carp. Animals 2025, 15, 1983. https://doi.org/10.3390/ani15131983
Yang Z, Sun G, Tao J, Tang W, Li W, Wei Z, Yu Q. Dietary Tea Polyphenols Improve Growth Performance and Intestinal Microbiota Under Chronic Crowding Stress in Hybrid Crucian Carp. Animals. 2025; 15(13):1983. https://doi.org/10.3390/ani15131983
Chicago/Turabian StyleYang, Zhe, Gege Sun, Jinsheng Tao, Weirong Tang, Wenpei Li, Zehong Wei, and Qifang Yu. 2025. "Dietary Tea Polyphenols Improve Growth Performance and Intestinal Microbiota Under Chronic Crowding Stress in Hybrid Crucian Carp" Animals 15, no. 13: 1983. https://doi.org/10.3390/ani15131983
APA StyleYang, Z., Sun, G., Tao, J., Tang, W., Li, W., Wei, Z., & Yu, Q. (2025). Dietary Tea Polyphenols Improve Growth Performance and Intestinal Microbiota Under Chronic Crowding Stress in Hybrid Crucian Carp. Animals, 15(13), 1983. https://doi.org/10.3390/ani15131983






