Antimicrobial Resistance and Virulence Determinants of Escherichia coli Isolates from Raw Milk of Dairy Cows with Subclinical Mastitis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Mastitis Screening
2.2. Bacterial Isolation and Identification
2.3. DNA Extraction from E. coli Isolates
2.4. Molecular Identification of E. coli Using uidA PCR Assay
2.5. Detection of Virulence Factors, O Serogroups, and Antibiotic-Resistant Genes
2.6. Phenotypic Antimicrobial Resistance
3. Results
3.1. California Mastitis Test (CMT) and Somatic Cell Counts (SCCs)
3.2. Identification of E. coli
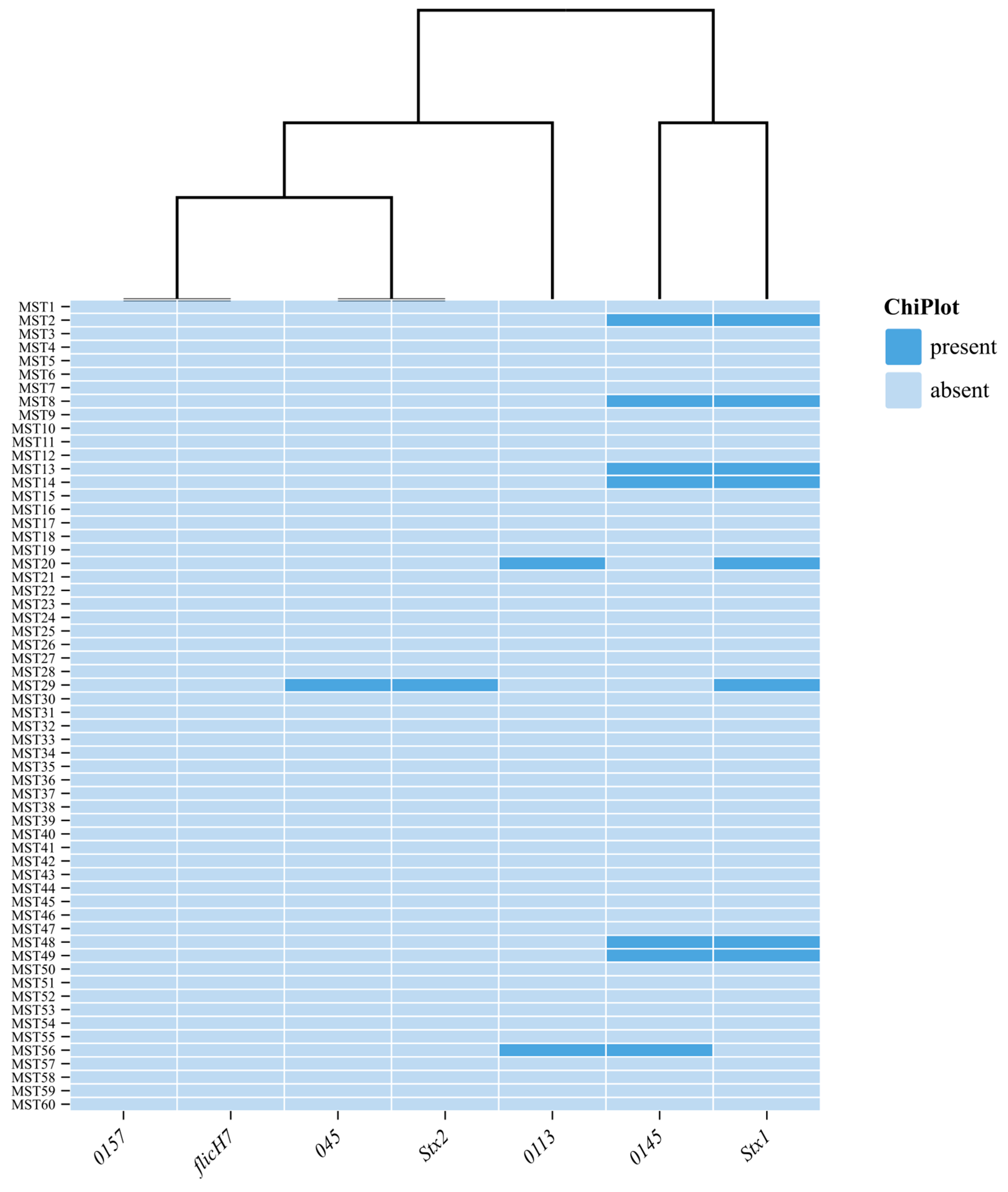
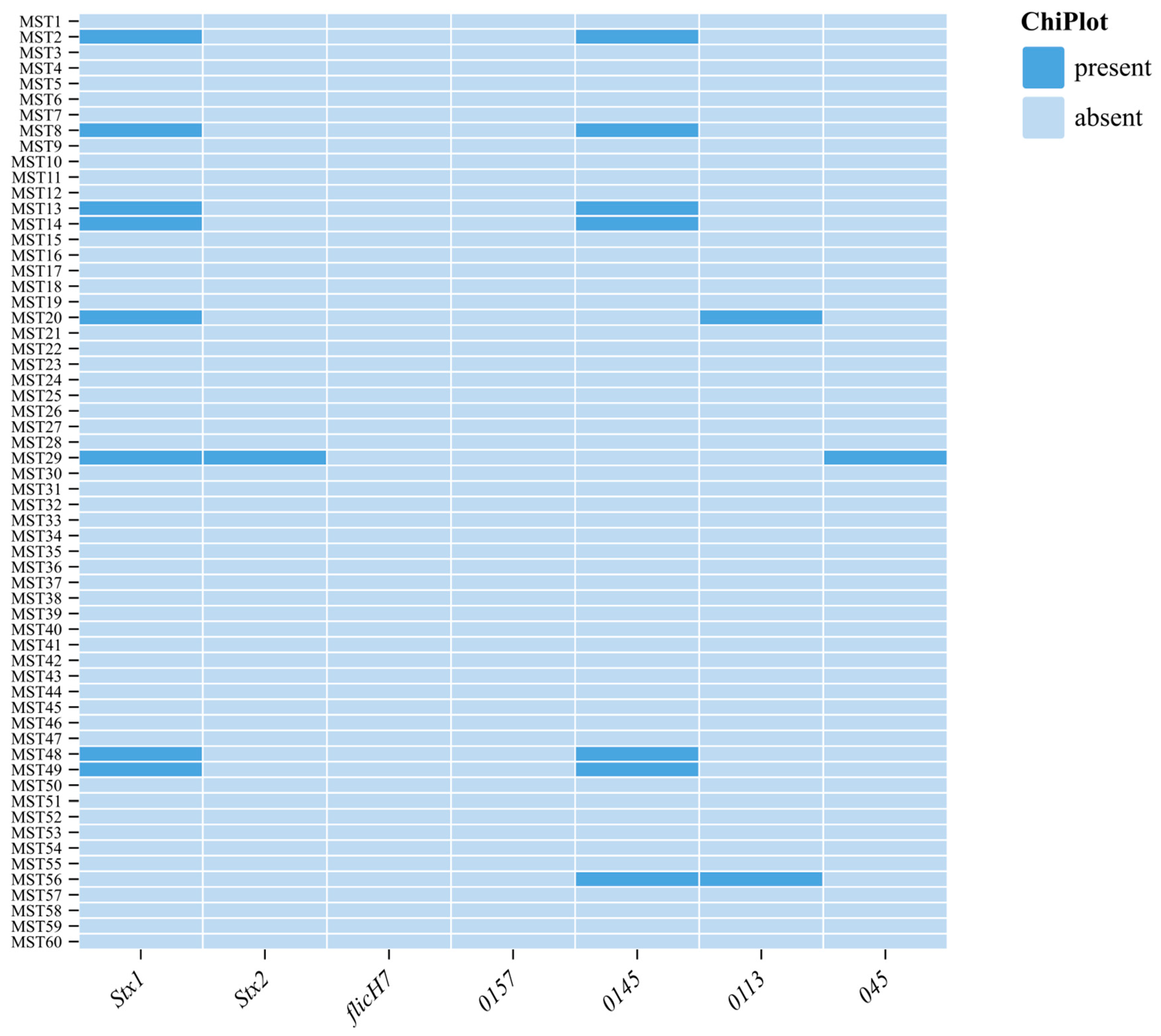
3.3. Detection of Virulent Genes and O Serogroups
3.4. Antibiotic Sensitivity and Resistance Genes Detected in E. coli Isolates
3.5. Multidrug-Resistant E. coli Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine mastitis: Prevalence, risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed, South Ethiopia. BMC Veter. Res. 2016, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed]
- González, R.N.; Wilson, D.J. Mycoplasmal mastitis in dairy herds. Veter. Clin. Food Anim. Pract. 2003, 19, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, G.; Nehring, R.; Hallahan, C.; Sandretto, C. Pasture-based dairy systems: Who are the producers and are their operations more profitable than conventional dairies? J. Agric. Resour. Econ. 2009, 34, 412–427. [Google Scholar]
- Oliver, S.P.; Jayarao, B.M.; Almeida, R.A. Foodborne pathogens, mastitis, milk quality, and dairy food safety. In NMC Annual Meeting Proceedings; National Mastitis Council: New Prague, MN, USA, 2005; Volume 1, pp. 3–27. [Google Scholar]
- Hennekinne, J.-A.; De Buyser, M.-L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef]
- Burvenich, C.; Van Merris, V.; Mehrzad, J.; Diez-Fraile, A.; Duchateau, L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef]
- Abebe, M.; Hailelule, A.; Abrha, B.; Nigus, A.; Birhanu, M.; Adane, H.; Genene, T.; Daniel, H.; Getachew, G.; Merga, G.; et al. Antibiogram of Escherichia coli strains isolated from food of bovine origin in selected Woredas of Tigray, Ethiopia. J. Bacteriol. Res. 2014, 6, 17–22. [Google Scholar]
- CfD Control. Prevention. Outbreaks of Escherichia coli O157: H7 infections among children associated with farm visits–Pennsylvania and Washington, 2000. MMWR Morb. Mortal. Wkly. Rep. 2001, 50, 293–297. [Google Scholar]
- Boerlin, P.; McEwen, S.A.; Boerlin-Petzold, F.; Wilson, J.B.; Johnson, R.P.; Gyles, C.L. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. J. Clin. Microbiol. 1999, 37, 497–503. [Google Scholar] [CrossRef]
- Bag, M.S.; Khan, M.R.; Sami, M.H.; Begum, F.; Islam, M.; Rahman, M.; Rahman, M.; Hassan, J. Virulence determinants and antimicrobial resistance of E. coli isolated from bovine clinical mastitis in some selected dairy farms of Bangladesh. Saudi J. Biol. Sci. 2021, 28, 6317–6323. [Google Scholar] [CrossRef]
- Pereira, E.d.S.; Crippa, B.L.; Morasi, R.M.; de Almeida, J.M.; Gebara, C.; Langoni, H.; Neto, A.T.; Gonçalves, M.C.; Silva, N.C.C. Identification of enteropathogenic Escherichia coli as the cause of mastitis in cows from Brazil. Pesqui. Veter. Bras. 2024, 44, e07430. [Google Scholar] [CrossRef]
- Goulart, D.B.; Mellata, M. Escherichia coli mastitis in dairy cattle: Etiology, diagnosis, and treatment challenges. Front. Microbiol. 2022, 13, 928346. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Eisenberger, D.; Carl, A.; Balsliemke, J.; Kämpf, P.; Nickel, S.; Schulze, G.; Valenza, G. Molecular characterization of extended-spectrum β-lactamase-producing Escherichia coli isolates from milk samples of dairy cows with mastitis in Bavaria, Germany. Microb. Drug Resist. 2018, 24, 505–510. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, J.; Ho, H.; Wang, Y.; Huang, S.; Han, R. Prevalence and antimicrobial-resistance phenotypes and genotypes of Escherichia coli isolated from raw milk samples from mastitis cases in four regions of China. J. Glob. Antimicrob. Resist. 2020, 22, 94–101. [Google Scholar] [CrossRef]
- Saidani, M.; Messadi, L.; Soudani, A.; Daaloul-Jedidi, M.; Châtre, P.; Ben Chehida, F.; Mamlouk, A.; Mahjoub, W.; Madec, J.-Y.; Haenni, M. Epidemiology, antimicrobial resistance, and extended-spectrum beta-lactamase-producing enterobacteriaceae in clinical bovine mastitis in Tunisia. Microb. Drug Resist. 2018, 24, 1242–1248. [Google Scholar] [CrossRef]
- Karzis, J.; Donkin, E.F.; Webb, E.C.; Etter, E.M.; Petzer, I.M. Somatic cell count thresholds in composite and quarter milk samples as indicator of bovine intramammary infection status. Onderstepoort J. Veter. Res. 2017, 84, 1–10. [Google Scholar] [CrossRef]
- Khasapane, N.G.; Koos, M.; Nkhebenyane, S.J.; Khumalo, Z.T.H.; Ramatla, T.; Thekisoe, O. Detection of Staphylococcus isolates and their antimicrobial resistance profiles and virulence genes from subclinical mastitis cattle milk using MALDI-TOF MS, PCR and sequencing in free state province, South Africa. Animals 2024, 14, 154. [Google Scholar] [CrossRef]
- Cameron, M.; Perry, J.; Middleton, J.; Chaffer, M.; Lewis, J.; Keefe, G. Evaluation of MALDI-TOF mass spectrometry and a custom reference spectrum expanded database for the identification of bovine-associated coagulase-negative staphylococci. J. Dairy Sci. 2018, 101, 590–595. [Google Scholar] [CrossRef]
- Nessa, K.; Ahmed, D.; Islam, J.; Kabir, F.L.; Hossain, M.A. Usefulness of a multiplex PCR for detection of diarrheagenic Escherichia coli in a diagnostic microbiology laboratory setting. Bangladesh J. Med. Microbiol. 2007, 1, 38–42. [Google Scholar] [CrossRef]
- Ramatla, T.; Tutubala, M.; Motlhaping, T.; de Wet, L.; Mokgokong, P.; Thekisoe, O.; Lekota, K. Molecular detection of Shiga toxin and extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolates from sheep and goats. Mol. Biol. Rep. 2024, 51, 57. [Google Scholar] [CrossRef] [PubMed]
- Abdlla, Y.; Sheet, O.H.; Alsanjary, R.A.; Plötz, M.; Abdulmawjood, A.A. Isolation and Identification of Escherichia coli from Buffalo’s Milk using PCR Technique in Nineveh Governorate. Egypt. J. Veter. Sci. 2024, 55, 1881–1887. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 6th ed.; CLSI Document VET01-S; CLSI: Pittsburgh, PA, USA, 2023. [Google Scholar]
- Dehkordi, F.S.; Yazdani, F.; Mozafari, J.; Valizadeh, Y. Virulence factors, serogroups and antimicrobial resistance properties of Escherichia coli strains in fermented dairy products. BMC Res. Notes 2014, 7, 217. [Google Scholar] [CrossRef]
- Ranjbar, R.; Dehkordi, F.S.; Shahreza, M.H.S.; Rahimi, E. Prevalence, identification of virulence factors, O-serogroups and antibiotic resistance properties of Shiga-toxin producing Escherichia coli strains isolated from raw milk and traditional dairy products. Antimicrob. Resist. Infect. Control. 2018, 7, 53. [Google Scholar] [CrossRef]
- Thrusfield, M. Veterinary Epidemiology; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- FAO; GDP; IFCN. Dairy Development’s Impact on Poverty Reduction; FAO: Chicago, IL, USA, 2018. [Google Scholar]
- Mekonnen, S.; Koop, G.; Melkie, S.; Getahun, C.; Hogeveen, H.; Lam, T. Prevalence of subclinical mastitis and associated risk factors at cow and herd level in dairy farms in North-West Ethiopia. Prev. Veter. Med. 2017, 145, 23–31. [Google Scholar] [CrossRef]
- Fahim, K.M.; Ismael, E.; Khalefa, H.S.; Farag, H.S.; Hamza, D.A. Isolation and characterization of E. coli strains causing intramammary infections from dairy animals and wild birds. Int. J. Veter. Sci. Med. 2019, 7, 61–70. [Google Scholar] [CrossRef]
- Zuo, J.; Lv, Z.; Lian, L.; Wu, Z.; Fu, S.; Zhang, H.; Wu, J.; Pan, Z.; Yu, Y.; Chen, W.; et al. Difference Analysis on Virulence Genes, Biofilms and Antimicrobial Susceptibility of Escherichia coli from Clinical and Subclinical Bovine Mastitis. Veter. Sci. 2025, 12, 132. [Google Scholar] [CrossRef]
- Naseer, M.A.; Aqib, A.I.; Ashar, A.; Saleem, M.I.; Shoaib, M.; Kulyar, M.F.-E.; Ashfaq, K.; Bhutta, Z.A.; Nighat, S. Detection of Altered Pattern of Antibiogram and Biofilm Character in Staphylococcus aureus Isolated from Dairy Milk. Pak. J. Zool. 2021, 53, 191–199. [Google Scholar] [CrossRef]
- Javed; Umar, M.; Ijaz, M.; Fatima, Z.; Anjum, A.A.; Aqib, A.I.; Ali, M.M.; Rehman, A.; Ahmed, A.; Ghaffar, A. Frequency and antimicrobial susceptibility of methicillin and vancomycin-resistant Staphylococcus aureus from bovine milk. Pak. Veter. J. 2021, 41, 463–468. [Google Scholar] [CrossRef]
- Abed, A.H.; Menshawy, A.M.S.; Zeinhom, M.M.A.; Hossain, D.; Khalifa, E.; Wareth, G.; Awad, M.F. Subclinical mastitis in selected bovine dairy herds in North Upper Egypt: Assessment of prevalence, causative bacterial pathogens, antimicrobial resistance and virulence-associated genes. Microorganisms 2021, 9, 1175. [Google Scholar] [CrossRef]
- Hope, A. Laboratory Handbook on Bovine Mastitis. Aust. Veter- J. 2000, 78, 488. [Google Scholar] [CrossRef]
- Islam, K.; Ahad, A.; Barua, M.; Islam, A.; Chakma, S.; Dorji, C.; Uddin, M.A.; Islam, S.; Ahasan, A.S.M.L. Isolation and epidemiology of multidrug resistant Escherichia coli from goats in Cox’s Bazar, Bangladesh. J. Adv. Veter. Anim. Res. 2016, 3, 166–172. [Google Scholar] [CrossRef]
- Bhoomika; Shakya, S.; Patyal, A.; Gade, N.E. Occurrence and characteristics of extended-spectrum β-lactamases producing Escherichia coli in foods of animal origin and human clinical samples in Chhattisgarh, India. Veter. World 2016, 9, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Ntuli, V.; Njage, P.; Buys, E. Characterization of Escherichia coli and other Enterobacteriaceae in producer-distributor bulk milk. J. Dairy Sci. 2016, 99, 9534–9549. [Google Scholar] [CrossRef]
- Liu, H.; Meng, L.; Dong, L.; Zhang, Y.; Wang, J.; Zheng, N. Prevalence, antimicrobial susceptibility, and molecular characterization of Escherichia coli isolated from raw milk in dairy herds in Northern China. Front. Microbiol. 2021, 12, 730656. [Google Scholar] [CrossRef]
- Ramírez-Castillo, F.Y.; Moreno-Flores, A.C.; Avelar-González, F.J.; Márquez-Díaz, F.; Harel, J.; Guerrero-Barrera, A.L. An evaluation of multidrug-resistant Escherichia coli isolates in urinary tract infections from Aguascalientes, Mexico: Cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 34. [Google Scholar] [CrossRef]
- Ashraf, A.; Imran, M.; Chang, Y.-F. Antimicrobial resistance of Escherichia coli isolates from mastitic milk and its possible relationship with resistance and virulence genes. Pak. J. Zool. 2018, 50, 1435–1441. [Google Scholar] [CrossRef]
- Momtaz, H.; Rahimi, E.; Moshkelani, S. Molecular detection of antimicrobial resistance genes in E. coli isolated from slaughtered commercial chickens in Iran. Veter. Med. 2012, 57, 193–197. [Google Scholar] [CrossRef]
- Tavakoli, M.; Pourtaghi, H. Molecular detection of virulence genes and multi-drug resistance patterns in Escherichia coli (STEC) in clinical bovine mastitis: Alborz province, Iran. Iran. J. Vet. Res. 2017, 18, 208–211. [Google Scholar]
- Mashak, Z. Prevalence and antibiotic resistance of Escherichia coli O157:H7 isolated from raw meat samples of ruminants and poultry. J. Food Nutr. Res. 2018, 6, 96–102. [Google Scholar] [CrossRef]
- Zafarane, S.; Houri, H.; Kazemian, H.; Heidari, H.; Amiri, P.; Tabarraei, B. Characterization of virulence genes, serogroups and antimicrobial susceptibility of Shiga toxin producing Escherichia coli isolated from bovine mastitic milk in Tehran, Iran. Trop Biomed. 2017, 34, 295–304. [Google Scholar] [PubMed]
- Brenjchi, M.; Jamshidi, A.; Farzaneh, N.; Bassami, M.R. Short paper: Identification of shiga toxin producing Escherichia coli o157: H7 in raw cow milk samples from dairy farms in mashhad using multiplex pcr assay. Iran. J. Vet. Res. 2011, 12, 145–149. [Google Scholar]
- Ullah, S.; Khan, S.U.H.; Ali, T.; Zeb, M.T.; Riaz, M.H.; Khan, S.; Goyal, S.M.; Rouby, S.R. Molecular characterization and antibiotic susceptibility of Shiga toxin- producing Escherichia coli (STEC) isolated from raw milk of dairy bovines in Khyber Pakhtunkhwa, Pakistan. PLoS ONE 2024, 19, e0307830. [Google Scholar] [CrossRef] [PubMed]
- Amézquita-López, B.A.; Quiñones, B.; Cooley, M.B.; León-Félix, J.; Campo, N.C.-D.; Mandrell, R.E.; Jiménez, M.; Chaidez, C.; Ibekwe, A.M. Genotypic analyses of shiga toxin-producing Escherichia coli O157 and Non-O157 recovered from feces of domestic animals on rural farms in Mexico. PLoS ONE 2012, 7, e51565. [Google Scholar] [CrossRef]
- Okoche, D.; Asiimwe, B.B.; Katabazi, F.A.; Kato, L.; Najjuka, C.F.; Zhang, Q. Prevalence and characterization of carbapenem-resistant Enterobacteriaceae isolated from Mulago National Referral Hospital, Uganda. PLoS ONE 2015, 10, e0135745. [Google Scholar] [CrossRef]
- Hoelle, J.; Johnson, J.R.; Johnston, B.D.; Kinkle, B.; Boczek, L.; Ryu, H.; Hayes, S. Survey of US wastewater for carbapenem-resistant Enterobacteriaceae. J. Water Health 2019, 17, 219–226. [Google Scholar] [CrossRef]
- Tahar, S.; Nabil, M.M.; Safia, T.; Ngaiganam, E.P.; Omar, A.; Hafidha, C.; Hanane, Z.; Rolain, J.-M.; Diene, S.M. Molecular characterization of multidrug-resistant Escherichia coli isolated from milk of dairy cows with clinical mastitis in Algeria. J. Food Prot. 2020, 83, 2173–2178. [Google Scholar] [CrossRef]
- Ameen, F.; Reda, S.A.; El-Shatoury, S.A.; Riad, E.M.; Enany, M.E.; Alarfaj, A.A. Prevalence of antibiotic resistant mastitis pathogens in dairy cows in Egypt and potential biological control agents produced from plant endophytic actinobacteria. Saudi J. Biol. Sci. 2019, 26, 1492–1498. [Google Scholar] [CrossRef]
- Rangel, P.M.; Marin, J.M. Antimicrobial resistance in brazilian isolates of Shiga toxin-encoding Escherichia coli from cows with mastitis. Ars Vet. Jaboticabal SP 2009, 25, 18–23. [Google Scholar]
- Barbour, E.K.; Kassabian, T.J.; Shaib, H.; Kassaify, Z.; Iyer, A.; Azhar, E.; Harakeh, S.; Kumosani, T. The significance of Escherichia coli-induced mastitis in cows associated with the presence of virulence genes and wide range-resistance to twenty antimicrobials. Int. J. Appl. Res. Vet. Med. 2015, 13, 51–63. [Google Scholar]
- Fazel, F.; Jamshidi, A.; Khoramian, B. Phenotypic and genotypic study on antimicrobial resistance patterns of E. coli isolates from bovine mastitis. Microb. Pathog. 2019, 132, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Ngaywa, C.; Aboge, G.O.; Obiero, G.; Omwenga, I.; Ngwili, N.; Wamwere, G.; Wainaina, M.; Bett, B. Antimicrobial resistant Escherichia coli isolates detected in raw milk of livestock in pastoral areas of northern Kenya. Food Control. 2019, 102, 173–178. [Google Scholar] [CrossRef]
- Dowidar, H.A.; Khalifa, M.I. Molecular Isolation and Identification of Multidrug-resistant Escherichia coli from Milk, Meat, and Product Samples. J. Adv. Vet. Res. 2023, 13, 643–646. Available online: https://advetresearch.com/index.php/AVR/article/view/1267 (accessed on 15 March 2025).
| Antibiotic Disks | Number of Isolates | Percentage |
|---|---|---|
| P, CIP, MEM | 1 | 1.6 |
| P, TET, IMP | 1 | 1.6 |
| P, CIP, CN | 8 | 13.3 |
| P, CIP, AMP | 3 | 5 |
| P, CIP, AMP, CN | 1 | 1.6 |
| P, CIP, AMP, E | 2 | 3.3 |
| P, CIP, AMP, TET | 4 | 6.6 |
| P, CIP, E, TET | 1 | 1.6 |
| P, CIP, CN, MEM | 1 | 1.6 |
| P, AMP, MEM, CN | 2 | 3.3 |
| P, AMP, MEM, IMP | 1 | 1.6 |
| E, AMP, CIP | 1 | 1.6 |
| E, CN, CIP, MEM | 1 | 1.6 |
| AMP, CN, CIP | 1 | 1.6 |
| P, CIP, CN, IMP, MEM | 1 | 1.6 |
| P, E, TET, CIP, IMP, MEM | 1 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khasapane, N.G.; de Smidt, O.; Lekota, K.E.; Nkhebenyane, J.; Thekisoe, O.; Ramatla, T. Antimicrobial Resistance and Virulence Determinants of Escherichia coli Isolates from Raw Milk of Dairy Cows with Subclinical Mastitis. Animals 2025, 15, 1980. https://doi.org/10.3390/ani15131980
Khasapane NG, de Smidt O, Lekota KE, Nkhebenyane J, Thekisoe O, Ramatla T. Antimicrobial Resistance and Virulence Determinants of Escherichia coli Isolates from Raw Milk of Dairy Cows with Subclinical Mastitis. Animals. 2025; 15(13):1980. https://doi.org/10.3390/ani15131980
Chicago/Turabian StyleKhasapane, Ntelekwane George, Olga de Smidt, Kgaugelo Edward Lekota, Jane Nkhebenyane, Oriel Thekisoe, and Tsepo Ramatla. 2025. "Antimicrobial Resistance and Virulence Determinants of Escherichia coli Isolates from Raw Milk of Dairy Cows with Subclinical Mastitis" Animals 15, no. 13: 1980. https://doi.org/10.3390/ani15131980
APA StyleKhasapane, N. G., de Smidt, O., Lekota, K. E., Nkhebenyane, J., Thekisoe, O., & Ramatla, T. (2025). Antimicrobial Resistance and Virulence Determinants of Escherichia coli Isolates from Raw Milk of Dairy Cows with Subclinical Mastitis. Animals, 15(13), 1980. https://doi.org/10.3390/ani15131980







