Comparative Assessment of Functional and Morphological Markers in Guinea Pig (Cavia porcellus) Oocytes Collected at Different Estrous Cycle Phases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Animals and Synchronization Protocol
2.3. Oocyte Collection and Classification
2.4. Brilliant Cresyl Blue Test
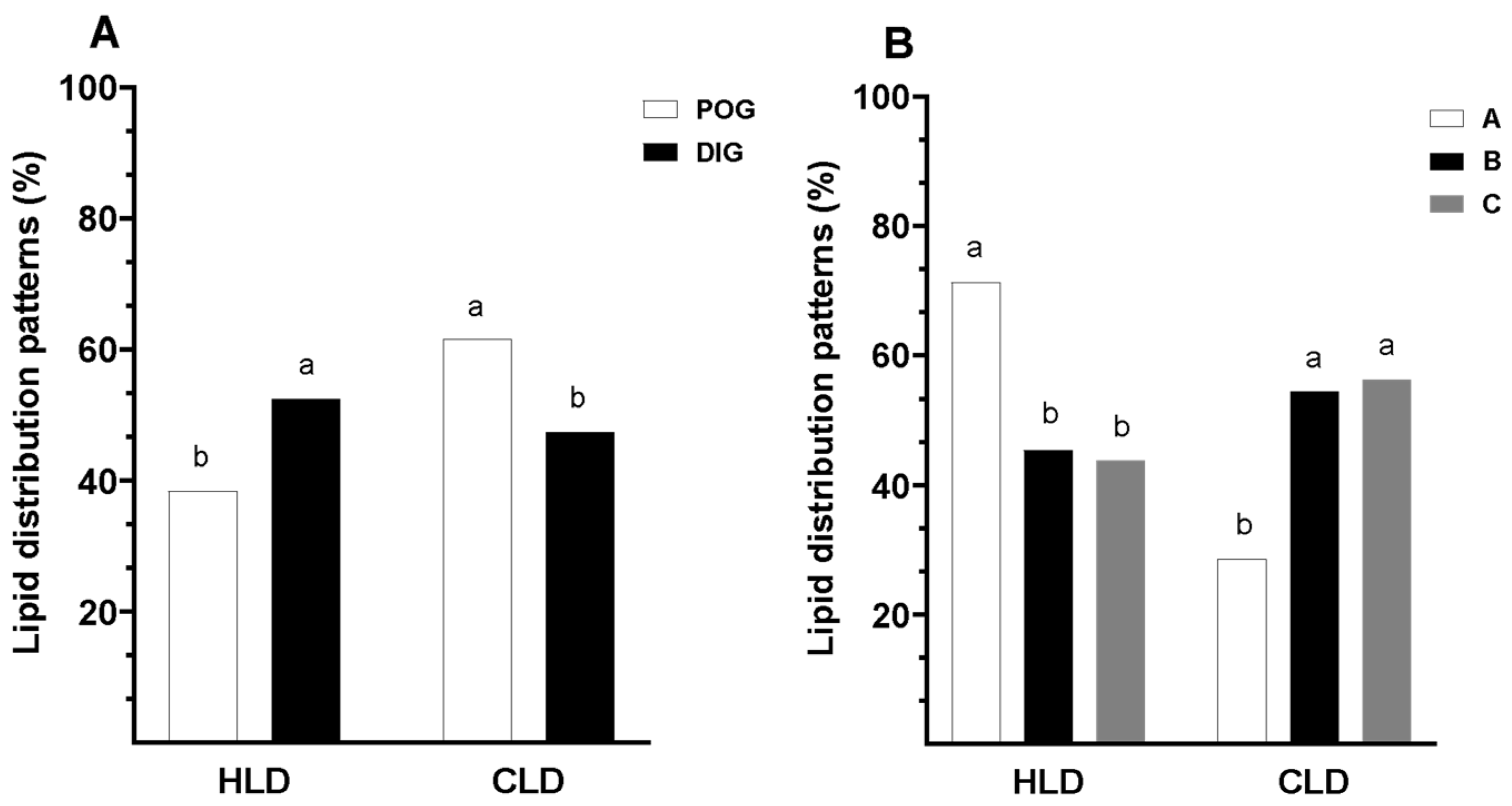
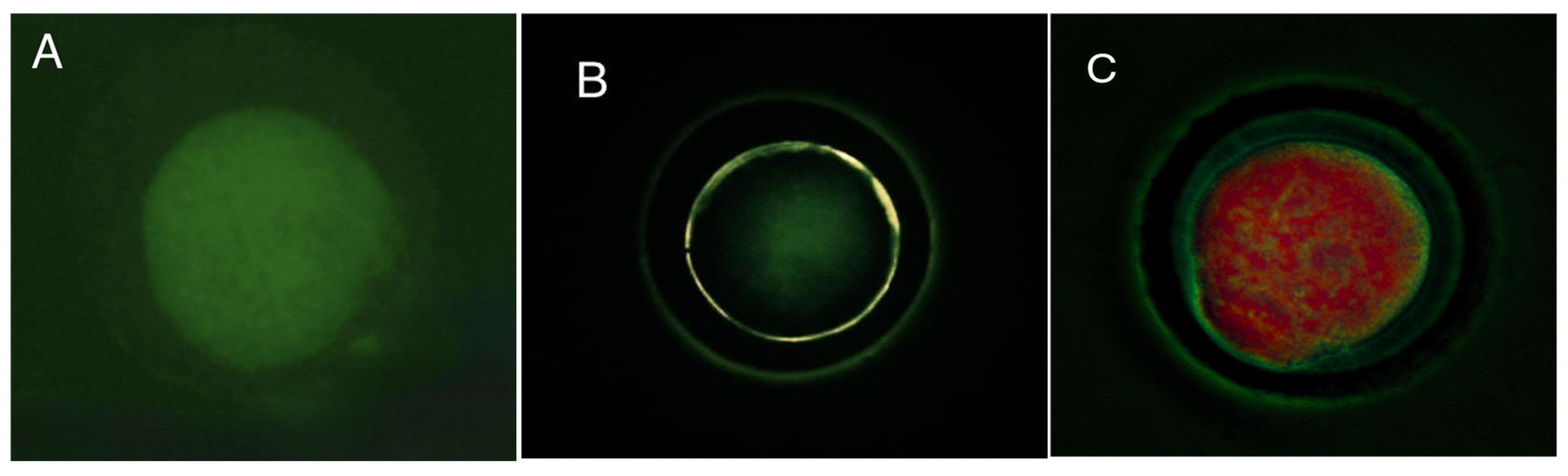
2.5. Lipid Distribution Patterns
2.6. In Vitro Maturation (IVM) of COCs
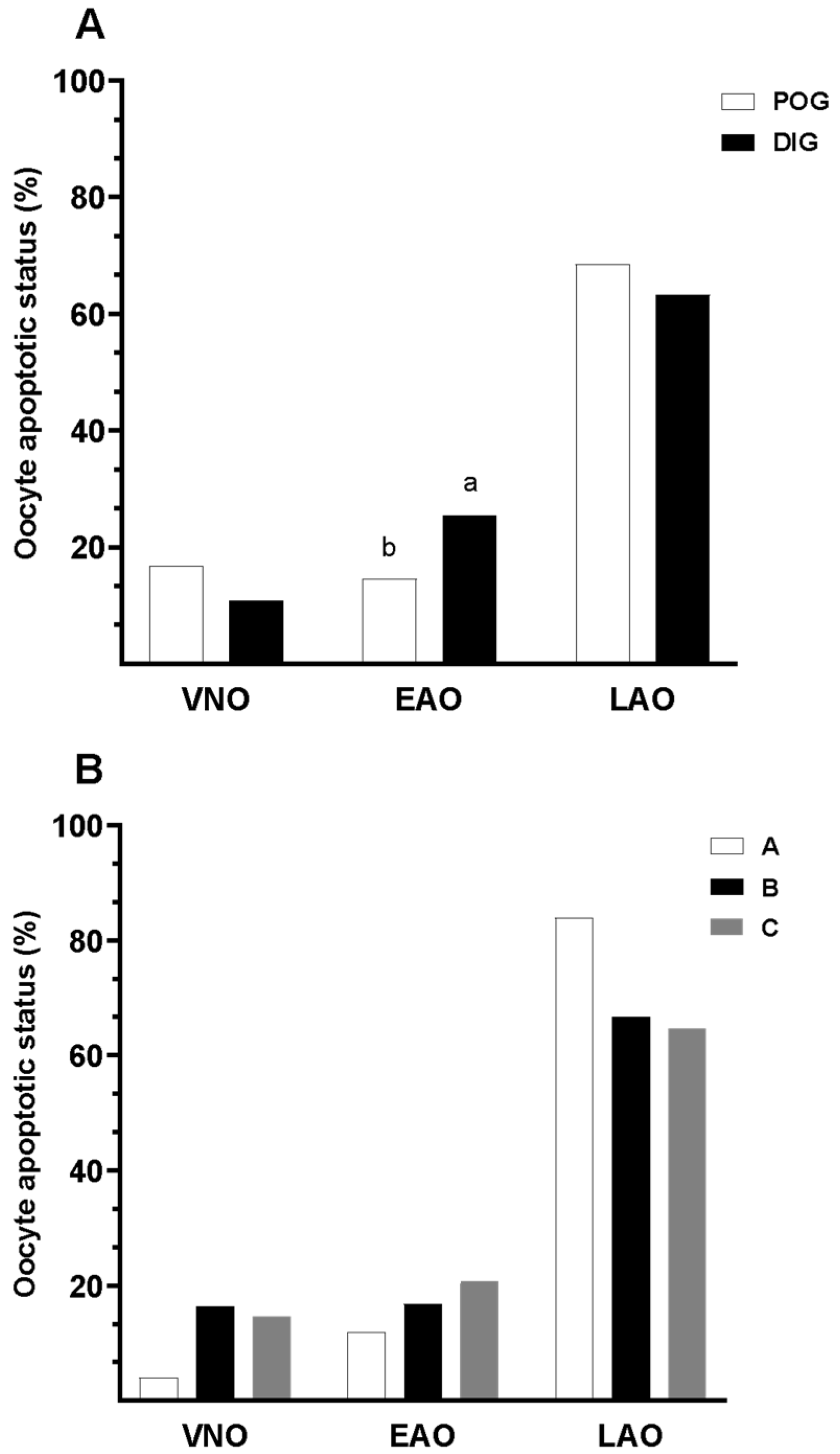
2.7. Apoptosis Assessment
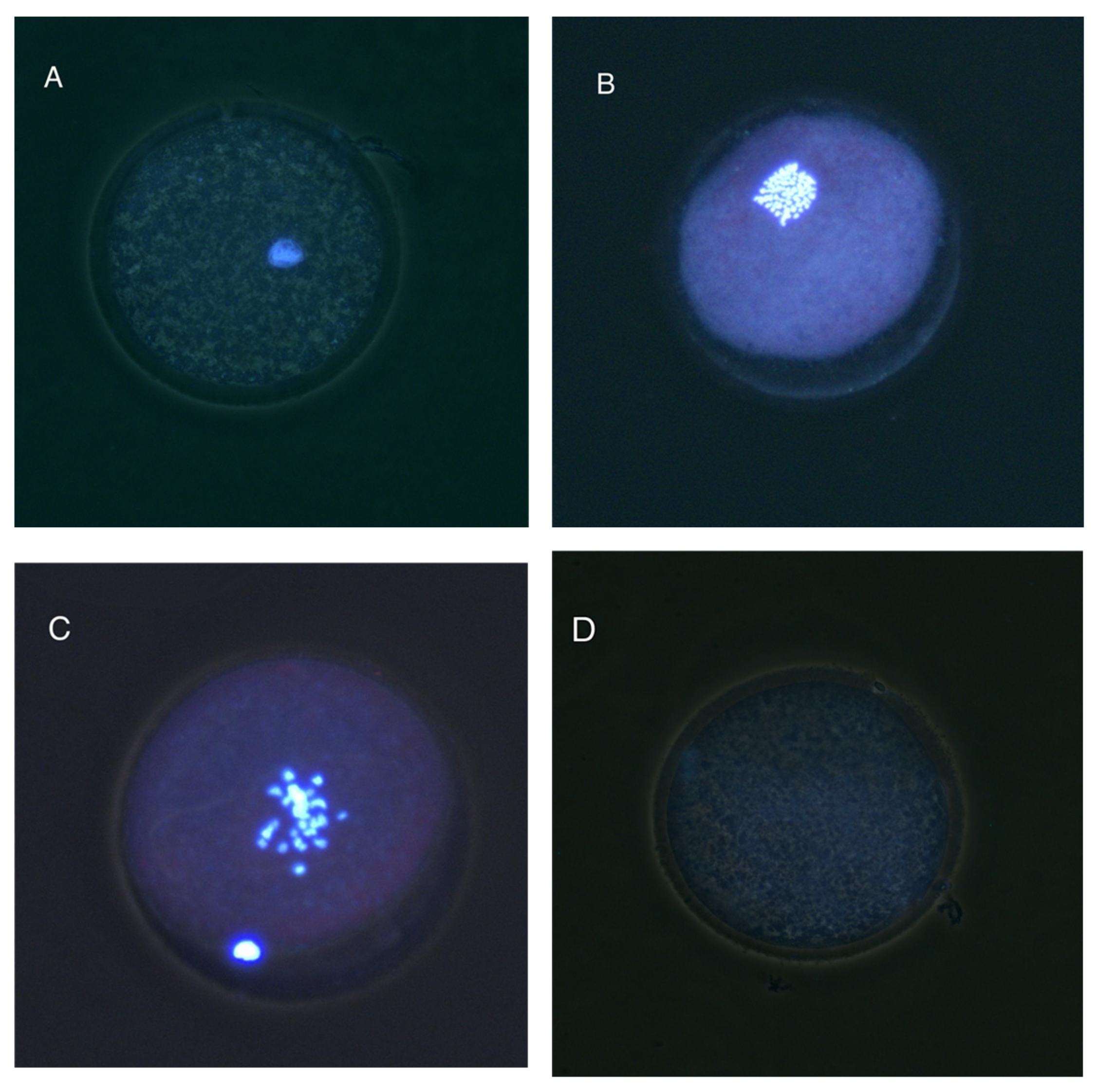
2.8. Nuclear Progression Assessment
- Immature: Germinal vesicle (GV) present or oocyte in Metaphase I (MI).
- Mature—Metaphase II (MII): First polar body visible and metaphase present.
- Degenerated (DEG): Absence of discernible nuclear structures or evidence of chromatin fragmentation.
2.9. Determination of Oocyte Diameter
2.10. Statistical Analysis
3. Results
3.1. Evaluation of Brilliant Cresyl Blue Staining
3.2. Lipid Distribution Patterns in Guinea Pig Oocytes
3.3. Apoptotic Status
3.4. Nuclear Maturation
3.5. Evaluation of Morphology in Guinea Pig Oocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IVM | In Vitro Maturation |
| COC | Cumulus–Oocyte Complex |
| BCB | Brilliant Cresyl Blue |
| G6PD | Glucose-6-Phosphate Dehydrogenase |
| PBS | Phosphate-Buffered Saline |
| FBS | Fetal Bovine Serum |
| FSH | Follicle-Stimulating Hormone |
| LH | Luteinizing Hormone |
| EGF | Epidermal Growth Factor |
| GV | Germinal Vesicle |
| M-II | Metaphase II |
| DEG | Degenerated Oocyte |
| OR | Odds ratio |
| CI | Confidence Interval |
| HLD | Homogeneous Lipid Distribution |
| CLD | Centralized Lipid Distribution |
| VNO | Viable Non-Apoptotic Oocyte |
| EAO | Early Apoptotic Oocyte |
| LAO | Late Apoptotic Oocyte |
| POG | Periovulatory Stage in Guinea Pigs |
| DIG | Diestrus Stage in Guinea Pigs |
| egpS | Estrous Guinea Pig Serum |
| egpFF | Estrous Guinea Pig Follicular Fluid |
References
- Sandweiss, D.; Wing, E. Ritual Rodents: The Guinea Pigs of Chincha, Peru. J. Field Archaeol. 1997, 24, 47–58. [Google Scholar] [CrossRef]
- Avilés, D.F.; Martínez, A.M.; Landi, V.; Delgado, J.V. El Cuy (Cavia porcellus): Un Recurso Andino de Interés Agroalimentario The Guinea Pig (Cavia porcellus): An Andean Resource of Interest as an Agricultural Food Source. Anim. Genet. Resour./Resour. Génétiques Anim./Recur. Genéticos Anim. 2014, 55, 87–91. [Google Scholar] [CrossRef]
- Aphrodita, A.; Sentono, D.N.; Fitria, L. Analysis of Carcass Weight and Proximate Composition as Guinea Pig [Cavia porcellus (Linnaeus, 1758)] Meat Quality Indicator. BIO Web Conf. 2024, 94, 06004. [Google Scholar] [CrossRef]
- Pinchao-Pinchao, Y.; Serna-Cock, L.; Osorio-Mora, O.; Tirado, D.F. Guinea Pig Breeding and Its Relation to Sustainable Food Security and Sovereignty in South America: Nutrition, Health, and Production Challenges. CyTA-J. Food 2024, 22, 2392886. [Google Scholar] [CrossRef]
- Suzuki, O.; Koura, M.; Noguchi, Y.; Takano, K.; Yamamoto, Y.; Matsuda, J. Optimization of Superovulation Induction by Human Menopausal Gonadotropin in Guinea Pigs Based on Follicular Waves and FSH-Receptor Homologies. Mol. Reprod. Dev. 2003, 64, 219–225. [Google Scholar] [CrossRef]
- Grégoire, A.; Allard, A.; Huamán, E.; León, S.; Silva, R.M.; Buff, S.; Berard, M.; Joly, T. Control of the Estrous Cycle in Guinea-Pig (Cavia porcellus). Theriogenology 2012, 78, 842–847. [Google Scholar] [CrossRef]
- Shi, F.; Watanabe, G.; Trewin, A.L.; Hutz, R.J.; Taya, K. Localization of Ovarian Inhibin/Activin Subunits in Follicular Dominance during the Estrous Cycle of Guinea Pigs. Zoolog. Sci. 2000, 17, 1311–1320. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Sun, Q.; Xia, S.; Cui, J.; Yang, L.; An, L.; Zhang, J.; Su, L.; Su, Y.; et al. Combined Treatment with Cysteamine and Leukemia Inhibitory Factor Promotes Guinea Pig Oocyte Meiosis in Vitro. Am. J. Transl. Res. 2019, 11, 7479–7491. [Google Scholar]
- Demetrio, D.; Benedetti, E.; Demetrio, C.G.B.; Fonseca, J.; Oliveira, M.; Magalhaes, A.; dos Santos, R.M. How Can We Improve Embryo Production and Pregnancy Outcomes of Holstein Embryos Produced in Vitro? (12 Years of Practical Results at a California Dairy Farm). Anim. Reprod. 2020, 17, e20200053. [Google Scholar] [CrossRef]
- Lonergan, P.; Fair, T. Maturation of Oocytes in Vitro. Annu. Rev. Anim. Biosci. 2016, 4, 255–268. [Google Scholar] [CrossRef]
- Cañón-Beltrán, K.; García-García, R.M.; Cajas, Y.N.; Fierro, N.; Lorenzo, P.L.; Arias-Álvarez, M. Improvement of Oocyte Competence and in Vitro Oocyte Maturation with EGF and IGF-I in Guinea Pig Model. Theriogenology 2024, 214, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Cheng, W.; Liu, L.; Zheng, H.; Gu, W.; Miao, F.; Zhang, J.; Wang, L.; Su, Y.; Liu, Y.; et al. Relationship between Chromatin Configuration and in Vitro Maturation Ability in Guinea Pig Oocytes. Vet. Med. Sci. 2021, 7, 2410–2417. [Google Scholar] [CrossRef]
- Samaniego, J.X.; Pesantez, J.L.; Ayala, L.E.; Perea, F.P.; Galarza, D.A.; Dutan, J.B.; Ruiz, S. Effects of Follicular Fluid and Serum Supplementation on Cumulus Cell Expansion and Nuclear Progression of Guinea Pig Oocytes, Using a Baseline Medium Established with Bovine Oocytes. Animals 2025, 15, 666. [Google Scholar] [CrossRef]
- Revel, F.; Mermillod, P.; Peynot, N.; Renard, J.P.; Heyman, Y. Low Developmental Capacity of in Vitro Matured and Fertilized Oocytes from Calves Compared with That of Cows. J. Reprod. Fertil. 1995, 103, 115–120. [Google Scholar] [CrossRef]
- Hendriksen, P.J.M.; Vos, P.L.A.M.; Steenweg, W.N.M.; Bevers, M.M.; Dieleman, S.J. Bovine Follicular Development and Its Effect on the in Vitro Competence of Oocytes. Theriogenology 2000, 53, 11–20. [Google Scholar] [CrossRef]
- Hyttel, P.; Fair, T.; Callesen, H.; Greve, T. Oocyte Growth, Capacitation and Final Maturation in Cattle. Theriogenology 1997, 47, 23–32. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Vireque, A.A.; Adona, P.R.; Meirelles, F.V.; Ferriani, R.A.; Navarro, P.A.A.S. Cytoplasmic Maturation of Bovine Oocytes: Structural and Biochemical Modifications and Acquisition of Developmental Competence. Theriogenology 2009, 71, 836–848. [Google Scholar] [CrossRef]
- Wongsrikeao, P.; Otoi, T.; Yamasaki, H.; Agung, B.; Taniguchi, M.; Naoi, H.; Shimizu, R.; Nagai, T. Effects of Single and Double Exposure to Brilliant Cresyl Blue on the Selection of Porcine Oocytes for in Vitro Production of Embryos. Theriogenology 2006, 66, 366–372. [Google Scholar] [CrossRef]
- Alm, H.; Torner, H.; Löhrke, B.; Viergutz, T.; Ghoneim, I.M.; Kanitz, W. Bovine Blastocyst Development Rate in Vitro Is Influenced by Selection of Oocytes by Brillant Cresyl Blue Staining before IVM as Indicator for Glucose-6-Phosphate Dehydrogenase Activity. Theriogenology 2005, 63, 2194–2205. [Google Scholar] [CrossRef]
- Torner, H.; Ghanem, N.; Ambros, C.; Hölker, M.; Tomek, W.; Phatsara, C.; Alm, H.; Sirard, M.A.; Kanitz, W.; Schellander, K.; et al. Molecular and Subcellular Characterisation of Oocytes Screened for Their Developmental Competence Based on Glucose-6-Phosphate Dehydrogenase Activity. Reproduction 2008, 135, 197–212. [Google Scholar] [CrossRef]
- Jia, L.; Ding, B.; Shen, C.; Luo, S.; Zhang, Y.; Zhou, L.; Ding, R.; Qu, P.; Liu, E. Use of Oocytes Selected by Brilliant Cresyl Blue Staining Enhances Rabbit Cloned Embryo Development in Vitro. Zygote 2019, 27, 166–172. [Google Scholar] [CrossRef]
- Sturmey, R.G.; Reis, A.; Leese, H.J.; McEvoy, T.G. Role of Fatty Acids in Energy Provision during Oocyte Maturation and Early Embryo Development. Reprod. Domest. Anim. 2009, 44 (Suppl. 3), 50–58. [Google Scholar] [CrossRef]
- Prates, E.G.; Nunes, J.T.; Pereira, R.M. A Role of Lipid Metabolism during Cumulus-Oocyte Complex Maturation: Impact of Lipid Modulators to Improve Embryo Production. Mediat. Inflamm. 2014, 2014, 692067. [Google Scholar] [CrossRef]
- Krisher, R.L. The Effect of Oocyte Quality on Development. J. Anim. Sci. 2004, 82, E14–E23. [Google Scholar]
- Lonergan, P.; Monaghan, P.; Rizos, D.; Boland, M.P.; Gordon, I. Effect of Follicle Size on Bovine Oocyte Quality and Developmental Competence Following Maturation, Fertilization, and Culture in Vitro. Mol. Reprod. Dev. 1994, 37, 48–53. [Google Scholar] [CrossRef]
- Yoon, K.W.; Shin, T.Y.; Park, J.I.; Roh, S.; Lim, J.M.; Lee, B.C.; Hwang, W.S.; Lee, E.S. Development of Porcine Oocytes from Preovulatory Follicles of Different Sizes after Maturation in Media Supplemented with Follicular Fluids. Reprod. Fertil. Dev. 2000, 12, 133–139. [Google Scholar] [CrossRef]
- He, M.; Zhang, T.; Yang, Y.; Wang, C. Mechanisms of Oocyte Maturation and Related Epigenetic Regulation. Front. Cell Dev. Biol. 2021, 9, 654028. [Google Scholar] [CrossRef]




| Category | Description |
|---|---|
| A | Oocytes surrounded by four or more layers of cumulus cells. |
| B | Few layers of cumulus coating, homogeneous or irregular cytoplasm. |
| C | Expanded cumulus cell coating, partial or nonexistent. |
| Classification | Annexin V (+) | Ethidium Bromide (+) | Interpretation |
|---|---|---|---|
| Viable Non-Apoptotic Oocyte (VNO) | − | − | Oocyte with no signs of apoptosis. |
| Early Apoptotic Oocyte (EAO) | + | − | Oocyte in early stages of apoptosis. |
| Late Apoptotic Oocyte (LAO) | + | + | Oocyte in late apoptosis or necrotic stage. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaniego, J.X.; Pesántez, J.L.; Perea, F.P.; Pazmiño, A.P.; Dután, J.B.; Ruiz, S. Comparative Assessment of Functional and Morphological Markers in Guinea Pig (Cavia porcellus) Oocytes Collected at Different Estrous Cycle Phases. Animals 2025, 15, 1953. https://doi.org/10.3390/ani15131953
Samaniego JX, Pesántez JL, Perea FP, Pazmiño AP, Dután JB, Ruiz S. Comparative Assessment of Functional and Morphological Markers in Guinea Pig (Cavia porcellus) Oocytes Collected at Different Estrous Cycle Phases. Animals. 2025; 15(13):1953. https://doi.org/10.3390/ani15131953
Chicago/Turabian StyleSamaniego, Jorge X., José L. Pesántez, Fernando P. Perea, Andrea P. Pazmiño, Jorge B. Dután, and Salvador Ruiz. 2025. "Comparative Assessment of Functional and Morphological Markers in Guinea Pig (Cavia porcellus) Oocytes Collected at Different Estrous Cycle Phases" Animals 15, no. 13: 1953. https://doi.org/10.3390/ani15131953
APA StyleSamaniego, J. X., Pesántez, J. L., Perea, F. P., Pazmiño, A. P., Dután, J. B., & Ruiz, S. (2025). Comparative Assessment of Functional and Morphological Markers in Guinea Pig (Cavia porcellus) Oocytes Collected at Different Estrous Cycle Phases. Animals, 15(13), 1953. https://doi.org/10.3390/ani15131953





