An Overview of Advancements in Proteomic Approaches to Enhance Livestock Production and Aquaculture
Simple Summary
Abstract
1. Introduction
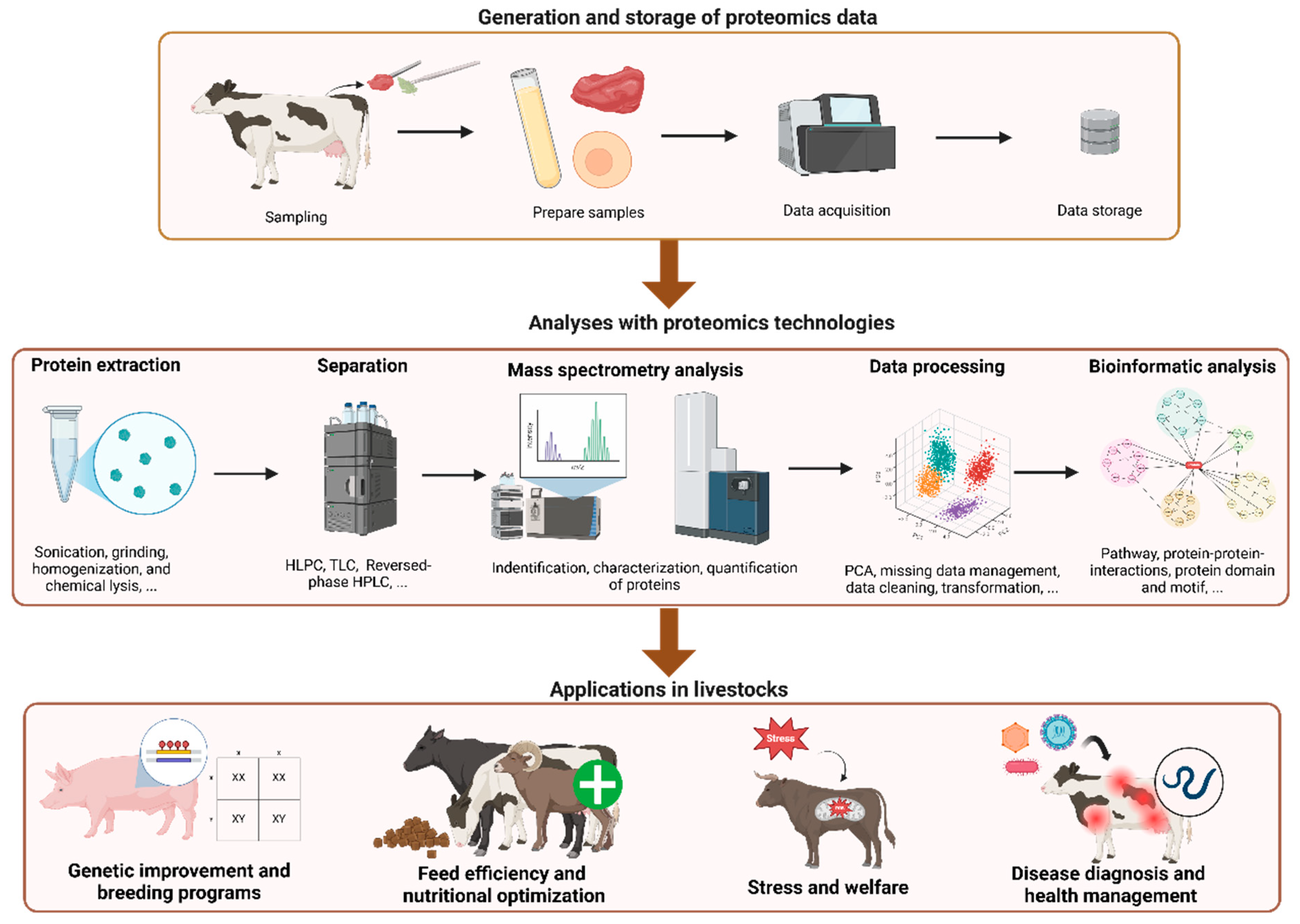
2. Applications of Proteomics in Livestock Production
2.1. Genetic Improvement and Breeding Programs
2.2. Feed Efficiency and Nutritional Optimization
2.3. Stress and Welfare
| Indicators | Proteomics Techniques/Method | Species | Findings | References |
|---|---|---|---|---|
| Stress response | LC-MS/MS | Swine | Label-free quantitative proteomics identified 66 differentially abundant proteins between non-stressed and stressed pigs, with 30 increased and 36 decreased in the non-stressed group. | [67] |
| Pre-slaughter stress | OFFGEL, SDS-PAGE and LC-MS | Bovine | Five protein bands showed significant differences between normal and DFD meat samples, containing actin, phosphoglucomutase-1, alpha-crystallin B, heat shock protein beta-6, and heat shock protein beta-1. | [68] |
| Chronic lameness | LC-MS/MS and SDS-PAGE | Bovine | Chronic inflammatory lameness in dairy cows is associated with increased expression of stress proteins with chaperone, metabolism, redox, and structural functions in the dorsal horn of the spinal cord. | [69] |
| Heat stress | LC-MS/MS | Swine | Overexpression of HSP70 in intestinal epithelial cells led to changes in the expression of many proteins involved in cell–extracellular matrix interactions, cell adhesion, and apoptosis. | [70] |
| Environmental enrichment | iTRAQ and LC-MS/MS | Swine | Pigs in enriched environments had lower plasma cortisol and lactate levels, indicating a reduced stress response. Pigs in enriched environments showed changes in neurotransmitter levels in the brain, including decreased noradrenaline and dopamine, and increased serotonin, also suggesting a lower stress response. | [71] |
| Slaughter methods | MALDI-TOF MS | Poultry | Ritual slaughter without stunning resulted in significantly elevated stress indicators like cortisol and triiodothyronine compared to commercial slaughter with electrical stunning. | [72] |
| Electrical stunning | 2-DE and MALDI-TOF/TOF MS | Sheep | The study found 243 proteins that were significantly differentially expressed between stunned and non-stunned (halal) slaughter, with 119 being upregulated and 124 being downregulated. | [73] |
| Chronic circadian disruption | LC-MS/MS | Bovine | Dairy cows exposed to circadian rhythm disruption during late gestation showed increased markers of oxidative stress and metabolism in their muscle tissue. | [74] |
| Response of bovine granulosa cells to acute heat stress | LC-MS/MS | Bovine | Heat stress triggered oxidative stress-mediated apoptosis in bovine granulosa cells (bGCs), but the cells exhibited a time-dependent recovery of proliferation potential by 48 h. The study identified 37 differentially regulated metabolites in bGCs in response to acute heat stress, which were involved in bioenergetics support mechanisms and cellular adaptations. | [75] |
2.4. Disease Diagnosis and Health Management
| Indicators | Proteomics Techniques/Method | Species | Findings | References |
|---|---|---|---|---|
| Neosporosis | LC-MS/MS | Bovine | Neospora caninum infection primarily impacts the host cell’s mitochondrial processes and metabolism. The low-virulence isolate Nc-Spain1H had a greater influence on the host cell proteome compared to the high-virulence isolate Nc-Spain7. | [92] |
| Salmonella infection | LC-MS/MS | Poultry | Salmonella infection increased the abundance of proteins involved in the host’s response to oxidative stress, amino acid metabolism, and lysosomal activity in the spleen of broiler chickens. Salmonella infection decreased the abundance of proteins involved in cell cycle progression, RNA binding, and cytoskeletal development in the spleen of broiler chickens. | [93] |
| Pneumonia and mastitis | 2-DE and MALDI-TOF mass spectrometry | Bovine | Identified 60 secreted proteins from Mycoplasma bovis, a pathogen that causes pneumonia and mastitis in cattle, and 8 of these proteins were predicted to be virulence-related factors. | [94] |
| Mycobacterium avium paratuberculosis infection | label-free LC-MS/MS | Bovine | Cows resistant to MAP infection showed higher abundance of TLR2 and MHC class II proteins in their PBMCs, indicating a successful defensive immune response. Cows persistently infected with MAP showed higher abundance of ITGA2B and KCNMA1 in their PBMCs, suggesting an unsuccessful immune response. | [95] |
| Ketosis | LC-MS | Bovine | The metabolomic analysis showed that cows with clinical ketosis had significant alterations in pathways related to amino acid, carbohydrate, and nucleotide metabolism compared to healthy controls, and these changes were consistent across the transition from prepartum to postpartum. | [96] |
| Toxoplasma gondii infection | iTRAQ labeling, and LC-MS/MS | Pig | Overexpression of two potential anti-T. gondii proteins, HSP70.2 and PDIA3, in swine macrophage cells enhanced resistance to T. gondii infection. | [97] |
| Mastitis | MALDI-TOF mass spectrometry | Goat | Identified the Staphylococcus species present in 19 isolates from subclinical caprine mastitis, with S. epidermidis being the most common at 47.36%. Henotypic resistance testing showed high resistance to penicillin G (58%), but lower resistance to cefoxitin and oxacillin (both 26.31%). All strains were susceptible to amoxicillin + clavulanic acid. | [98] |
| Coinfection with Marek’s disease virus and reticuloendotheliosis virus | Tandem mass tag (TMT) labeling and LC-MS/MS | Chicken | MDV and REV coinfection increased viral replication compared to single infections. Coinfection led to differential expression of 98 proteins, with 38 upregulated and 60 downregulated. | [99] |
| Rotavirus infection | iTRAQ and LC-MS/MS | Pig | Identified 223 differentially accumulated proteins (DAPs) in porcine rotavirus (PoRV)-infected IPEC-J2 cells compared to mock-infected cells, with 125 being up-accumulated and 98 being down-accumulated. | [100] |
| Deltacoronavirus | iTRAQ and LC-MS/MS | Pig | A total of 78 differentially expressed proteins (DEPs) were identified in IPEC-J2 cells infected with porcine deltacoronavirus (PDCoV), with 23 being upregulated and 55 being downregulated. | [101] |
| Mastitis | MALDI-TOF MS | Bovine | MALDI-TOF MS fingerprinting was superior to 16S rRNA gene sequencing for discriminating between different streptococcal species and subspecies involved in bovine mastitis. MALDI-TOF MS analysis identified three specific protein biomarkers characteristic of the Streptococcus genus and showed variability at both the species and subspecies level. | [102] |
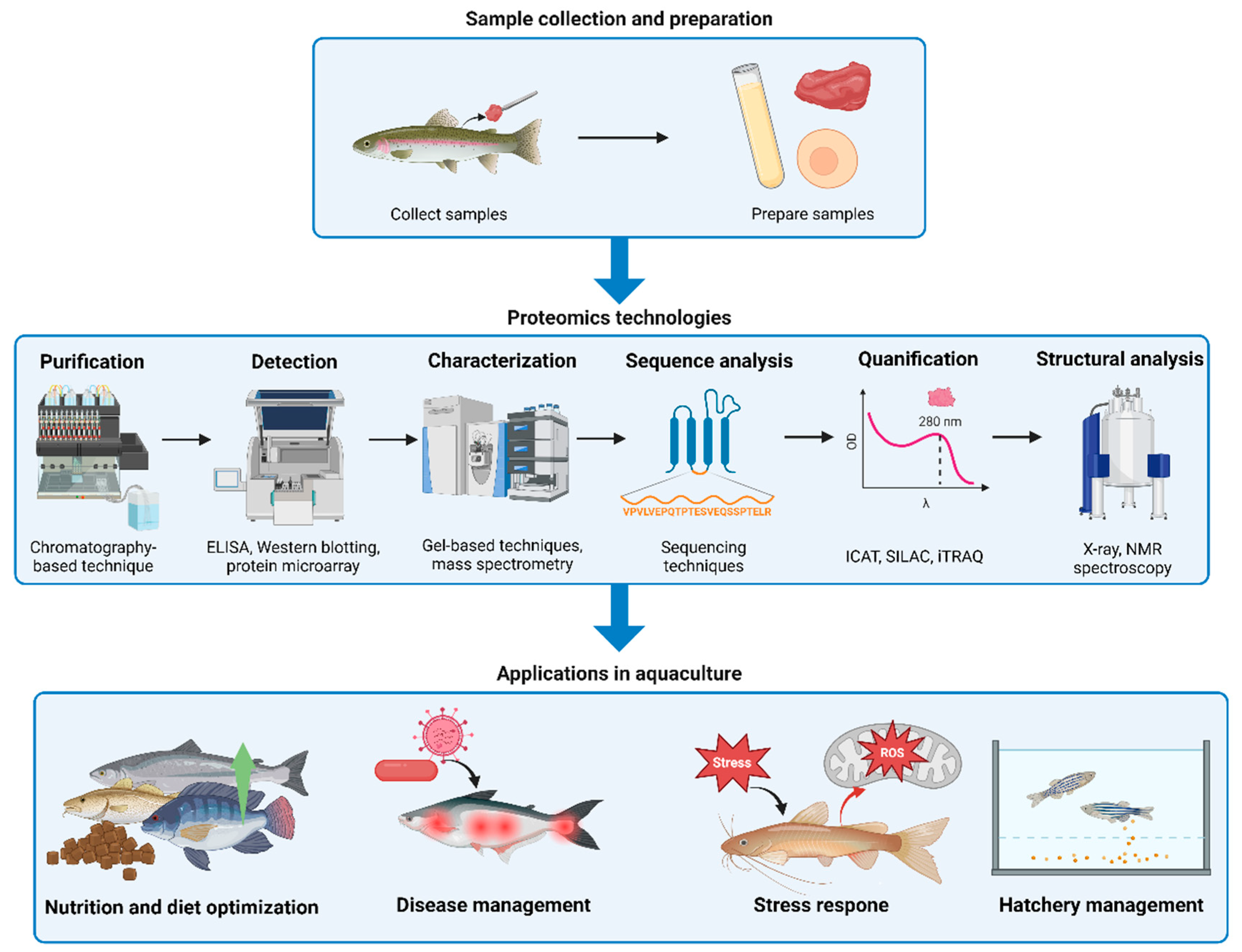
3. Applications of Proteomics in Aquaculture
3.1. Proteomic Insights into Fish Physiology
| Indicators | Technique Used | Species | Findings | References |
|---|---|---|---|---|
| Immune and stress biomarkers in skin mucus | 2D-PAGE, LC-MS/MS | Gadus morhua, Cyclopterus lumpus | Identified immune-related proteins such as galectin-1, cystatin B, heat shock proteins, and peroxiredoxin1. | [106,110] |
| Innate immunity and physiological status | LC-MS/MS, 2-DE | Sparus aurata | Key proteins (e.g., actins, glycolytic enzymes, heat shock proteins) linked to immune defense, metabolism, and stress responses. | [111,112] |
| Antimicrobial and proteolytic activity | Nano-LC-MS/MS | Scorpaena plumieri | Identified 391 proteins with antimicrobial and venom-related activity, including proteolytic enzymes. | [113] |
| Feeding mechanisms of bloodsucking fish | LC-MS/MS, 1D SDS-PAGE | Lampetra morii | Novel proteins involved in blood coagulation suppression and host immune evasion during feeding were identified. | [114] |
| Reproductive processes in seminal plasma | HPLC-ESI-MS/MS, 2D-DIGE | Oncorhynchus mykiss, Cyprinus carpio | Identified proteins regulating sperm motility, membrane stability, antioxidative defense, and inflammation responses. | [107,115] |
| Growth and muscle development | DIGE, MALDI-TOF/TOF | Sparus aurata | Proteins such as parvalbumins and Wap65 were linked to growth, stress adaptation, and dietary influences. | [116,117] |
| Gill proteome analysis | Data-independent acquisition | Gasterosteus aculeatus | Explored molecular differences in gill proteins among ecotypes, revealing adaptation to environmental and morphological variations. | [118] |
| Lymphoid organ function | Shotgun proteomics | Oncorhynchus mykiss | Profiling of head kidney and spleen proteins provided insights into immune mechanisms and DNA repair processes. | [119] |
| Confirmation of specific proteins | LC-MS/MS | Gadus morhua | Verified the presence of natterin-like proteins in skin, liver, and kidney, linked to immune responses. | [120] |
3.2. Disease Resistance and Immunity
3.3. Environmental Adaptation and Stress Response
| Stressors | Technique Used | Species | Findings | References |
|---|---|---|---|---|
| Physical stress | ||||
| Overcrowding | LC-MS/MS, 2-DE | Sparus aurata | Liver and immune proteins showed significant changes under overcrowding compared to optimized rearing conditions. | [136,137] |
| 2-DE | Salmo salar | Proteins in muscle and plasma revealed secondary and tertiary stress responses. | [138] | |
| X-ray irradiation | LC-MS | Salmo salar | Persistent alterations in gill proteins observed in early-stage rainbow trout. | [139] |
| Freezing conditions | 2-DE, MALDI-TOF/TOF MS | Cyprinus carpio | Sperm proteins supported antioxidative defense, membrane stability, and motility control. | [140,141] |
| High temperature | LTQ-Orbitrap XL | Salmo salar | Proteomic changes in the liver indicated reduced energy expenditure to cope with oxidative stress. | [142] |
| Low temperature | iTRAQ | Takifugu fasciatus | Enhanced oxidative stress response and mitochondrial enzyme activity were linked to cold tolerance. | [143] |
| Hypersalinity | LC-MS | Unspecified | Differentially expressed proteins supported osmoregulation, digestion, and mineral regulation in response to salinity changes. | [144] |
| High CO2 and temperature | 2-DE, Nanoflow LC-MS/MS | Hippoglossus hippoglossus | Energy metabolism proteins in gills and immune proteins in blood plasma were significantly affected. | [145] |
| Chemical stress | ||||
| Copper | 2D-DIGE, iTRAQ | Oncorhynchus mykiss, Cyprinus carpio, Carassius auratus gibelio | Oxidative stress markers, such as superoxide dismutase and cytochrome c, were identified in gills. | [146] |
| Arsenic | 2D, MALDI-TOF/TOF MS | Labeo rohita | Identified biomarkers (Apo-A1, A2ML, Wap65) indicate arsenic-induced liver toxicity. | [147] |
| Benzotriazole | 2-DE, TOF/TOF MS/MS | Gobiocypris rarus | Neurological and liver alterations differed between male and female fish. | [148,149] |
| PAHs (Polycyclic Aromatics) | LC-MS/MS | Gadus morhua | An albumin-like protein in plasma was linked to PAH-induced stress. | [150] |
| PCB (Polychlorinated Biphenyl) | MALDI-TOF MS, MS/MS | Gadus morhua | Proteins linked to neurotoxicity and stress pathways (e.g., Notch signaling) were identified. | [151] |
| Bisphenol A | LC-MS/MS | Danio rerio | Proteomic changes in brain tissue suggested complex toxicity mechanisms involving metabolism and transport. | [152] |
| Pesticides (Permethrin, Terbufos) | LC-MS/MS | Pimephales promelas | Enrichment of proteins associated with proteasome systems and glycolysis was observed. | [153] |
| Pesticide (Dieldrin) | LC-MS/MS, iTRAQ | Danio rerio | Mitochondrial proteins showed links to diseases like Parkinson’s and Huntington’s. | [154] |
| Herbicide (Ametryn) | SDS-PAGE | Danio rerio | Induced proteins were linked to glycolysis and lipid transport, while suppressed proteins were associated with oxygen transport. | [155] |
3.4. Reproductive Biology and Hatchery Management
4. Challenges and Limitations in the Use of Proteomics in Livestock and Aquaculture Production
4.1. Management and Maintenance of Proteomics Data
4.2. Lack of Comprehensive Phenomics Data
4.3. Sample Preparation and Variability
4.4. Species-Specific and Tissue-Specific Challenges
4.5. Cost and Accessibility
5. Future Directions
5.1. Multi-Omics Integration and Systems Biology Approaches
5.2. Single-Cell and Spatial Proteomics
5.3. Proteogenomics and Proteotranscriptomics
5.4. Artificial Intelligence in Proteomics
5.5. Standardized Protocols and Data Sharing
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farmakis, D.; Papingiotis, G.; Parissis, J.; Filippatos, G. Ups and downs in heart failure: The case of proteomics. Eur. J. Heart Fail. 2018, 20, 63–66. [Google Scholar] [CrossRef]
- Alsagaby, S.A. Understanding the fundamentals of proteomics. Curr. Top. Pept. Protein. Res. 2019, 20, 25–33. [Google Scholar] [CrossRef]
- Sahayarayan, J.J.; Enogochitra, A.; Chandrasekaran, M. Basic Concepts in Proteomics and Applications. Comput. Bioinform. Multidiscip. Appl. 2021, 279–293. [Google Scholar]
- Dolomatov, S.; Kazakova, V.P.; Zukow, W. Proteomics. J. Educ. Health Sport 2021, 11, 158–189. [Google Scholar] [CrossRef]
- Yokota, H. Applications of proteomics in pharmaceutical research and development. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2019, 1867, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Manes, N.P.; Nita-Lazar, A. Application of targeted mass spectrometry in bottom-up proteomics for systems biology research. J. Proteom. 2018, 189, 75–90. [Google Scholar] [CrossRef]
- Channaveerappa, D.; Ngounou Wetie, A.G.; Darie, C.C. Bottlenecks in proteomics: An update. Adv. Mass Spectrom. Biomed. Res. 2019, 1140, 753–769. [Google Scholar]
- Mathpal, D. An analysis of proteomics and its applications. Asian J. Multidimens. Res. 2021, 10, 633–640. [Google Scholar] [CrossRef]
- Jaiswal, S.; Rasal, K.D.; Chandra, T.; Prabha, R.; Iquebal, M.A.; Rai, A.; Kumar, D. Proteomics in fish health and aquaculture productivity management: Status and future perspectives. Aquaculture 2023, 566, 739159. [Google Scholar] [CrossRef]
- Moreira, M.; Schrama, D.; Farinha, A.P.; Cerqueira, M.; Raposo de Magalhaes, C.; Carrilho, R.; Rodrigues, P. Fish pathology research and diagnosis in aquaculture of farmed fish; a proteomics perspective. Animals 2021, 11, 125. [Google Scholar] [CrossRef]
- Kumar, S.; Mohanty, A.; Kauhsik, J.; Mukesh, M. Proteomics-based advancements in research toward sustainable production from dairy livestock. In Advances in Animal Experimentation and Modeling; Elsevier: Amsterdam, The Netherlands, 2022; pp. 353–358. [Google Scholar]
- Chakraborty, D.; Sharma, N.; Kour, S.; Sodhi, S.S.; Gupta, M.K.; Lee, S.J.; Son, Y.O. Applications of omics technology for livestock selection and improvement. Front. Genet. 2022, 13, 774113. [Google Scholar] [CrossRef]
- YANG, Y.-l.; Rong, Z.; Kui, L. Future livestock breeding: Precision breeding based on multi-omics information and population personalization. J. Integr. Agric. 2017, 16, 2784–2791. [Google Scholar] [CrossRef]
- Al-Sharif, M.; Radwan, H.; Hendam, B.; Ateya, A. DNA polymorphisms of FGFBP1, leptin, κ-casein, and αs1-casein genes and their association with reproductive performance in dromedary she-camels. Theriogenology 2022, 178, 18–29. [Google Scholar] [CrossRef]
- Da Silva Diniz, W.J.; Ward, A.K. 282 Multi-omics approaches to improve animal production. J. Anim. Sci. 2021, 99, 20–21. [Google Scholar] [CrossRef]
- Mullen, A.; Stapleton, P.; Corcoran, D.; Hamill, R.; White, A. Understanding meat quality through the application of genomic and proteomic approaches. Meat Sci. 2006, 74, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Rexroad, C.; Vallet, J.; Matukumalli, L.K.; Reecy, J.; Bickhart, D.; Blackburn, H.; Boggess, M.; Cheng, H.; Clutter, A.; Cockett, N. Genome to phenome: Improving animal health, production, and well-being—A new USDA blueprint for animal genome research 2018–2027. Front. Genet. 2019, 10, 327. [Google Scholar] [CrossRef]
- Peddinti, D.; Nanduri, B.; Kaya, A.; Feugang, J.M.; Burgess, S.C.; Memili, E. Comprehensive proteomic analysis of bovine spermatozoa of varying fertility rates and identification of biomarkers associated with fertility. BMC Syst. Biol. 2008, 2, 1–13. [Google Scholar] [CrossRef]
- Itze-Mayrhofer, C.; Brem, G. Quantitative proteomic strategies to study reproduction in farm animals: Female reproductive fluids. J. Proteom. 2020, 225, 103884. [Google Scholar] [CrossRef]
- Kosteria, I.; Anagnostopoulos, A.K.; Kanaka-Gantenbein, C.; Chrousos, G.P.; Tsangaris, G.T. The use of proteomics in assisted reproduction. In Vivo 2017, 31, 267–283. [Google Scholar] [CrossRef]
- Braundmeier, A.; Miller, D. Invited review: The search is on: Finding accurate molecular markers of male fertility. J. Dairy Sci. 2001, 84, 1915–1925. [Google Scholar] [CrossRef]
- Arnold, G.J.; Frohlich, T. Dynamic proteome signatures in gametes, embryos and their maternal environment. Reprod. Fertil. Dev. 2010, 23, 81–93. [Google Scholar] [CrossRef]
- Lamy, J.; Labas, V.; Harichaux, G.; Tsikis, G.; Mermillod, P.; Saint-Dizier, M. Regulation of the bovine oviductal fluid proteome. Reproduction 2016, 152, 629–644. [Google Scholar] [CrossRef]
- Muñoz, M.; Corrales, F.J.; Caamaño, J.N.; Díez, C.; Trigal, B.; Mora, M.I.; Martín, D.; Carrocera, S.; Gómez, E. Proteome of the early embryo–maternal dialogue in the cattle uterus. J. Proteome Res. 2012, 11, 751–766. [Google Scholar] [CrossRef]
- Zachut, M.; Sood, P.; Levin, Y.; Moallem, U. Proteomic analysis of preovulatory follicular fluid reveals differentially abundant proteins in less fertile dairy cows. J. Proteom. 2016, 139, 122–129. [Google Scholar] [CrossRef]
- McIlveen, H.; Buchanan, J. The impact of sensory factors on beef purchase and consumption. Nutr. Food Sci. 2001, 31, 286–292. [Google Scholar] [CrossRef]
- Gagaoua, M.; Bonnet, M.; Picard, B. Protein array-based approach to evaluate biomarkers of beef tenderness and marbling in cows: Understanding of the underlying mechanisms and prediction. Foods 2020, 9, 1180. [Google Scholar] [CrossRef]
- Gagaoua, M.; Hughes, J.; Terlouw, E.C.; Warner, R.D.; Purslow, P.P.; Lorenzo, J.M.; Picard, B. Proteomic biomarkers of beef colour. Trends Food Sci. Technol. 2020, 101, 234–252. [Google Scholar] [CrossRef]
- Picard, B.; Gagaoua, M. Meta-proteomics for the discovery of protein biomarkers of beef tenderness: An overview of integrated studies. Food Res. Int. 2020, 127, 108739. [Google Scholar] [CrossRef]
- Gagaoua, M.; Monteils, V.; Couvreur, S.; Picard, B. Identification of biomarkers associated with the rearing practices, carcass characteristics, and beef quality: An integrative approach. J. Agric. Food Chem. 2017, 65, 8264–8278. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H. Application of proteomics to the areas of milk production, processing and quality control—A review. Int. J. Dairy Technol. 2014, 67, 153–166. [Google Scholar] [CrossRef]
- Manso, M.; Léonil, J.; Jan, G.; Gagnaire, V. Application of proteomics to the characterisation of milk and dairy products. Int. Dairy J. 2005, 15, 845–855. [Google Scholar] [CrossRef]
- Agregán, R.; Echegaray, N.; López-Pedrouso, M.; Kharabsheh, R.; Franco, D.; Lorenzo, J.M. Proteomic advances in milk and dairy products. Molecules 2021, 26, 3832. [Google Scholar] [CrossRef]
- Le, T.T.; Deeth, H.C.; Larsen, L.B. Proteomics of major bovine milk proteins: Novel insights. Int. Dairy J. 2017, 67, 2–15. [Google Scholar] [CrossRef]
- Elolimy, A.A.; Zeineldin, M.; Abdelmegeid, M.; Abdelatty, A.M.; Alharthi, A.S.; Bakr, M.H.; Elghandour, M.M.; Salem, A.Z.; Loor, J.J. Metabolomics and Proteomics Signatures in Feed-Efficient Beef and Dairy Cattle. Sustain. Agric. Rev. 54 Anim. Biotechnol. Livest. Prod. 1 2021, 54, 153–165. [Google Scholar]
- Pires, B.V.; Reolon, H.G.; Abduch, N.G.; Souza, L.L.; Sakamoto, L.S.; Mercadante, M.E.Z.; Silva, R.M.; Fragomeni, B.O.; Baldi, F.; Paz, C.C. Effects of feeding and drinking behavior on performance and carcass traits in beef cattle. Animals 2022, 12, 3196. [Google Scholar] [CrossRef]
- Taiwo, G.A.; Idowu, M.; Denvir, J.; Cervantes, A.P.; Ogunade, I.M. Identification of key pathways associated with residual feed intake of beef cattle based on whole blood transcriptome data analyzed using gene set enrichment analysis. Front. Vet. Sci. 2022, 9, 848027. [Google Scholar] [CrossRef]
- Mullins, Y.; Keogh, K.; Blackshields, G.; Kenny, D.A.; Kelly, A.K.; Waters, S.M. Transcriptome assisted label free proteomics of hepatic tissue in response to both dietary restriction and compensatory growth in cattle. J. Proteom. 2021, 232, 104048. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H.; Li, S.; Cao, Z.; Yang, H.; Wang, Y. Integrative hepatic metabolomics and proteomics reveal insights into the mechanism of different feed efficiency with high or low dietary forage levels in Holstein heifers. J. Proteom. 2019, 194, 1–13. [Google Scholar] [CrossRef]
- Chagas, L.; Bass, J.; Blache, D.; Burke, C.; Kay, J.; Lindsay, D.; Lucy, M.; Martin, G.; Meier, S.; Rhodes, F. Invited review: New perspectives on the roles of nutrition and metabolic priorities in the subfertility of high-producing dairy cows. J. Dairy Sci. 2007, 90, 4022–4032. [Google Scholar] [CrossRef]
- Mukiibi, R.; Vinsky, M.; Keogh, K.A.; Fitzsimmons, C.; Stothard, P.; Waters, S.M.; Li, C. Transcriptome analyses reveal reduced hepatic lipid synthesis and accumulation in more feed efficient beef cattle. Sci. Rep. 2018, 8, 7303. [Google Scholar] [CrossRef]
- Serna-García, M.; Fonseca, L.F.S.; Panadero Romero, J.J.; Carretero Asuncion, J.; dos Santos Silva, D.B.; Salatta, B.M.; Frezarim, G.B.; Mercadante, M.E.Z.; Bonilha, S.F.M.; Ferro, J.A. Transcriptome Profiling of the Liver in Nellore Cattle Phenotypically Divergent for RFI in Two Genetic Groups. Animals 2023, 13, 359. [Google Scholar] [CrossRef]
- Hiller, B. Recent developments in lipid metabolism in ruminants–the role of fat in maintaining animal health and performance. Anim. Prod. Sci. 2014, 54, 1549–1560. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Zhang, Z.; Zhao, H.; Huang, D.; Cheng, G.; Yang, Y. Proteomic analysis of physiological function response to hot summer in liver from lactating dairy cows. J. Therm. Biol. 2017, 65, 82–87. [Google Scholar] [CrossRef]
- Da Poian, A.T.; Da Poian, A.T.; Castanho, M.A.; Castanho, M.A. Integrative Human Biochemistry: A Textbook for Medical Biochemistry; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Moyes, K.; Bendixen, E.; Codrea, M.C.; Ingvartsen, K.L. Identification of hepatic biomarkers for physiological imbalance of dairy cows in early and mid lactation using proteomic technology. J. Dairy Sci. 2013, 96, 3599–3610. [Google Scholar] [CrossRef]
- Schäff, C.; Börner, S.; Hacke, S.; Kautzsch, U.; Albrecht, D.; Hammon, H.M.; Röntgen, M.; Kuhla, B.R. Increased anaplerosis, TCA cycling, and oxidative phosphorylation in the liver of dairy cows with intensive body fat mobilization during early lactation. J. Proteome Res. 2012, 11, 5503–5514. [Google Scholar]
- Alexandre, P.A.; Naval-Sanchez, M.; Porto-Neto, L.R.; Ferraz, J.B.S.; Reverter, A.; Fukumasu, H. Systems biology reveals NR2F6 and TGFB1 as key regulators of feed efficiency in beef cattle. Front. Genet. 2019, 10, 230. [Google Scholar] [CrossRef]
- Taussat, S.; Boussaha, M.; Ramayo-Caldas, Y.; Martin, P.; Venot, E.; Cantalapiedra-Hijar, G.; Hozé, C.; Fritz, S.; Renand, G. Gene networks for three feed efficiency criteria reveal shared and specific biological processes. Genet. Sel. Evol. 2020, 52, 1–14. [Google Scholar] [CrossRef]
- Xi, Y.; Yang, Z.; Wu, F.; Han, Z.; Wang, G. Gene expression profiling of hormonal regulation related to the residual feed intake of Holstein cattle. Biochem. Biophys. Res. Commun. 2015, 465, 19–25. [Google Scholar] [CrossRef]
- Lamy, E.; Mau, M. Saliva proteomics as an emerging, non-invasive tool to study livestock physiology, nutrition and diseases. J. Proteom. 2012, 75, 4251–4258. [Google Scholar] [CrossRef]
- Castillo-Lopez, E.; Pacífico, C.; Sener-Aydemir, A.; Hummel, K.; Nöbauer, K.; Ricci, S.; Rivera-Chacon, R.; Reisinger, N.; Razzazi-Fazeli, E.; Zebeli, Q. Diet and phytogenic supplementation substantially modulate the salivary proteome in dairy cows. J. Proteom. 2023, 273, 104795. [Google Scholar] [CrossRef]
- Schulz, B.L.; Cooper-White, J.; Punyadeera, C.K. Saliva proteome research: Current status and future outlook. Crit. Rev. Biotechnol. 2013, 33, 246–259. [Google Scholar] [CrossRef]
- Wang, J.; Liang, Y.; Wang, Y.; Cui, J.; Liu, M.; Du, W.; Xu, Y. Computational prediction of human salivary proteins from blood circulation and application to diagnostic biomarker identification. PLoS ONE 2013, 8, e80211. [Google Scholar] [CrossRef]
- Marco-Ramell, A.; Pato, R.; Peña, R.; Saco, Y.; Manteca, X.; De La Torre, J.R.; Bassols, A. Identification of serum stress biomarkers in pigs housed at different stocking densities. Vet. J. 2011, 190, e66–e71. [Google Scholar] [CrossRef]
- Hazard, D.; Fernandez, X.; Pinguet, J.; Chambon, C.; Letisse, F.; Portais, J.-C.; Wadih-Moussa, Z.; Rémignon, H.; Molette, C. Functional genomics of the muscle response to restraint and transport in chickens. J. Anim. Sci. 2011, 89, 2717–2730. [Google Scholar] [CrossRef]
- Cruzen, S.; Pearce, S.; Baumgard, L.; Gabler, N.; Huff-Lonergan, E.; Lonergan, S. Proteomic changes to the sarcoplasmic fraction of predominantly red or white muscle following acute heat stress. J. Proteom. 2015, 128, 141–153. [Google Scholar] [CrossRef]
- Zeng, T.; Jiang, X.; Li, J.; Wang, D.; Li, G.; Lu, L.; Wang, G. Comparative proteomic analysis of the hepatic response to heat stress in Muscovy and Pekin ducks: Insight into thermal tolerance related to energy metabolism. PLoS ONE 2013, 8, e76917. [Google Scholar] [CrossRef]
- Rakib, M.; Messina, V.; Gargiulo, J.; Lyons, N.; Garcia, S. Potential use of HSP70 as an indicator of heat stress in dairy cows—A review. J. Dairy Sci. 2024, 107, 11597–11610. [Google Scholar] [CrossRef]
- Baek, Y.-C.; Kim, M.; Jeong, J.-Y.; Oh, Y.-K.; Lee, S.-D.; Lee, Y.-K.; Ji, S.-Y.; Choi, H. Effects of short-term acute heat stress on physiological responses and heat shock proteins of Hanwoo steer (Korean cattle). J. Anim. Reprod. Biotechnol. 2019, 34, 173–182. [Google Scholar] [CrossRef]
- Chen, X.; Shu, H.; Sun, F.; Yao, J.; Gu, X. Impact of heat stress on blood, production, and physiological indicators in heat-tolerant and heat-sensitive dairy cows. Animals 2023, 13, 2562. [Google Scholar] [CrossRef]
- Humer, E.; Khol-Parisini, A.; Metzler-Zebeli, B.U.; Gruber, L.; Zebeli, Q. Alterations of the lipid metabolome in dairy cows experiencing excessive lipolysis early postpartum. PLoS ONE 2016, 11, e0158633. [Google Scholar] [CrossRef]
- Cairoli, F.; Battocchio, M.; Veronesi, M.C.; Brambilla, D.; Conserva, F.; Eberini, I.; Wait, R.; Gianazza, E. Serum protein pattern during cow pregnancy: Acute-phase proteins increase in the peripartum period. Electrophoresis 2006, 27, 1617–1625. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Bu, D.; Li, S.; Yuan, T.; Zhou, L.; Yang, J.; Sun, P. Comparative proteomics analysis of plasma proteins during the transition period in dairy cows with or without subclinical mastitis after calving. Czech J. Anim. Sci. 2012, 57, 481–489. [Google Scholar] [CrossRef]
- Lu, J.; Antunes Fernandes, E.; Páez Cano, A.E.; Vinitwatanakhun, J.; Boeren, S.; van Hooijdonk, T.; van Knegsel, A.; Vervoort, J.; Hettinga, K.A. Changes in milk proteome and metabolome associated with dry period length, energy balance, and lactation stage in postparturient dairy cows. J. Proteome Res. 2013, 12, 3288–3296. [Google Scholar] [CrossRef]
- Xu, W.; Van Knegsel, A.; Saccenti, E.; Van Hoeij, R.; Kemp, B.; Vervoort, J. Metabolomics of milk reflects a negative energy balance in cows. J. Proteome Res. 2020, 19, 2942–2949. [Google Scholar] [CrossRef]
- Di Luca, A.; Ianni, A.; Henry, M.; Martino, C.; Meleady, P.; Martino, G. Label-free quantitative proteomics and stress responses in pigs—The case of short or long road transportation. PLoS ONE 2022, 17, e0277950. [Google Scholar] [CrossRef]
- Fuente-García, C.; Sentandreu, M.A.; Aldai, N.; Oliván, M.; Sentandreu, E. Proteomic pipeline for biomarker hunting of defective bovine meat assisted by liquid chromatography-mass spectrometry analysis and chemometrics. J. Proteom. 2021, 238, 104153. [Google Scholar] [CrossRef]
- Herzberg, D.; Strobel, P.; Müller, H.; Meneses, C.; Werner, M.; Bustamante, H. Proteomic profiling of proteins in the dorsal horn of the spinal cord in dairy cows with chronic lameness. PLoS ONE 2020, 15, e0228134. [Google Scholar] [CrossRef]
- Yong, Y.; Li, J.; Yu, T.; Fang, B.; Liu, X.; Yu, Z.; Ma, X.; Gooneratne, R.; Abd El-Aty, A.; Ju, X. Overexpression of heat shock protein 70 induces apoptosis of intestinal epithelial cells in heat-stressed pigs: A proteomics approach. J. Therm. Biol. 2022, 108, 103289. [Google Scholar] [CrossRef]
- Arroyo, L.; Valent, D.; Carreras, R.; Pato, R.; Sabrià, J.; Velarde, A.; Bassols, A. Neurobiology of environmental enrichment in pigs: Hanges in monoaminergic neurotransmitters in several brain areas and in the hippocampal proteome. J. Proteom. 2020, 229, 103943. [Google Scholar] [CrossRef]
- Govindaiah, P.M.; Maheswarappa, N.B.; Banerjee, R.; Muthukumar, M.; Manohar, B.B.; Mishra, B.P.; Sen, A.R.; Biswas, A.K. Decoding halal and jhatka slaughter: Novel insights into welfare and protein biomarkers in slow-growing broiler chicken. J. Sci. Food Agric. 2024, 104, 9160–9168. [Google Scholar] [CrossRef]
- Kiran, M.; Maheswarappa, N.B.; Banerjee, R.; Ch, V.; Rapole, S. Impact of stunning before slaughter on expression of skeletal muscles proteome in sheep. Anim. Biotechnol. 2023, 34, 495–502. [Google Scholar] [CrossRef]
- McCabe, C.J.; Aryal, U.K.; Casey, T.; Boerman, J. Impact of exposure to chronic light–dark phase shifting circadian rhythm disruption on muscle proteome in periparturient dairy cows. Proteomes 2021, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Sammad, A.; Hu, L.; Luo, H.; Abbas, Z.; Umer, S.; Zhao, S.; Xu, Q.; Khan, A.; Wang, Y.; Zhu, H. Investigation of metabolome underlying the biological mechanisms of acute heat stressed granulosa cells. Int. J. Mol. Sci. 2022, 23, 2146. [Google Scholar] [CrossRef] [PubMed]
- Ceciliani, F.; Eckersall, D.; Burchmore, R.; Lecchi, C. Proteomics in veterinary medicine: Applications and trends in disease pathogenesis and diagnostics. Vet Pathol 2014, 51, 351–362. [Google Scholar] [CrossRef]
- Bilić, P.; Kuleš, J.; Galan, A.; Gomes de Pontes, L.; Guillemin, N.; Horvatić, A.; Festa Sabes, A.; Mrljak, V.; Eckersall, P.D. Proteomics in veterinary medicine and animal science: Neglected scientific opportunities with immediate impact. Proteomics 2018, 18, 1800047. [Google Scholar] [CrossRef]
- O’Reilly, E.L.; Viora, L.; Malcata, F.; Pepler, P.T.; Zadoks, R.; Brady, N.; Hanh, H.Q.; McLaughlin, M.; Horvatic, A.; Gelemanovic, A. Biomarker and proteome analysis of milk from dairy cows with clinical mastitis: Determining the effect of different bacterial pathogens on the response to infection. Res. Vet. Sci. 2024, 172, 105240. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.; Piras, C.; Kovačić, M.; Samardžija, M.; Ahmed, H.; De Canio, M.; Urbani, A.; Meštrić, Z.F.; Soggiu, A.; Bonizzi, L. Proteomics of inflammatory and oxidative stress response in cows with subclinical and clinical mastitis. J. Proteom. 2012, 75, 4412–4428. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.M.; Ibeagha, A.E.; Messier, S.; Zhao, X. Proteomics, genomics, and pathway analyses of Escherichia coli and Staphylococcus aureus infected milk whey reveal molecular pathways and networks involved in mastitis. J. Proteome Res. 2010, 9, 4604–4619. [Google Scholar] [CrossRef]
- Kim, Y.; Atalla, H.; Mallard, B.; Robert, C.; Karrow, N. Changes in Holstein cow milk and serum proteins during intramammary infection with three different strains of Staphylococcus aureus. BMC Vet. Res. 2011, 7, 1–13. [Google Scholar] [CrossRef]
- Lim, B.; Kim, S.; Lim, K.-S.; Jeong, C.-G.; Kim, S.-C.; Lee, S.-M.; Park, C.-K.; Te Pas, M.F.; Gho, H.; Kim, T.-H. Integrated time-serial transcriptome networks reveal common innate and tissue-specific adaptive immune responses to PRRSV infection. Vet. Res. 2020, 51, 1–18. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Zheng, H.; Shang, Y.; Guo, J.; Tian, H.; Lu, G.; Jin, Y.; He, J.; Cai, X. Proteomics analysis of porcine serum proteins by LC-MS/MS after foot-and-mouth disease virus (FMDV) infection. J. Vet. Med. Sci. 2011, 73, 1569–1572. [Google Scholar] [CrossRef]
- Sun, J.-f.; Shi, Z.-x.; Guo, H.-c.; Li, S.; Tu, C.-c. Proteomic analysis of swine serum following highly virulent classical swine fever virus infection. Virol. J. 2011, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Thongboonkerd, V.; Chiangjong, W.; Mares, J.; Moravec, J.; Tuma, Z.; Karvunidis, T.; Sinchaikul, S.; Chen, S.-T.; Opatrný Jr, K.; Matejovic, M. Altered plasma proteome during an early phase of peritonitis-induced sepsis. Clin. Sci. 2009, 116, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Sutton, K.; Nash, T.; Sives, S.; Borowska, D.; Mitchell, J.; Vohra, P.; Stevens, M.P.; Vervelde, L. Disentangling the innate immune responses of intestinal epithelial cells and lamina propria cells to Salmonella Typhimurium infection in chickens. Front. Microbiol. 2023, 14, 1258796. [Google Scholar] [CrossRef] [PubMed]
- Ducatelle, R.; Goossens, E.; De Meyer, F.; Eeckhaut, V.; Antonissen, G.; Haesebrouck, F.; Van Immerseel, F. Biomarkers for monitoring intestinal health in poultry: Present status and future perspectives. Vet. Res. 2018, 49, 43. [Google Scholar] [CrossRef]
- Baxter, M.F.; Latorre, J.D.; Dridi, S.; Merino-Guzman, R.; Hernandez-Velasco, X.; Hargis, B.M.; Tellez-Isaias, G. Identification of serum biomarkers for intestinal integrity in a broiler chicken malabsorption model. Front. Vet. Sci. 2019, 6, 144. [Google Scholar] [CrossRef]
- De Meyer, F.; Eeckhaut, V.; Ducatelle, R.; Dhaenens, M.; Daled, S.; Dedeurwaerder, A.; De Gussem, M.; Haesebrouck, F.; Deforce, D.; Van Immerseel, F. Host intestinal biomarker identification in a gut leakage model in broilers. Vet. Res. 2019, 50, 1–14. [Google Scholar] [CrossRef]
- Kaewsatuan, P.; Poompramun, C.; Kubota, S.; Yongsawatdigul, J.; Molee, W.; Uimari, P.; Molee, A. Comparative proteomics revealed duodenal metabolic function associated with feed efficiency in slow-growing chicken. Poult. Sci. 2022, 101, 101824. [Google Scholar] [CrossRef]
- Fonseca, L.D.; Eler, J.P.; Pereira, M.A.; Rosa, A.F.; Alexandre, P.A.; Moncau, C.T.; Salvato, F.; Rosa-Fernandes, L.; Palmisano, G.; Ferraz, J.B. Liver proteomics unravel the metabolic pathways related to feed efficiency in beef cattle. Sci. Rep. 2019, 9, 5364. [Google Scholar] [CrossRef]
- Regidor-Cerrillo, J.; Xia, D.; Jiménez-Pelayo, L.; García-Sánchez, M.; Collantes-Fernández, E.; Randle, N.; Wastling, J.; Ortega-Mora, L.-M.; Horcajo, P. Proteomic characterization of host-pathogen interactions during bovine trophoblast cell line infection by Neospora caninum. Pathogens 2020, 9, 749. [Google Scholar] [CrossRef]
- Adetunji, A.; Casey, T.; Franco, J.; Shah, D.; Fasina, Y. Proteomic analysis of the effect of Salmonella challenge on broiler chicken. Molecules 2022, 27, 7277. [Google Scholar] [CrossRef]
- Zubair, M.; Muhamed, S.A.; Khan, F.A.; Zhao, G.; Menghwar, H.; Faisal, M.; Zhang, H.; Zhu, X.; Rasheed, M.A.; Chen, Y. Identification of 60 secreted proteins for Mycoplasma bovis with secretome assay. Microb. Pathog. 2020, 143, 104135. [Google Scholar] [CrossRef] [PubMed]
- Korbonits, L.; Kleinwort, K.J.; Amann, B.; Didier, A.; Märtlbauer, E.; Hauck, S.M.; Deeg, C.A. Mycobacterium avium subsp. paratuberculosis infected cows reveal divergent immune response in bovine peripheral blood derived lymphocyte proteome. Metabolites 2022, 12, 924. [Google Scholar] [CrossRef]
- Wu, Z.-L.; Chen, S.-Y.; Hu, S.; Jia, X.; Wang, J.; Lai, S.-J. Metabolomic and proteomic profiles associated with ketosis in dairy cows. Front. Genet. 2020, 11, 551587. [Google Scholar] [CrossRef] [PubMed]
- He, J.-J.; Ma, J.; Wang, J.-L.; Zhang, F.-K.; Li, J.-X.; Zhai, B.-T.; Elsheikha, H.M.; Zhu, X.-Q. iTRAQ-based quantitative proteomics analysis identifies host pathways modulated during Toxoplasma gondii infection in swine. Microorganisms 2020, 8, 518. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Lu, M.; Aleem, M.T.; Zhang, Y.; Wang, M.; Wen, Z.; Song, X.; Xu, L.; Li, X.; Yan, R. Identification of excretory and secretory proteins from Haemonchus contortus inducing a Th9 immune response in goats. Vet. Res. 2022, 53, 36. [Google Scholar] [CrossRef]
- Du, X.; Zhou, D.; Zhou, J.; Xue, J.; Cheng, Z. Marek’s disease virus and Reticuloendotheliosis virus coinfection enhances viral replication and alters cellular protein profiles. Front. Vet. Sci. 2022, 9, 854007. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, S.; Fan, B.; Niu, B.; Guo, R.; Gu, J.; Gao, S.; Li, B. iTRAQ-based proteome analysis of porcine group A rotavirus-infected porcine IPEC-J2 intestinal epithelial cells. J. Proteom. 2021, 248, 104354. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Zhang, Y.; Yang, H. Quantitative proteomic analysis of porcine intestinal epithelial cells infected with porcine deltacoronavirus using iTRAQ-coupled LC-MS/MS. J. Proteome Res. 2020, 19, 4470–4485. [Google Scholar] [CrossRef]
- Alnakip, M.E.; Rhouma, N.R.; Abd-Elfatah, E.N.; Quintela-Baluja, M.; Böhme, K.; Fernández-No, I.; Bayoumi, M.A.; Abdelhafez, M.M.; Taboada-Rodriguez, A.; Calo-Mata, P. Discrimination of major and minor streptococci incriminated in bovine mastitis by MALDI-TOF MS fingerprinting and 16S rRNA gene sequencing. Res. Vet. Sci. 2020, 132, 426–438. [Google Scholar] [CrossRef]
- Rodrigues, P.M.; Martin, S.A.; Silva, T.S.; Boonanuntanasarn, S.; Schrama, D.; Moreira, M.; Raposo, C. Proteomics in fish and aquaculture research. Proteom. Domest. Anim. Farm Syst. Biol. 2018, 311–338. [Google Scholar]
- Carrera, M.; Piñeiro, C.; Martinez, I. Proteomic strategies to evaluate the impact of farming conditions on food quality and safety in aquaculture products. In Mass Spectrometry in Food Analysis; CRC Press: Boca Raton, FL, USA, 2022; pp. 43–59. [Google Scholar]
- Nissa, M.U.; Pinto, N.; Parkar, H.; Goswami, M.; Srivastava, S. Proteomics in fisheries and aquaculture: An approach for food security. Food Control 2021, 127, 108125. [Google Scholar] [CrossRef]
- Rajan, B.; Fernandes, J.M.; Caipang, C.M.; Kiron, V.; Rombout, J.H.; Brinchmann, M.F. Proteome reference map of the skin mucus of Atlantic cod (Gadus morhua) revealing immune competent molecules. Fish Shellfish Immunol. 2011, 31, 224–231. [Google Scholar] [CrossRef]
- Dietrich, M.A.; Arnold, G.J.; Nynca, J.; Fröhlich, T.; Otte, K.; Ciereszko, A. Characterization of carp seminal plasma proteome in relation to blood plasma. J. Proteom. 2014, 98, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Carrera, M.; Cañas, B.; Gallardo, J.M. The sarcoplasmic fish proteome: Pathways, metabolic networks and potential bioactive peptides for nutritional inferences. J. Proteom. 2013, 78, 211–220. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Hu, W. Changes in microbiological, physicochemical and muscle proteins of post mortem large yellow croaker (Pseudosciaena crocea). Food Control 2013, 34, 514–520. [Google Scholar] [CrossRef]
- Patel, D.M.; Brinchmann, M.F. Skin mucus proteins of lumpsucker (Cyclopterus lumpus). Biochem. Biophys. Rep. 2017, 9, 217–225. [Google Scholar] [CrossRef]
- Jurado, J.; Fuentes-Almagro, C.A.; Guardiola, F.A.; Cuesta, A.; Esteban, M.Á.; Prieto-Álamo, M.-J. Proteomic profile of the skin mucus of farmed gilthead seabream (Sparus aurata). J. Proteom. 2015, 120, 21–34. [Google Scholar] [CrossRef]
- Sanahuja, I.; Ibarz, A. Skin mucus proteome of gilthead sea bream: A non-invasive method to screen for welfare indicators. Fish Shellfish Immunol. 2015, 46, 426–435. [Google Scholar] [CrossRef]
- Borges, M.H.; Andrich, F.; Lemos, P.H.; Soares, T.G.; Menezes, T.N.; Campos, F.V.; Neves, L.X.; Castro-Borges, W.; Figueiredo, S.G. Combined proteomic and functional analysis reveals rich sources of protein diversity in skin mucus and venom from the Scorpaena plumieri fish. J. Proteom. 2018, 187, 200–211. [Google Scholar] [CrossRef]
- Li, B.; Gou, M.; Han, J.; Yuan, X.; Li, Y.; Li, T.; Jiang, Q.; Xiao, R.; Li, Q. Proteomic analysis of buccal gland secretion from fasting and feeding lampreys (Lampetra morii). Proteome Sci. 2018, 16, 9. [Google Scholar] [CrossRef]
- Nynca, J.; Arnold, G.J.; Fröhlich, T.; Otte, K.; Flenkenthaler, F.; Ciereszko, A. Proteomic identification of rainbow trout seminal plasma proteins. Proteomics 2014, 14, 133–140. [Google Scholar] [CrossRef]
- Matos, E.; Silva, T.S.; Wulff, T.; Valente, L.M.; Sousa, V.; Sampaio, E.; Gonçalves, A.; Silva, J.M.; de Pinho, P.G.; Dinis, M.T. Influence of supplemental maslinic acid (olive-derived triterpene) on the post-mortem muscle properties and quality traits of gilthead seabream. Aquaculture 2013, 396, 146–155. [Google Scholar] [CrossRef]
- Addis, M.F.; Cappuccinelli, R.; Tedde, V.; Pagnozzi, D.; Porcu, M.C.; Bonaglini, E.; Roggio, T.; Uzzau, S. Proteomic analysis of muscle tissue from gilthead sea bream (Sparus aurata L.) farmed in offshore floating cages. Aquaculture 2010, 309, 245–252. [Google Scholar] [CrossRef]
- Li, J.; Levitan, B.; Gomez-Jimenez, S.; Kültz, D. Development of a gill assay library for ecological proteomics of threespine sticklebacks (Gasterosteus aculeatus). Mol. Cell. Proteom. 2018, 17, 2146–2163. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Hummel, K.; Razzazi-Fazeli, E.; El-Matbouli, M. Proteome profiles of head kidney and spleen of rainbow trout (Oncorhynchus mykiss). Proteomics 2018, 18, 1800101. [Google Scholar] [CrossRef]
- Rajan, B.; Patel, D.M.; Kitani, Y.; Viswanath, K.; Brinchmann, M.F. Novel mannose binding natterin-like protein in the skin mucus of Atlantic cod (Gadus morhua). Fish Shellfish Immunol. 2017, 68, 452–457. [Google Scholar] [CrossRef]
- Jennings, S.; Stentiford, G.D.; Leocadio, A.M.; Jeffery, K.R.; Metcalfe, J.D.; Katsiadaki, I.; Auchterlonie, N.A.; Mangi, S.C.; Pinnegar, J.K.; Ellis, T. Aquatic food security: Insights into challenges and solutions from an analysis of interactions between fisheries, aquaculture, food safety, human health, fish and human welfare, economy and environment. Fish Fish. 2016, 17, 893–938. [Google Scholar] [CrossRef]
- Saleh, M.; Kumar, G.; Abdel-Baki, A.-A.; Dkhil, M.A.; El-Matbouli, M.; Al-Quraishy, S. Quantitative shotgun proteomics distinguishes wound-healing biomarker signatures in common carp skin mucus in response to Ichthyophthirius multifiliis. Vet. Res. 2018, 49, 1–12. [Google Scholar] [CrossRef]
- Wu, N.; Song, Y.-L.; Wang, B.; Zhang, X.-Y.; Zhang, X.-J.; Wang, Y.-L.; Cheng, Y.-Y.; Chen, D.-D.; Xia, X.-Q.; Lu, Y.-S. Fish gut-liver immunity during homeostasis or inflammation revealed by integrative transcriptome and proteome studies. Sci. Rep. 2016, 6, 36048. [Google Scholar] [CrossRef]
- Causey, D.R.; Kim, J.-H.; Stead, D.A.; Martin, S.A.; Devlin, R.H.; Macqueen, D.J. Proteomic comparison of selective breeding and growth hormone transgenesis in fish: Unique pathways to enhanced growth. J. Proteom. 2019, 192, 114–124. [Google Scholar] [CrossRef]
- Liu, P.-f.; Du, Y.; Meng, L.; Li, X.; Yang, D.; Liu, Y. Phosphoproteomic analyses of kidneys of Atlantic salmon infected with Aeromonas salmonicida. Sci. Rep. 2019, 9, 2101. [Google Scholar] [CrossRef]
- Babaheydari, S.B.; Keyvanshokooh, S.; Dorafshan, S.; Johari, S.A. Proteomic analysis of skeletal deformity in diploid and triploid rainbow trout (Oncorhynchus mykiss) larvae. Comp. Biochem. Physiol. Part D Genom. Proteom. 2016, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Fu, J.P.; Li, N.; Liu, Z.-X.; Qin, T.; Zhang, X.; Nie, P. Immunogenic proteins and their vaccine development potential evaluation in outer membrane proteins (OMPs) of Flavobacterium columnare. Aquac. Fish. 2016, 1, 1–8. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Z.; Zang, M.; Liu, Y.; Lu, C. Identification of Omp38 by immunoproteomic analysis and evaluation as a potential vaccine antigen against Aeromonas hydrophila in Chinese breams. Fish Shellfish Immunol. 2013, 34, 74–81. [Google Scholar] [CrossRef]
- Pang, H.Y.; Li, Y.; Wu, Z.H.; Jian, J.C.; Lu, Y.S.; Cai, S.H. Immunoproteomic analysis and identification of novel immunogenic proteins from Vibrio harveyi. J. Appl. Microbiol. 2010, 109, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Shahin, K.; Thompson, K.D.; Inglis, N.F.; Mclean, K.; Ramirez-Paredes, J.G.; Monaghan, S.J.; Hoare, R.; Fontaine, M.; Metselaar, M.; Adams, A. Characterization of the outer membrane proteome of Francisella noatunensis subsp. orientalis. J. Appl. Microbiol. 2018, 125, 686–699. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Wolfenden, D.C.; Thomson, J.S. Stress management and welfare. In Fish physiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 35, pp. 463–539. [Google Scholar]
- Barreto, M.O.; Rey Planellas, S.; Yang, Y.; Phillips, C.; Descovich, K. Emerging indicators of fish welfare in aquaculture. Rev. Aquac. 2022, 14, 343–361. [Google Scholar] [CrossRef]
- Rise, M.L.; Martyniuk, C.J.; Chen, M. Comparative physiology and aquaculture: Toward Omics-enabled improvement of aquatic animal health and sustainable production. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 31, 100603. [Google Scholar] [CrossRef]
- Ye, H.; Lin, Q.; Luo, H. Applications of transcriptomics and proteomics in understanding fish immunity. Fish Shellfish Immunol. 2018, 77, 319–327. [Google Scholar] [CrossRef]
- Karim, M.; Puiseux-Dao, S.; Edery, M. Toxins and stress in fish: Proteomic analyses and response network. Toxicon 2011, 57, 959–969. [Google Scholar] [CrossRef]
- Alves, R.N.; Cordeiro, O.; Silva, T.S.; Richard, N.; de Vareilles, M.; Marino, G.; Di Marco, P.; Rodrigues, P.M.; Conceição, L.E. Metabolic molecular indicators of chronic stress in gilthead seabream (Sparus aurata) using comparative proteomics. Aquaculture 2010, 299, 57–66. [Google Scholar] [CrossRef]
- Cordero, H.; Morcillo, P.; Cuesta, A.; Brinchmann, M.F.; Esteban, M.A. Differential proteome profile of skin mucus of gilthead seabream (Sparus aurata) after probiotic intake and/or overcrowding stress. J. Proteom. 2016, 132, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Veiseth-Kent, E.; Grove, H.; Færgestad, E.M.; Fjæra, S.O. Changes in muscle and blood plasma proteomes of Atlantic salmon (Salmo salar) induced by crowding. Aquaculture 2010, 309, 272–279. [Google Scholar] [CrossRef]
- Smith, R.W.; Moccia, R.D.; Seymour, C.B.; Mothersill, C.E. Irradiation of rainbow trout at early life stages results in a proteomic legacy in adult gills. Part A; proteomic responses in the irradiated fish and in non-irradiated bystander fish. Environ. Res. 2018, 163, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, M.A.; Ciereszko, A. Proteomic characterization of fresh spermatozoa and supernatant after cryopreservation in relation to freezability of carp (Cyprinus carpio L.) semen. PLoS ONE 2018, 13, e0192972. [Google Scholar] [CrossRef]
- Dietrich, M.A.; Irnazarow, I.; Ciereszko, A. Proteomic identification of seminal plasma proteins related to the freezability of carp semen. J. Proteom. 2017, 162, 52–61. [Google Scholar] [CrossRef]
- Nuez-Ortín, W.G.; Carter, C.G.; Nichols, P.D.; Cooke, I.R.; Wilson, R. Liver proteome response of pre-harvest Atlantic salmon following exposure to elevated temperature. BMC Genom. 2018, 19, 1–13. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, X.; Hu, Y.; Xu, J.; Wang, T.; Yin, S. iTRAQ-based quantitative proteomic analysis of Takifugu fasciatus liver in response to low-temperature stress. J. Proteom. 2019, 201, 27–36. [Google Scholar] [CrossRef]
- Schauer, K.L.; Reddam, A.; Xu, E.G.; Wolfe, L.M.; Grosell, M. Interrogation of the Gulf toadfish intestinal proteome response to hypersalinity exposure provides insights into osmoregulatory mechanisms and regulation of carbonate mineral precipitation. Comp. Biochem. Physiol. Part D: Genom. Proteom. 2018, 27, 66–76. [Google Scholar] [CrossRef]
- Bresolin de Souza, K.; Jutfelt, F.; Kling, P.; Förlin, L.; Sturve, J. Effects of increased CO2 on fish gill and plasma proteome. PLoS ONE 2014, 9, e102901. [Google Scholar] [CrossRef]
- Eyckmans, M.; Benoot, D.; Van Raemdonck, G.A.; Zegels, G.; Van Ostade, X.W.; Witters, E.; Blust, R.; De Boeck, G. Comparative proteomics of copper exposure and toxicity in rainbow trout, common carp and gibel carp. Comp. Biochem. Physiol. Part D Genom. Proteom. 2012, 7, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mahanty, A.; Mohanty, S.; Mazumder, D.G.; Cash, P.; Mohanty, B.P. Identification of potential biomarkers of hepatotoxicity by plasma proteome analysis of arsenic-exposed carp Labeo rohita. J. Hazard. Mater. 2017, 336, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Martyniuk, C.J.; Zha, J.; Wang, Z. Brain quantitative proteomic responses reveal new insight of benzotriazole neurotoxicity in female Chinese rare minnow (Gobiocypris rarus). Aquat. Toxicol. 2016, 181, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zha, J.; Martyniuk, C.J.; Wang, Z.; Zhao, J. Histopathological and proteomic responses in male Chinese rare minnow (Gobiocypris rarus) indicate hepatotoxicity following benzotriazole exposure. Environ. Pollut. 2017, 229, 459–469. [Google Scholar] [CrossRef]
- Enerstvedt, K.S.; Sydnes, M.O.; Pampanin, D.M. Study of the plasma proteome of Atlantic cod (Gadus morhua): Effect of exposure to two PAHs and their corresponding diols. Chemosphere 2017, 183, 294–304. [Google Scholar] [CrossRef]
- Berg, K.; Puntervoll, P.; Klungsøyr, J.; Goksøyr, A. Brain proteome alterations of Atlantic cod (Gadus morhua) exposed to PCB 153. Aquat. Toxicol. 2011, 105, 206–217. [Google Scholar] [CrossRef]
- Wu, X.; Lu, C.; Dong, X.; Zhang, Z.; Yang, M.; Xu, H. Proteomics analysis of zebrafish brain following chronically exposed to bisphenol A. Toxicol. Environ. Chem. 2017, 99, 469–481. [Google Scholar] [CrossRef]
- Biales, A.D.; Bencic, D.C.; Flick, R.L.; Blocksom, K.A.; Lazorchak, J.M.; Lattier, D.L. Proteomic analysis of a model fish species exposed to individual pesticides and a binary mixture. Aquat. Toxicol. 2011, 101, 196–206. [Google Scholar] [CrossRef]
- Simmons, D.B.; Cowie, A.M.; Koh, J.; Sherry, J.P.; Martyniuk, C.J. Label-free and iTRAQ proteomics analysis in the liver of zebrafish (Danio rerio) following dietary exposure to the organochlorine pesticide dieldrin. J. Proteom. 2019, 202, 103362. [Google Scholar] [CrossRef]
- Lin, H.D.; Hsu, L.S.; Chien, C.C.; Chen, S.C. Proteomic analysis of ametryn toxicity in zebrafish embryos. Environ. Toxicol. 2018, 33, 579–586. [Google Scholar] [CrossRef]
- Sonesson, A.K.; Hallerman, E.; Humphries, F.; Hilsdorf, A.; Leskien, D.; Rosendal, K.; Bartley, D.; Hu, X.; Garcia Gomez, R.; Mair, G. Sustainable management and improvement of genetic resources for aquaculture. J. World Aquac. Soc. 2023, 54, 364–396. [Google Scholar] [CrossRef]
- Engdaw, F.; Geremew, A. Broodstock nutrition in Nile tilapia and its implications on reproductive efficiency. Front. Aquac. 2024, 3, 1281640. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Fostier, A.; Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010, 165, 516–534. [Google Scholar] [CrossRef]
- Uddin, M.N.; Das, A.K.; Sarker, M.A.; Roy, D.; Mithun, M.N.A.S.; Rahman, S.; Uddin, M.S. Problems and Its Related Factors Affecting the Hatchery Owners in Producing Fish Seeds in Rural Bangladesh. Agric. Res. 2024, 1–12. [Google Scholar] [CrossRef]
- Yilmaz, O.; Patinote, A.; Nguyen, T.V.; Com, E.; Lavigne, R.; Pineau, C.; Sullivan, C.V.; Bobe, J. Scrambled eggs: Proteomic portraits and novel biomarkers of egg quality in zebrafish (Danio rerio). PLoS ONE 2017, 12, e0188084. [Google Scholar] [CrossRef]
- Nynca, J.; Arnold, G.J.; Fröhlich, T.; Ciereszko, A. Shotgun proteomics of rainbow trout ovarian fluid. Reprod. Fertil. Dev. 2015, 27, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Kohn, Y.Y.; Symonds, J.E.; Kleffmann, T.; Nakagawa, S.; Lagisz, M.; Lokman, P.M. Proteomic analysis of early-stage embryos: Implications for egg quality in hapuku (Polyprion oxygeneios). Fish Physiol. Biochem. 2015, 41, 1403–1417. [Google Scholar] [CrossRef]
- Gombar, R.; Pitcher, T.E.; Lewis, J.A.; Auld, J.; Vacratsis, P.O. Proteomic characterization of seminal plasma from alternative reproductive tactics of Chinook salmon (Oncorhynchus tswatchysha). J. Proteom. 2017, 157, 1–9. [Google Scholar] [CrossRef]
- Nynca, J.; Arnold, G.J.; Fröhlich, T.; Ciereszko, A. Cryopreservation-induced alterations in protein composition of rainbow trout semen. Proteomics 2015, 15, 2643–2654. [Google Scholar] [CrossRef]
- Vilgis, T.A. The physics of the mouthfeel of caviar and other fish roe. Int. J. Gastron. Food Sci. 2020, 19, 100192. [Google Scholar] [CrossRef]
- Keyvanshokooh, S.; Vaziri, B. Proteome analysis of Persian sturgeon (Acipenser persicus) ova. Anim. Reprod. Sci. 2008, 109, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, M.D.; Tiplady, K.; Lopdell, T.; Law, T.A.; Scott, A.; Harland, C.; Sherlock, R.; Henty, K.; Obolonkin, V.; Lehnert, K. Expression variants of the lipogenic AGPAT6 gene affect diverse milk composition phenotypes in Bos taurus. PLoS ONE 2014, 9, e85757. [Google Scholar] [CrossRef]
- Janjanam, J.; Singh, S.; Jena, M.K.; Varshney, N.; Kola, S.; Kumar, S.; Kaushik, J.K.; Grover, S.; Dang, A.K.; Mukesh, M. Comparative 2D-DIGE proteomic analysis of bovine mammary epithelial cells during lactation reveals protein signatures for lactation persistency and milk yield. PLoS ONE 2014, 9, e102515. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Sabherwal, M.; Duncan, E.; Stevens, S.; Stockwell, P.; McConnell, M.; Bekhit, A.E.-D.; Carne, A. In-depth characterization of sheep (Ovis aries) milk whey proteome and comparison with cow (Bos taurus). PLoS ONE 2015, 10, e0139774. [Google Scholar] [CrossRef]
- Ali, A.; Shaalan, W.M.; Al-Tobasei, R.; Salem, M. Coding and noncoding genes involved in atrophy and compensatory muscle growth in Nile Tilapia. Cells 2022, 11, 2504. [Google Scholar] [CrossRef]
- Oliveira, J.; Oliva-Teles, A.; Couto, A. Tracking Biomarkers for the Health and Welfare of Aquaculture Fish. Fishes 2024, 9, 289. [Google Scholar] [CrossRef]
- Hartman, S.O. Genomic Studies of Novel Behavior Traits in Lactating Sows and their Relationship to Heat Stress Resilience and Maternal Performance. Master’s Thesis, Purdue University, West Lafayette, IN, USA, 2024. [Google Scholar]
- da Silva, J.; Andrade, L.; Rodrigues, P.; Cordeiro, L.; Lima, G.; Lopes, J.; Castillo, E.; Martins, R.; Assunção, A.; Vieira, J. Plasma Proteome Alterations of Laying Hens Subjected to Heat Stress and Fed a Diet Supplemented with Pequi Oil (Caryocar brasiliense Camb.): New Insights in the Identification of Heat Stress Biomarkers. Biomolecules 2024, 14, 1424. [Google Scholar] [CrossRef]
- Houston, R.D.; Kriaridou, C.; Robledo, D. Animal board invited review: Widespread adoption of genetic technologies is key to sustainable expansion of global aquaculture. Animal 2022, 16, 100642. [Google Scholar] [CrossRef]
- Purushothaman, K.; Das, P.P.; Presslauer, C.; Lim, T.K.; Johansen, S.D.; Lin, Q.; Babiak, I. Proteomics analysis of early developmental stages of zebrafish embryos. Int. J. Mol. Sci. 2019, 20, 6359. [Google Scholar] [CrossRef]
- Yan, J.; Ding, Y.; Peng, Z.; Qin, L.; Gu, J.; Wan, C. Systematic Proteomics Study on the Embryonic Development of Danio rerio. J. Proteome Res. 2023, 22, 2814–2826. [Google Scholar] [CrossRef]
- Adnane, M.; de Almeida, A.M.; Chapwanya, A. Unveiling the power of proteomics in advancing tropical animal health and production. Trop. Anim. Health Prod. 2024, 56, 182. [Google Scholar] [CrossRef]
- Eckersall, P.D. Proteins, proteomics, and the dysproteinemias. Clin. Biochem. Domest. Anim. 2008, 6, 117–155. [Google Scholar]
- Plews, M.; Lamoureux, L.; Simon, S.L.; Graham, C.; Ruddat, V.; Czub, S.; Knox, J.D. Factors affecting the accuracy of urine-based biomarkers of BSE. Proteome Sci. 2011, 9, 1–11. [Google Scholar] [CrossRef]
- Gajahin Gamage, N.T.; Miyashita, R.; Takahashi, K.; Asakawa, S.; Senevirathna, J.D.M. Proteomic applications in aquatic environment studies. Proteomes 2022, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, C.; Arena, S.; Talamo, F.; Ledda, L.; Renzone, G.; Ferrara, L.; Scaloni, A. Comparative proteomic analysis of mammalian animal tissues and body fluids: Bovine proteome database. J. Chromatogr. B 2005, 815, 157–168. [Google Scholar] [CrossRef]
- Rodrigues, P.M.; Silva, T.S.; Dias, J.; Jessen, F. Proteomics in aquaculture: Applications and trends. J. Proteom. 2012, 75, 4325–4345. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.; Franco, C.; Pires, E.; Ventosa, M.; Palhinhas, R.; Koci, K.; de Almeida, A.M.; Coelho, A.V. Mass spectrometry and animal science: Protein identification strategies and particularities of farm animal species. J. Proteom. 2012, 75, 4190–4206. [Google Scholar] [CrossRef] [PubMed]
- Couvillion, S.P.; Mostoller, K.E.; Williams, J.E.; Pace, R.M.; Stohel, I.L.; Peterson, H.K.; Nicora, C.D.; Nakayasu, E.S.; Webb-Robertson, B.-J.M.; McGuire, M.A. Interrogating the role of the milk microbiome in mastitis in the multi-omics era. Front. Microbiol. 2023, 14, 1105675. [Google Scholar] [CrossRef]
- Kusumawati, A.; Mustopa, A.; Wibawan, I.; Setiyono, A.; Sudarwanto, M. Metagenomic analysis of pathogen mastitis in cow’s milk from Cicurug, Sukabumi, West Java, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 762, 012064. [Google Scholar] [CrossRef]
- Luo, M.; Zhu, W.; Liang, Z.; Feng, B.; Xie, X.; Li, Y.; Liu, Y.; Shi, X.; Fu, J.; Miao, L. High-temperature stress response: Insights into the molecular regulation of American shad (Alosa sapidissima) using a multi-omics approach. Sci. Total Environ. 2024, 916, 170329. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Liu, X.; Feng, W.; Liu, S.; Zhuang, Z. Analyses of the molecular mechanisms associated with salinity adaption of Trachidermus fasciatus through combined iTRAQ-based proteomics and RNA sequencing-based transcriptomics. Prog. Biophys. Mol. Biol. 2018, 136, 40–53. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Liu, C.; Qin, R.; Gong, D.; Wang, R.; Zhang, D.; Che, L.; Chen, D.; Xin, G. Multi-omics profiling highlights lipid metabolism alterations in pigs fed low-dose antibiotics. BMC Genet. 2020, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bagger, F.O.; Probst, V. Single cell sequencing in cancer diagnostics. Single-Cell Seq. Methylation: Methods Clin. Appl. 2020, 1255, 175–193. [Google Scholar]
- Kania, S.; Reed, S.; Thomford, J.; BonDurant, R.; Hirata, K.; Corbeil, R.; North, M.; Corbeil, L. Degradation of bovine complement C3 by trichomonad extracellular proteinase. Vet. Immunol. Immunopathol. 2001, 78, 83–96. [Google Scholar] [CrossRef]
- Codeluppi, S.; Borm, L.E.; Zeisel, A.; La Manno, G.; van Lunteren, J.A.; Svensson, C.I.; Linnarsson, S. Spatial organization of the somatosensory cortex revealed by osmFISH. Nat. Methods 2018, 15, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Fancello, L.; Burger, T. An analysis of proteogenomics and how and when transcriptome-informed reduction of protein databases can enhance eukaryotic proteomics. Genome Biol. 2022, 23, 132. [Google Scholar] [CrossRef]
- Menschaert, G.; Fenyö, D. Proteogenomics from a bioinformatics angle: A growing field. Mass Spectrom. Rev. 2017, 36, 584–599. [Google Scholar] [CrossRef]
- Dhindwal, P.; Thompson, C.; Kos, D.; Planedin, K.; Jain, R.; Jelinski, M.; Ruzzini, A. A neglected and emerging antimicrobial resistance gene encodes for a serine-dependent macrolide esterase. Proc. Natl. Acad. Sci. USA 2023, 120, e2219827120. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Byrne, K.A.; Reverter, A.; Harper, G.S.; Taniguchi, M.; McWilliam, S.M.; Mannen, H.; Oyama, K.; Lehnert, S.A. Transcriptional profiling of skeletal muscle tissue from two breeds of cattle. Mamm. Genome 2005, 16, 201–210. [Google Scholar] [CrossRef]
- Mohanty, B.P.; Mohanty, S.; Mitra, T.; Mahanty, A.; Ganguly, S.; Singh, S. Omics technology in fisheries and aquaculture. Adv. Fish Res. 2019, 7, 1–30. [Google Scholar]
- Vitorino, R. Transforming clinical research: The power of high-throughput omics integration. Proteomes 2024, 12, 25. [Google Scholar] [CrossRef]
- Quazi, S. Artificial intelligence and machine learning in precision and genomic medicine. Med. Oncol. 2022, 39, 120. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Kumar, C.; Zeng, W.-F.; Strauss, M.T. Artificial intelligence for proteomics and biomarker discovery. Cell Syst. 2021, 12, 759–770. [Google Scholar] [CrossRef]
- Singh, A.; Shannon, C.P.; Gautier, B.; Rohart, F.; Vacher, M.; Tebbutt, S.J.; Lê Cao, K.-A. DIABLO: An integrative approach for identifying key molecular drivers from multi-omics assays. Bioinformatics 2019, 35, 3055–3062. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, W.; Lv, C.; Guo, M.; Yang, M.; Fu, Q.; Liu, X. Advancements in artificial intelligence technology for improving animal welfare: Current applications and research progress. Anim. Res. One Health 2024, 2, 93–109. [Google Scholar] [CrossRef]
- Hughes, C.S.; Sorensen, P.H.; Morin, G.B. A standardized and reproducible proteomics protocol for bottom-up quantitative analysis of protein samples using SP3 and mass spectrometry. Proteom. Biomark. Discov. Methods Protoc. 2019, 1959, 65–87. [Google Scholar]
- Gawor, A.; Bulska, E. A Standardized Protocol for Assuring the Validity of Proteomics Results from Liquid Chromatography–High-Resolution Mass Spectrometry. Int. J. Mol. Sci. 2023, 24, 6129. [Google Scholar] [CrossRef]
- Griss, J.; Jones, A.R.; Sachsenberg, T.; Walzer, M.; Gatto, L.; Hartler, J.; Thallinger, G.G.; Salek, R.M.; Steinbeck, C.; Neuhauser, N. The mzTab data exchange format: Communicating mass-spectrometry-based proteomics and metabolomics experimental results to a wider audience. Mol. Cell. Proteom. 2014, 13, 2765–2775. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jitjumnong, J.; Taweechaipaisankul, A.; Lin, J.-C.; Wongchanla, S.; Chuwatthanakhajorn, S.; Lin, C.-J.; Khang, L.T.P.; Linh, N.V.; Sangsawad, P.; Dinh-Hung, N.; et al. An Overview of Advancements in Proteomic Approaches to Enhance Livestock Production and Aquaculture. Animals 2025, 15, 1946. https://doi.org/10.3390/ani15131946
Jitjumnong J, Taweechaipaisankul A, Lin J-C, Wongchanla S, Chuwatthanakhajorn S, Lin C-J, Khang LTP, Linh NV, Sangsawad P, Dinh-Hung N, et al. An Overview of Advancements in Proteomic Approaches to Enhance Livestock Production and Aquaculture. Animals. 2025; 15(13):1946. https://doi.org/10.3390/ani15131946
Chicago/Turabian StyleJitjumnong, Jakree, Anukul Taweechaipaisankul, Jou-Ching Lin, Supatirada Wongchanla, Schwann Chuwatthanakhajorn, Chih-Jen Lin, Luu Tang Phuc Khang, Nguyen Vu Linh, Papungkorn Sangsawad, Nguyen Dinh-Hung, and et al. 2025. "An Overview of Advancements in Proteomic Approaches to Enhance Livestock Production and Aquaculture" Animals 15, no. 13: 1946. https://doi.org/10.3390/ani15131946
APA StyleJitjumnong, J., Taweechaipaisankul, A., Lin, J.-C., Wongchanla, S., Chuwatthanakhajorn, S., Lin, C.-J., Khang, L. T. P., Linh, N. V., Sangsawad, P., Dinh-Hung, N., Tang, P.-C., & Moonmanee, T. (2025). An Overview of Advancements in Proteomic Approaches to Enhance Livestock Production and Aquaculture. Animals, 15(13), 1946. https://doi.org/10.3390/ani15131946







