1. Introduction
Cashmere goats are an important economic livestock species renowned for producing high-quality cashmere. They are widely distributed globally and have a long history of breeding, particularly in countries such as China, Mongolia, and Iran [
1]. They have a medium build and strong adaptability, enabling them to survive and reproduce in harsh environmental conditions [
1]. The cashmere produced by cashmere goats has a soft texture and excellent warmth retention [
2]. It is praised as the “soft gold” or “fiber gemstone” and serves as a precious raw material for high-end textiles [
2]. Textiles made from cashmere goat hair, including cashmere sweaters, scarves, and coats, are favored by consumers for their lightweight, warm, and comfortable wearing experience, and enjoy a great reputation in the international market [
3]. In recent years, with the increasing demand for high-quality natural fibers, the market demand for cashmere has continued to rise, driving the rapid development of the cashmere goat industry [
3]. However, the production and quality of cashmere goat hair are influenced by various factors, with the morphological development of secondary hair follicles (SHFs) during the embryonic period being crucial in determining cashmere yield [
3]. Therefore, in-depth research into the regulatory mechanisms of SHF development in cashmere goats is of great significance for improving cashmere yield and quality and promoting the sustainable development of the cashmere goat industry.
Hair follicles are crucial appendages of the skin, located within the dermis, and serve as key organs regulating hair growth, shedding, and regeneration [
1]. SHFs produce cashmere, and their degree of development directly determines the yield and quality of cashmere [
2]. The development of cashmere goat hair follicles begins in the embryonic period and is a highly complex and orderly process [
3]. Studies have shown that the development of primary hair follicles (PHFs) in cashmere goats precedes that of SHFs [
3]. Specifically, by embryonic day 45, the fetal skin has formed a complete epidermal structure; by day 55, PHFs begin to develop, with hair bud structures emerging; by day 65, PHF hair buds continue to grow and penetrate deeper into the dermal layer of the skin; and by day 75, SHFs begin to develop, with SHF hair bud structures appearing for the first time [
3,
4]. Notably, the developmental state of SHFs during the embryonic period directly determines the cashmere density and yield of cashmere goats after birth [
4]. Therefore, in-depth research into the developmental process of SHFs in cashmere goats during the embryonic period is of great significance for revealing the molecular mechanisms of cashmere formation, screening for key regulatory factors, and improving the yield and quality of cashmere.
Hair follicle development relies on precise signaling between the dermis and epidermis. The dermal papilla (DP), which serves as the signaling hub of hair follicles, is derived from the proliferation and differentiation of dermal fibroblasts and is a crucial structure for ensuring normal hair follicle growth [
2]. Studies have shown that embryonic hair follicle development is precisely regulated by various signaling pathways and regulatory factors, including Wnt, BMP, and FGF signaling pathways, as well as miRNAs and lncRNAs [
5]. LncRNAs, which were once considered “noise” in the transcription process, have recently been found to play important regulatory roles in hair follicle development and hair growth [
5]. For example, lncRNA MSTRG.20890.1 and lncRNA MSTRG.14227.1 can inhibit the expression of the
ADAMTS3 gene by targeting miRNAs, thereby inhibiting the proliferation and migration of dermal fibroblasts in cashmere goats [
1,
2]. LncRMA-627.1 can competitively bind to miR-29a-5p, which is also targeted by
EDAR, thereby regulating the formation of the hair follicle placode [
6]. LncRNA FABP_AS promotes
DKK1 gene expression by competitively binding to chi-miR-335-5p, inhibiting the activity of the Wnt/β-catenin signaling pathway, and thereby regulating the proliferation of hair follicle stem cells [
7]. In addition, Zhang et al. identified 521 differentially expressed lncRNAs in the embryonic skin of cashmere goats through high-throughput sequencing technology, with lncRNA H19, in particular, significantly promoting the viability and proliferation of DP cells through the chi-miR-214-3p/β-catenin axis [
8]. Although studies have gradually revealed some key signaling molecules regulating hair follicle development in cashmere goats, the specific mechanisms of lncRNAs in SHF development remain poorly understood and require further exploration.
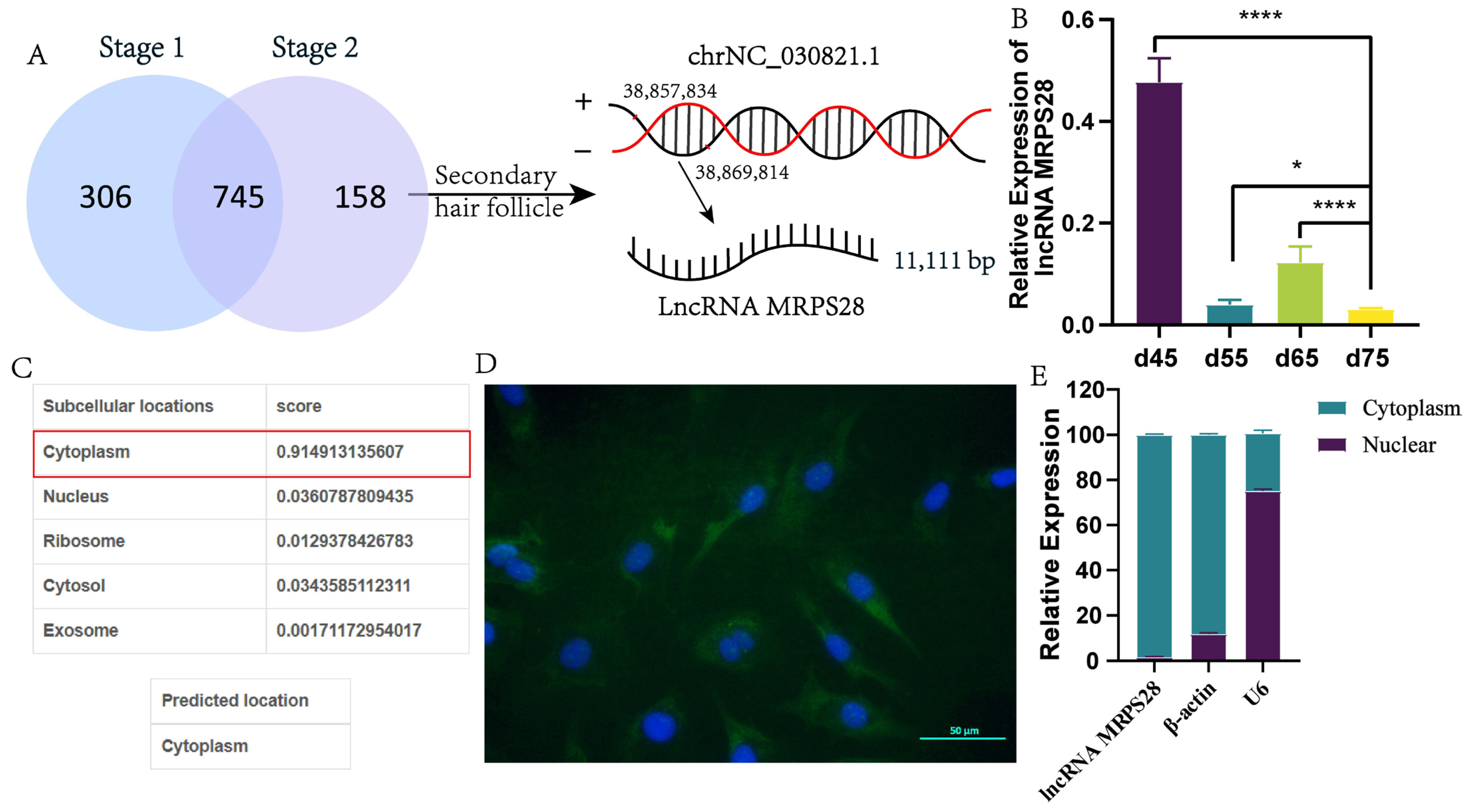
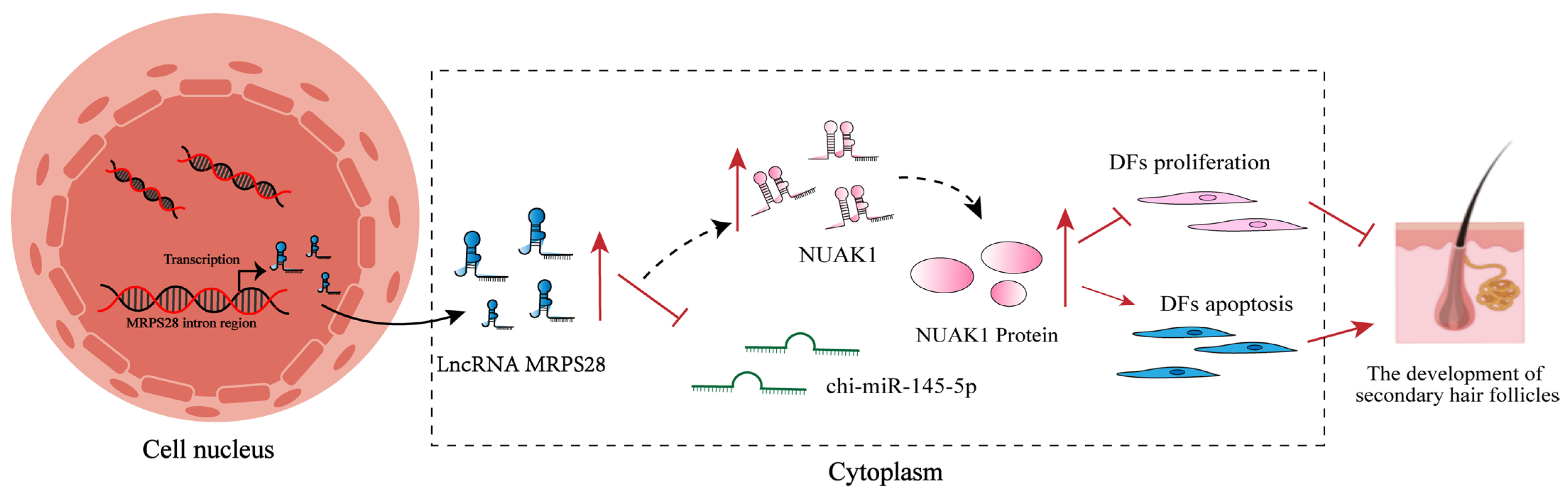
Based on the embryonic skin transcriptome database of cashmere goats previously constructed by our research group [
3], this study screened 158 lncRNAs related to SHF morphogenesis. Further analysis revealed that lncRNA MRPS28, transcribed from the intron region of the
MRPS28 gene, is significantly underexpressed at the critical stage of SHF development (embryonic day 75). Functional studies showed that lncRNA MRPS28 can sponge chi-miR-145-5p, relieving its inhibitory effect on the
NUAK1 gene, thereby upregulating
NUAK1 expression and inhibiting the proliferation and migration of dermal fibroblasts. However, this inhibitory effect may be achieved by reducing the proportion of cells in the S-phase of the cell cycle. This study aims to deeply explore the molecular mechanism by which lncRNA MRPS28 regulates the proliferation and migration of dermal fibroblasts through the chi-miR-145-5p/NUAK1 axis, and thereby affects the morphogenesis of SHFs in cashmere goats, so as to enrich the regulatory network of lncRNA in the development of SHFs in cashmere goats and provide a theoretical basis and molecular targets for improving the quality and yield of cashmere from cashmere goats.
2. Materials and Methods
2.1. Skin Sample Collection
The experimental animals utilized in the study were obtained from the Inner Mongolia Jinlai Animal Husbandry Technology Co., Ltd. (Hohhot, China). Twelve three-year-old ewes of Inner Mongolia cashmere goats with good production performance and the same growth environment were selected for synchronous estrus treatment. According to the insemination records of the ewes, skin tissue samples were collected from the fetuses of cashmere goats at different developmental stages (45, 55, 65, and 75 days), with three fetuses sampled at each stage. To ensure the preservation of their biological integrity and to maintain the quality of the samples for future analysis, these skin samples were promptly stored at −80 °C.
2.2. Screening of LncRNAs Related to Secondary Hair Follicle Morphogenesis
We utilized the established transcriptome database of cashmere goat skin from different embryonic stages (45, 55, 65, and 75 days) to identify lncRNAs that were differentially expressed with a |log2foldchange| ≥ 1 and
p ≤ 0.05 [
3]. Based on the characteristics of SHFs’ morphogenesis [
4], we designated d55vsd45, d65vsd45, and d65vsd55 as Stage 1, which is related to the development of PHFs. Similarly, we designated d75vsd45, d75vsd55, and d75vsd65 as Stage 2, all of which are associated with the development of both PHFs and SHFs. Subsequently, we identified the intersection of Stage 1 and Stage 2 and excluded this intersection from the definition of Stage 2, leaving the remaining part of Stage 2 as the important lncRNAs specifically related to the development of SHFs.
2.3. Subcellular Localization
LncLocator 1.0 software [
9] was used to predict the subcellular location of the lncRNA MRPS28. Subsequently, the cytoplasm and nucleus of the dermal fibroblasts were separated using a cytoplasmic and nuclear RNA purification kit (Norgen Biotek, Thorold, ON, Canada), and RNA was extracted from both. Following extraction, qRT-PCR was performed to detect the expression levels of lncRNA MRPS28 in the cytoplasmic and nuclear fractions.
2.4. FISH Assay
The dermal fibroblasts were adjusted to the desired density. Subsequently, the cells were fixed with 4% paraformaldehyde for 20 min, treated with Hyb buffer reagent for 1 h, and incubated overnight with the mixture of Hyb buffer and the probe. Afterward, the prehybridization solution was removed, and blocking serum was added. The cells were then incubated with BSA for 30 min at room temperature. Lastly, the cells were stained with DAPI solution for 8 min, washed to remove the staining solution, and examined under a fluorescence microscope.
2.5. Dual-Luciferase Report Detection
The dual-luciferase reporting system was used to verify the targeting relationship between a miRNA and its target gene. Specifically, miRNA mimics were co-transfected with psiCHECK2 vectors containing either wild-type (WT) or mutant (MUT) sequences of the target gene, using LipoFiter as the transfection reagent (Hanheng, Shanghai, China). Luciferase activity was measured 48 h post-transfection to assess the interaction between the miRNA and its target sequence. In the dual-luciferase assay, the wild-type plasmid refers to the one containing the original sequence of the target gene, which is used to simulate the normal function of the gene. The mutant plasmids are artificially modified sequences of the target gene (such as point mutations or deletions), which are employed to investigate the impact of sequence alterations on gene function.
2.6. Cell Culture and Transfection
Dermal fibroblasts were cultured in a medium containing 10% fetal bovine serum, essential for their growth. To ensure optimal conditions for cell growth and proliferation, all cell lines were maintained at 37 °C with 5% CO2. Based on the sequence information of lncRNA MRPS28 and NUAK1, the interference vectors targeting lncRNA MRPS28 and NUAK1 were constructed, respectively (Hanheng, Shanghai, China). Then, these interference vectors were transfected into dermal fibroblasts using lentivirus-mediated transfection. After transfection, the successfully transfected dermal fibroblasts were screened with purinomycin. During the experiment, we regarded the dermal fibroblasts that were not transfected as the blank control (NC). Meanwhile, we considered the dermal fibroblasts transfected with the empty vector as the negative control (sh-NC), and those transfected with the target interference sequence as the experimental group.
2.7. Cell Proliferation Assays
The proliferation of cells was evaluated through two methods: CCK8 and EdU. The CCK8 kit (Solarbio, Beijing, China) was utilized to assess cell proliferation, with the absorbance value of each well determined using an enzyme-labeling instrument at a wavelength of 450 nm. Additionally, the EdU assay was performed according to the guidelines provided by the BeyoClick™ EdU-555 Cell Proliferation Detection Kit (Beyotime, Shanghai, China). This assay determined the proliferation rate by calculating the ratio of cells labeled with EdU to those labeled with Hoechst.
2.8. DNA Staining
The cell cycle was determined using the DNA content quantification method (Solarbio, Beijing, China). To prepare the cells for analysis, they were first fixed in 70% ethanol, resuspended, and incubated at 4 °C. After a 24-hour incubation, RNase A was added, and the cells were further incubated at 37 °C for 30 min. Subsequently, a propidium iodide staining solution was added to stain the DNA. The cells were then incubated at 4 °C in the dark for an additional 30 min to ensure adequate staining, and the cell cycle was detected by flow cytometry (CytoFLEX, Beckman, Brea, CA, USA).
2.9. Cell Apoptosis Detection
Cell apoptosis was determined using the Annexin V-APC/PI Kit (Elabscience Biotechnology, Wuhan, China). A single-cell suspension was prepared and stained with reagents. After incubating in the dark for 20 min, cell apoptosis was detected by flow cytometry (CytoFLEX, Beckman, Brea, CA, USA).
2.10. Cell Scratch Assay
The cell scratch assay was conducted by scraping a monolayer of cells to create a wound [
1]. Images of the wounded area were captured immediately after scraping (0 h post-scratch) and again after a 24-hour incubation period. Before scraping, the cells were washed with PBS to remove any debris or unattached cells. Following the scratch, fresh serum-free medium was added to the cells, which were then incubated under standard conditions for 24 h. Cell migration into the wounded area was evaluated by comparing the images taken at 0 h and 24 h post-scratch. The cell migration rate was calculated using the formula: [(0 h area − 24 h area)/0 h area] × 100%.
2.11. Statistical Analysis
Statistical analysis was conducted using SPSS 26.0 (IBM, SPSS, Chicago, IL, USA) and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA). All data are presented as mean ± SD from ≥3 independent experiments. Student’s t-tests determined significance with p < 0.05 or p < 0.01.
4. Discussion
Cashmere goats are an important economic animal, and the quality and yield of their cashmere are directly related to the financial benefits of the breeding industry [
3]. The growth and development of cashmere is a complex biological process that involves the regulation of multiple genes and signaling pathways [
2]. In recent years, thanks to the rapid development of molecular biology techniques, the role of lncRNA in hair follicle development has increasingly become a research focus [
5]. Studies have demonstrated that lncRNA-HOTAIR is expressed throughout both the growth and resting phases of SHFs in cashmere goats, with expression levels significantly higher during the growth phase compared to the resting phase [
16]. This suggests its potential involvement in the reconstruction of SHFs and the formation and growth of cashmere [
16]. Additionally, lncRNA-H19 significantly promotes the viability and proliferation of DP cells, and this regulatory effect is mediated through the chi-miR-214-3p/β-catenin axis [
8]. Furthermore, lncRNA PlncRNA-1 may enhance the proliferation and differentiation of hair follicle stem cells by upregulating the Wnt/β-catenin signaling pathway mediated by
TGF-β1 [
11].
In this study, we screened 158 lncRNAs related to SHF morphogenesis from a transcriptome database of skin samples collected from cashmere goats at different embryonic stages, which we had previously constructed [
3]. Among these, lncRNA MRPS28, transcribed from the intron region of the
MRPS28 gene, exhibited significantly low expression during SHF morphogenesis. Through experimental analysis and validation, we discovered that lncRNA MRPS28 can act as a sponge for chi-miR-145-5p, effectively alleviating the inhibitory effect of chi-miR-145-5p on
NUAK1 expression in dermal fibroblasts.
Hair follicles, which are important appendages of mammalian skin, are composed of various cell types and play a crucial role in hair growth, replacement, and the maintenance of skin’s physiological functions [
2]. During hair follicle development, dermal fibroblasts are the most crucial participants, ultimately differentiating into the DP, a structure that serves as the “signaling hub” of hair follicles [
2]. This structure directly regulates hair follicle development, cyclic growth, and hair production through intercellular interactions and signal transmission [
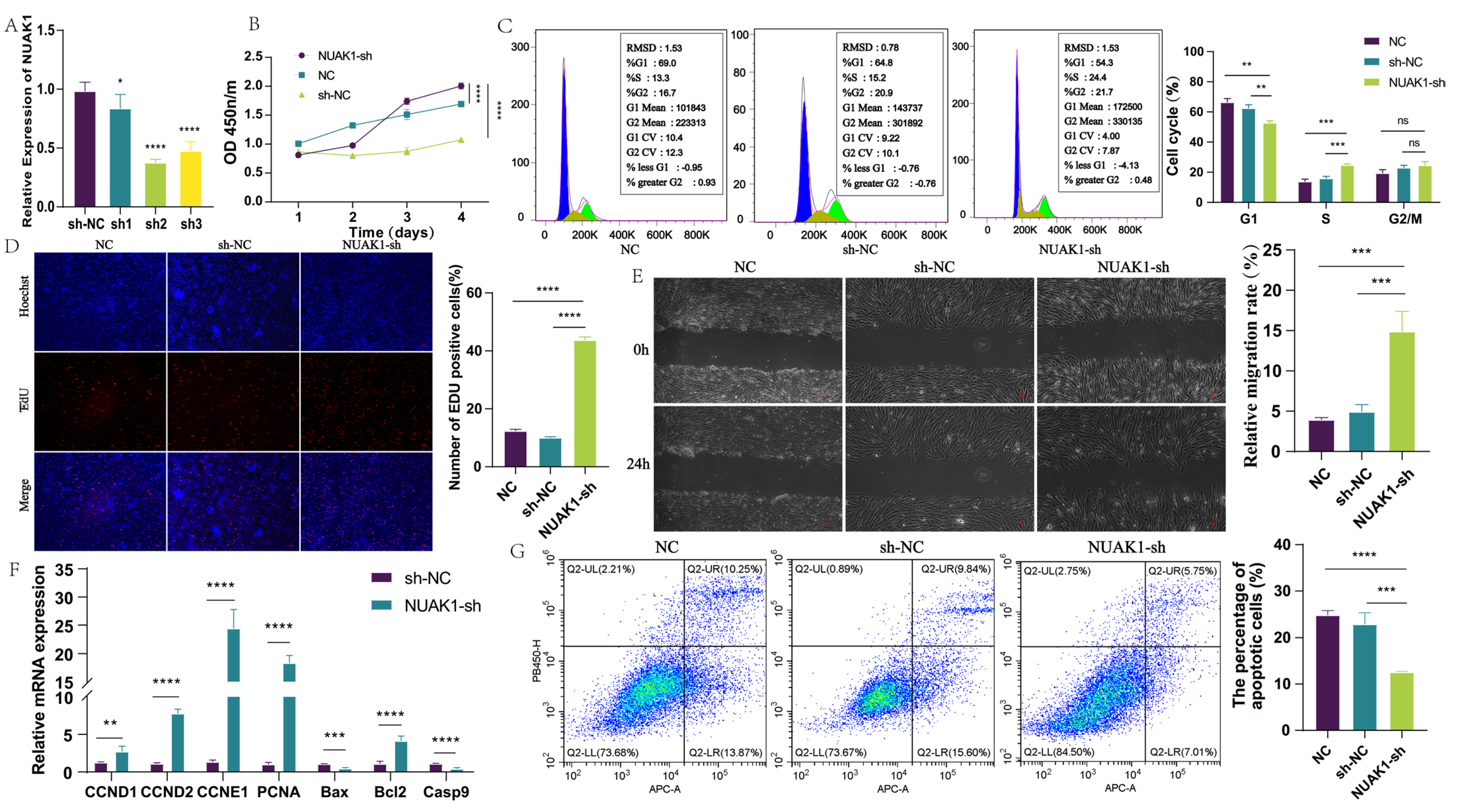
2]. In light of this, we investigated the effects of lncRNA MRPS28 on the phenotypes of dermal fibroblasts and found that it is primarily located in the cytoplasm. Interference with lncRNA MRPS28 significantly enhanced the proliferation and migration capabilities of dermal fibroblasts. In recent years, an increasing number of studies have shown that lncRNAs play extensive regulatory roles in organisms, one of which is their important function as sponge molecules that regulate miRNAs. For example, lncRNA MSTRG.20890.1 upregulates
ADAMTS3 expression by competitively binding to chi-miR-24-3p, thereby promoting the proliferation of dermal fibroblasts [
1]; lncRNA-XIST competes with
Shh for binding to miR-424, activating the Hedgehog signaling pathway and promoting dermal papilla-mediated hair follicle regeneration [
12]. Additionally, lncRNA FABP_AS competitively binds to chi-miR-335-5p, which promotes
DKK1 gene expression and reduces Wnt/β-catenin signaling pathway activity, thereby inhibiting the proliferation of hair follicle stem cells [
7]. These studies all indicate that lncRNAs can indirectly regulate hair follicle development by interacting with various key factors and signaling pathways.
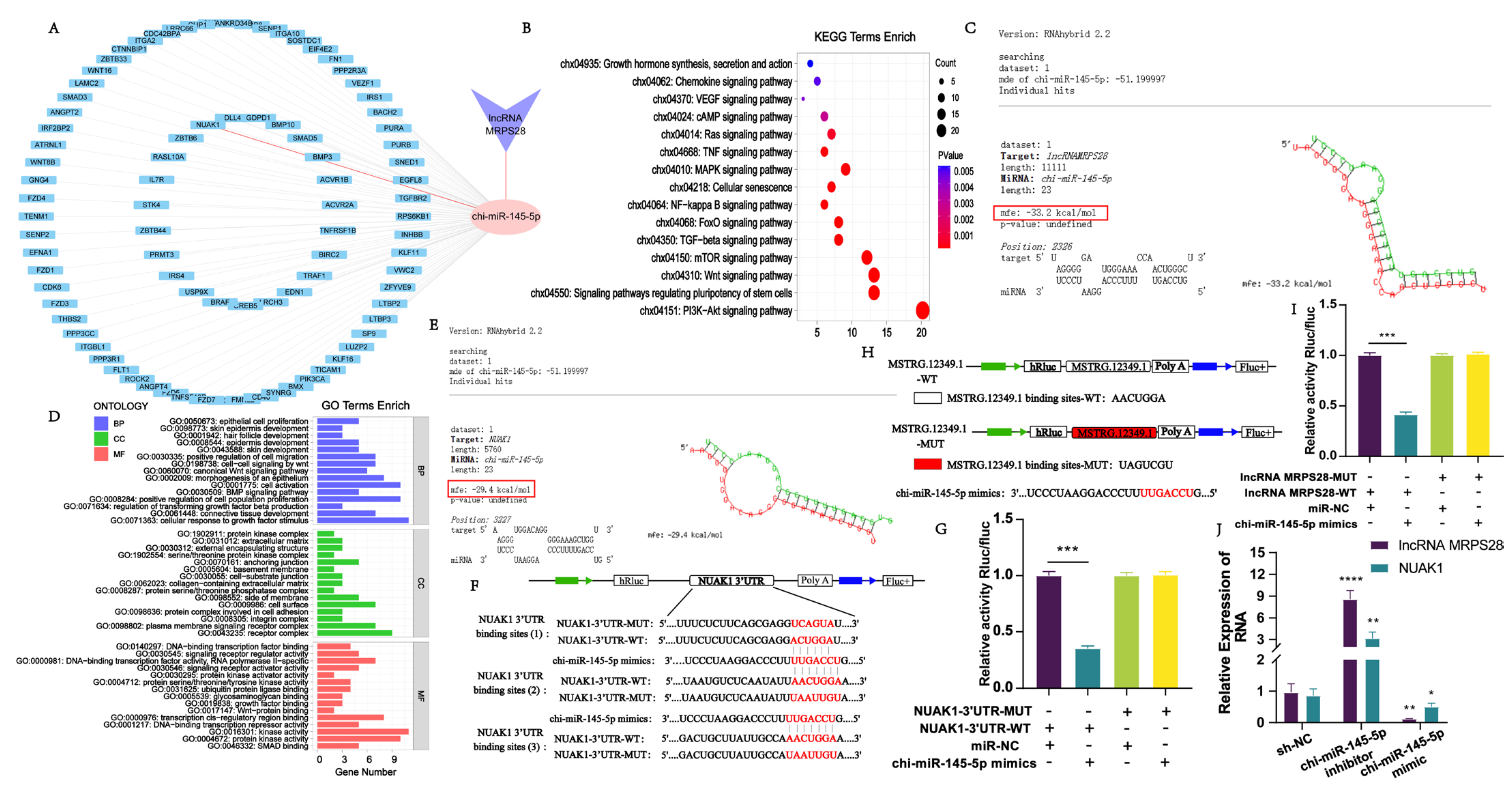
In this study, we employed bioinformatics methods to predict the miRNAs that are targeted by lncRNA MRPS28, as well as the genes targeted by these miRNAs. Through analysis, we found that lncRNA MRPS28 has a targeted binding site for chi-miR-145-5p. chi-miR-145-5p is one of the important miRNAs during the growth and development of hair follicles. Previous studies have demonstrated that chi-miR-145-5p can promote the differentiation of hair follicle stem cells by regulating the expression of the
NANOG and
Sox9 genes [
13]. Given these findings, in this study, we targeted chi-miR-145-5p as the miRNA of interest to further predict its target genes. Through bioinformatics analysis, it was revealed that chi-miR-145-5p has potential binding sites in 88 genes. Enrichment analysis of the target genes of chi-miR-145-5p found that they are mainly enriched in Wnt, TGF-β, PI3K-Akt, and FoxO signaling pathways, which are related to hair follicles. The FoxO signaling pathway is crucial in organisms, widely participating in multiple important physiological processes such as cell cycle regulation, cell apoptosis, and metabolic regulation [
14]. Studies have shown that among the Fox family,
FOXM1,
FOXN1,
FOXE1, and
FOXI3 are associated with hair follicle growth, development, and hair formation [
15,
17,
18,
19]. Among the 88 target genes, we found that the
NUAK1 gene is enriched in the FoxO signaling pathway and plays an important role in this pathway. Therefore, we selected this gene for further research. The results of qRT-PCR and dual-luciferase reporter assays showed that both lncRNA MRPS28 and
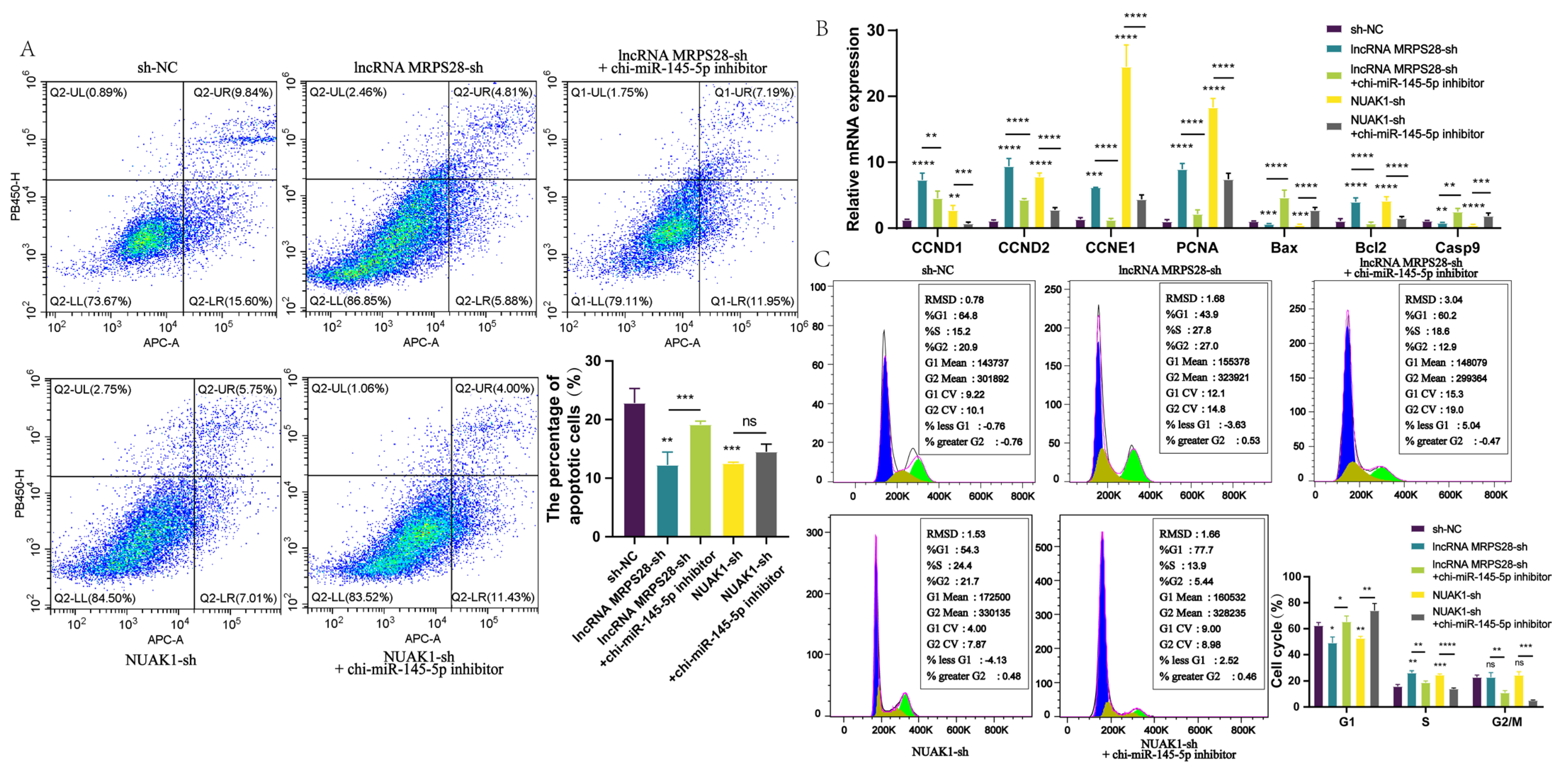
NUAK1 have binding sites for chi-miR-145-5p, indicating the existence of the lncRNA MRPS28/chi-miR-145-5p/NUAK1 regulatory network. Furthermore, we validated this regulatory network at the cellular level. By adding chi-miR-145-5p inhibitors to both the lncRNA MRPS28-sh and NUAK1-sh cell lines, we found that the promotional effects on dermal fibroblast proliferation and migration, as well as the inhibitory effect on apoptosis, caused by lncRNA MRPS28-sh and NUAK1-sh, could be counteracted by the chi-miR-145-5p inhibitor. This further demonstrates that in cashmere goat dermal fibroblasts, lncRNA MRPS28 indeed inhibits the proliferation of dermal fibroblasts and promotes their apoptosis through the chi-miR-145-5p/NUAK1 signaling axis, thereby regulating the formation of the DP structure and the morphogenesis of SHFs.
In summary, this study reveals the crucial role of lncRNA MRPS28 in the morphogenesis of SHFs, effectively regulating the proliferation and migration capabilities of dermal fibroblasts. The DP, as the “command center” for hair follicle development, is formed through continuous proliferation and differentiation of dermal fibroblasts and plays a decisive role in the normal growth and development of hair follicles [
2]. We further confirm that lncRNA MRPS28, as a ceRNA for chi-miR-145-5p, can upregulate the expression of
NUAK1, thereby indirectly affecting the development of SHFs. This discovery not only enriches the regulatory network of lncRNAs in SHF development but also provides new theoretical support and potential molecular targets for improving the quality and yield of cashmere in cashmere goats.