Supplementation of a Homeopathic Complex in the Diet of Castrated Male and Female Nursery Piglets and Its Effects on Behavior
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
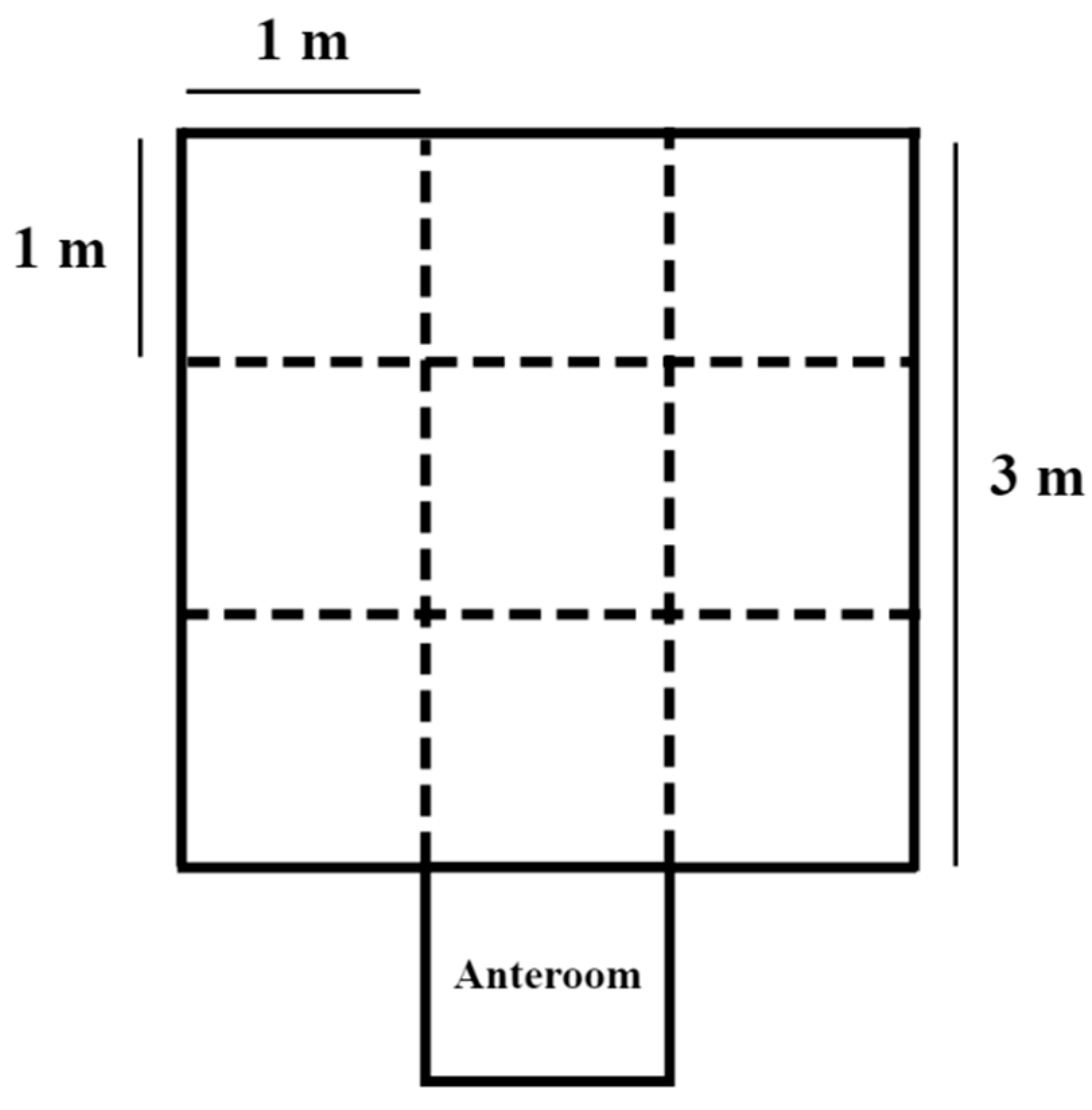
2.1. Animals, Experimental Design, and Housing
2.2. Composition of the Homeopathic Complex
2.3. Pre-Dietary Treatments Observations
2.4. Behavioral Tests
2.5. Open Field and Novel Object Tests
2.6. Sociability Test
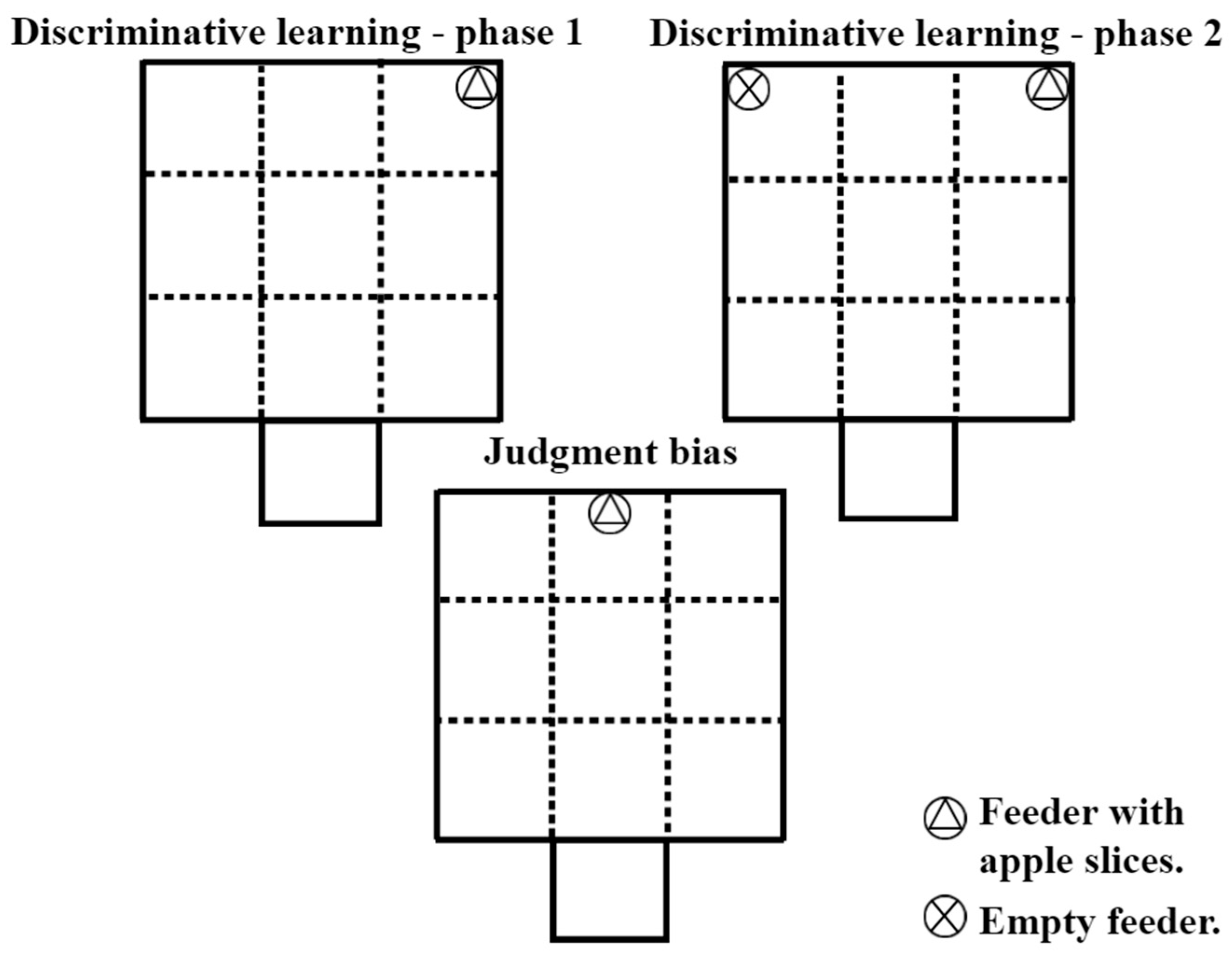
2.7. Discriminative Learning
2.8. Judgment Bias Test
2.9. Reactivity During Weighing
2.10. Statistical Analysis
3. Results
3.1. Open-Field Test
3.2. Novel Object Test
3.3. Sociability
3.4. Discriminative Learning and Judgment Bias Test
3.5. Reactivity During Weighing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The Biological Stress of Early Weaned Piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Kye, Y.C.; Kim, G.; Kim, H.W.; Gu, M.J.; Umboh, J.; Maaruf, K.; Kim, S.W.; Yun, C.H. Stress, Nutrition, and Intestinal Immune Responses in Pigs—A Review. Asian-Australas. J. Anim. Sci. 2016, 29, 1075. [Google Scholar] [CrossRef]
- Levine, S. Developmental Determinants of Sensitivity and Resistance to Stress. Psychoneuroendocrinology 2005, 30, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Cukaci, C.; Freissmuth, M.; Mann, C.; Marti, J.; Sperl, V. Against All Odds-the Persistent Popularity of Homeopathy. Wien. Klin. Wochenschr. 2020, 132, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.S.; Genova, J.L.; Barbosa, K.A.; Rupolo, P.E.; Azevedo, L.B.; Gregory, C.R.; Carvalho, S.T.; Nunes, R.V.; Carvalho, P.L.O.; Faveri, J.C. Addition of Homeopathic Products to Pig Diets in the Finishing Phase Promotes Improvement in Growth Performance. S. Afr. J. Anim. Sci. 2023, 52, 482–491. [Google Scholar] [CrossRef]
- Soto, F.R.M.; Vuaden, E.R.; Coelho, C.; Benites, N.R.; Bonamin, L.V.; de Azevedo, S.S. A Randomized Controlled Trial of Homeopathic Treatment of Weaned Piglets in a Commercial Swine Herd. Homeopathy 2008, 97, 202–205. [Google Scholar] [CrossRef]
- Camerlink, I.; Ellinger, L.; Bakker, E.J.; Lantinga, E.A. Homeopathy as Replacement to Antibiotics in the Case of Escherichia coli Diarrhoea in Neonatal Piglets. Homeopathy 2010, 99, 57–62. [Google Scholar] [CrossRef]
- Zigovski, G.; Bez, I.C.C.; Garrido, L.F.C.; Rodrigues, C.C.; de Oliveira, A.C.d.F.; Rupolo, P.E.; de Azevedo, L.B.; Hernandez, E.M.E.; Genova, J.L.; Weber, S.H.; et al. Ultra-Diluted Complex Additive in the Diet Reveals Benefits in the Intestinal Tract of Nursery-Phase Piglets. Vet. Res. Commun. 2024, 48, 2385–2395. [Google Scholar] [CrossRef]
- Gordinho Pinto, S.A.; Bohland, E.; de Paula Coelho, C.; Furquim de Azevedo Morgulis, M.S.; Bonamin, L.V. An Animal Model for the Study of Chamomilla in Stress and Depression: Pilot Study. Homeopathy 2008, 97, 141–144. [Google Scholar] [CrossRef]
- Marzotto, M.; Conforti, A.; Magnani, P.; Zanolin, M.E.; Bellavite, P. Effects of Ignatia Amara in Mouse Behavioural Models. Homeopathy 2012, 101, 57–67. [Google Scholar] [CrossRef]
- Mutlu, O.; Ulak, G.; Kokturk, S.; Celikyurt, I.K.; Akar, F.; Erden, F. Effects of Homeopathic Anax Imperator on Behavioural and Pain Models in Mice. Homeopathy 2015, 104, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.S.L.D.S.; Pardo, P.E.; Oba, E.; Kronka, S.D.N.; Frazatti-Gallina, N.M. Matricaria Chamomilla CH12 Decreases Handling Stress in Nelore Calves. J. Vet. Sci. 2006, 7, 189–192. [Google Scholar] [CrossRef]
- Anser, H.; Ikram, R.; Khatoon, H.; Naeem, S.; Khan, S.S.; Nazim, U.; Imam, S.; Shafiq, Y.; Ishaque, S. Comparison of the Antidepressant like Activity of Homeopathic Remedies (Argentum nitricum, Staphysagria and Ignatia amara) and Their Effect on the Behavior of Rodents. Pak. J. Pharm. Sci. 2020, 33, 937–945. [Google Scholar] [CrossRef]
- Lakshmipathy, R.; Ruckmani, A.; Venkatesan, D.; Madhusudhanan, N.; Pavithra, R. Anxiolytic Effect of Homeopathic Preparation of Pulsatilla Nigricans in Swiss Albino Mice. Homeopathy 2012, 101, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Clouard, C.; Resmond, R.; Prunier, A.; Tallet, C.; Merlot, E. Exploration of Early Social Behaviors and Social Styles in Relation to Individual Characteristics in Suckling Piglets. Sci. Rep. 2022, 12, 2318. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.A.; Dilger, R.N. Young Pigs Exhibit Differential Exploratory Behavior during Novelty Preference Tasks in Response to Age, Sex, and Delay. Behav. Brain Res. 2017, 321, 50–60. [Google Scholar] [CrossRef]
- Mieloch, F.J.; Nietfeld, S.; Straßburg, C.; Krieter, J.; Grosse Beilage, E.; Czycholl, I. Factors of Potential Influence on Different Behavioural Tests in Fattening Pigs. Appl. Anim. Behav. Sci. 2020, 222, 104900. [Google Scholar] [CrossRef]
- Li, Y.Z.; Kerr, B.J.; Kidd, M.T.; Gonyou, H.W. Use of Supplementary Tryptophan to Modify the Behavior of Pigs. J. Anim. Sci. 2006, 84, 212–220. [Google Scholar] [CrossRef]
- Pond, W.G.; Mersmann, H.J.; Su, D.; McGlone, J.J.; Wheeler, M.B.; Smith, E.O.B. Neonatal Dietary Cholesterol and Alleles of Cholesterol 7-α Hydroxylase Affect Piglet Cerebrum Weight, Cholesterol Concentration, and Behavior. J. Nutr. 2008, 138, 282–286. [Google Scholar] [CrossRef]
- Clouard, C.; Reimert, I.; Fleming, S.A.; Koopmans, S.J.; Schuurman, T.; Hauser, J. Dietary Sialylated Oligosaccharides in Early-Life May Promote Cognitive Flexibility during Development in Context of Obesogenic Dietary Intake. Nutr. Neurosci. 2022, 25, 2461–2478. [Google Scholar] [CrossRef]
- Rostagno, H.S.; Teixeira Albino, L.F.; Hannas, E.I.; Lopes Donzele, J.; Sakomura, N.K.; Perazzo, F.G.; Saraiva, A.; Lobão Teixeira, M.; Borges Rodrigues, P.; De Oliveira, R.F.; et al. Tabelas Brasileiras Para Aves e Suínos. In Composição de Alimentos e Exigências Nutricionais; Departamento de Zootecnia, Universidade Federal de Viçosa (UFV): Viçosa, Brazil, 2017; p. 488. ISBN 9788560249725. [Google Scholar]
- Fels, M.; Hoy, S.; Hartung, J. Influence of Origin Litter on Social Rank, Agonistic Behaviour and Growth Performance of Piglets after Weaning. Appl. Anim. Behav. Sci. 2012, 139, 225–232. [Google Scholar] [CrossRef]
- Puppe, B.; Ernst, K.; Schön, P.C.; Manteuffel, G. Cognitive Enrichment Affects Behavioural Reactivity in Domestic Pigs. Appl. Anim. Behav. Sci. 2007, 105, 75–86. [Google Scholar] [CrossRef]
- Haigh, A.; Chou, J.Y.; O’Driscoll, K. Variations in the Behavior of Pigs During an Open Field and Novel Object Test. Front. Vet. Sci. 2020, 7, 607. [Google Scholar] [CrossRef] [PubMed]
- Colpoys, J.D.; Abell, C.E.; Gabler, N.K.; Keating, A.F.; Millman, S.T.; Siegford, J.M.; Young, J.M.; Johnson, A.K. Feed Efficiency Effects on Barrow and Gilt Behavioral Reactivity to Novel Stimuli Tests. J. Anim. Sci. 2015, 93, 1267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dalmau, A.; Fabrega, E.; Velarde, A. Fear Assessment in Pigs Exposed to a Novel Object Test. Appl. Anim. Behav. Sci. 2009, 117, 173–180. [Google Scholar] [CrossRef]
- Leliveld, L.M.C.; Düpjan, S.; Tuchscherer, A.; Puppe, B. Vocal Correlates of Emotional Reactivity within and across Contexts in Domestic Pigs (Sus scrofa). Physiol. Behav. 2017, 181, 117–126. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- Bünger, B.; Schrader, L.; Schrade, H.; Zacharias, B. Agonistic Behaviour, Skin Lesions and Activity Pattern of Entire Male, Female and Castrated Male Finishing Pigs. Appl. Anim. Behav. Sci. 2015, 171, 64–68. [Google Scholar] [CrossRef]
- Cronin, G.M.; Dunshea, F.R.; Butler, K.L.; McCauley, I.; Barnett, J.L.; Hemsworth, P.H. The Effects of Immuno- and Surgical-Castration on the Behaviour and Consequently Growth of Group-Housed, Male Finisher Pigs. Appl. Anim. Behav. Sci. 2003, 81, 111–126. [Google Scholar] [CrossRef]
- Pilkington, K.; Kirkwood, G.; Rampes, H.; Fisher, P.; Richardson, J. Homeopathy for Anxiety and Anxiety Disorders: A Systematic Review of the Research. Homeopathy 2006, 95, 151–162. [Google Scholar] [CrossRef]
- Bacqué-cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef]
- Shri, R.; Bhutani, K.K.; Sharma, A. A New Anxiolytic Fatty Acid from Aethusa cynapium. Fitoterapia 2010, 81, 1053–1057. [Google Scholar] [CrossRef] [PubMed]
- Fels, M.; Hartung, J.; Hoy, S. Social Hierarchy Formation in Piglets Mixed in Different Group Compositions after Weaning. Appl. Anim. Behav. Sci. 2014, 152, 17–22. [Google Scholar] [CrossRef]
- Carreras, R.; Mainau, E.; Rodriguez, P.; Llonch, P.; Dalmau, A.; Manteca, X.; Velarde, A. Cognitive Bias in Pigs: Individual Classification and Consistency over Time. J. Vet. Behav. 2015, 10, 577–581. [Google Scholar] [CrossRef]
- Asher, L.; Friel, M.; Griffin, K.; Collins, L.M. Mood and Personality Interact to Determine Cognitive Biases in Pigs. Biol. Lett. 2016, 12, 20160402. [Google Scholar] [CrossRef]
- Carreras, R.; Arroyo, L.; Mainau, E.; Peña, R.; Bassols, A.; Dalmau, A.; Faucitano, L.; Manteca, X.; Velarde, A. Effect of Gender and Halothane Genotype on Cognitive Bias and Its Relationship with Fear in Pigs. Appl. Anim. Behav. Sci. 2016, 177, 12–18. [Google Scholar] [CrossRef]
- Gois, F.D.; Cairo, P.L.G.; de Souza Cantarelli, V.; do Bomfim Costa, L.C.; Fontana, R.; Allaman, I.B.; Sbardella, M.; de Carvalho Júnior, F.M.; Costa, L.B. Effect of Brazilian Red Pepper (Schinus terebinthifolius Raddi) Essential Oil on Performance, Diarrhea and Gut Health of Weanling Pigs. Livest. Sci. 2016, 183, 24–27. [Google Scholar] [CrossRef]
| Behavior | Description | Tests |
|---|---|---|
| Explored quadrants | Number of quadrants explored (quantity) | Open Field |
| Time exploring the arena | Time spent exploring the arena (seconds) | Open Field |
| Vocalizations | Screams, squeals, or grunt-squeals (quantity) | Open Field and Reactivity during weighing |
| Frequency | Number of times the piglet touched the object (quantity) | Novel Object |
| Duration of contact | Contact time with the object (seconds) | Novel Object |
| Fighting | Fight between two piglets (seconds) | Sociability |
| Negative interactions | Ear or tail biting (quantity) | Sociability |
| Eliminatory behaviors | Defecating or urinating (yes or no) | Sociability |
| Escape attempts | Number of times the animal jumped/raised its front legs against the sides of the weighing scale (quantity) | Sociability and Reactivity during weighing |
| Type of interaction with the feeder | Interaction or lack of interaction with the feeder/apple (did not touch the feeder; touched the feeder; ate the apple) | Discriminative learning (phase one and two) and Judgement bias |
| Number of punishments | Number of times the punishment was applied (quantity) | Discriminative learning (phase two) |
| Resistance to movement | No movement of any portion of the piglet’s body visible for a period > 2 s. The duration comprises the beginning of the standstill to any movement of the body (seconds) | Reactivity during weighing |
| Items | Treatments 1 | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| NC | 4.5 | 6.0 | 7.5 | 9.0 | T 2 | S 3 | T × S | |
| Open Field (n = 35) | ||||||||
| Vocalizations (Quantity) * | 41.1 (±9.1) | 18.6 (±9.1) | 25.4 (±9.1) | 36.6 (±9.1) | 27.6 (±9.1) | 0.435 | 0.016 | - |
| Explored quadrants (Quantity) | 14.4 (±2.6) | 17.3 (±2.6) | 13.7 (±2.6) | 19.7 (±2.6) | 14.0 (±2.6) | 0.429 | 0.349 | - |
| Time exploring the arena (Seconds) | 48.9 (±5.5) | 59.6 (±5.5) | 51.2 (±5.5) | 61.2 (±5.5) | 49.4 (±5.5) | 0.358 | 0.364 | - |
| Novel Object (n = 35) | ||||||||
| Frequency (Quantity) | 1.6 (±0.6) | 0.8 (±0.6) | 1.1 (±0.6) | 1.4 (±0.6) | 1.4 (±0.6) | 0.876 | 0.591 | - |
| Duration of contact (Seconds) | 7.5 (±4.3) | 4.9 (±4.3) | 6.0 (±4.3) | 12.9 (±4.3) | 10.1 (±4.3) | 0.711 | 0.896 | - |
| Reactivity During Weighing (n = 105) | ||||||||
| Escape attempts (Quantity) | 0.9 (±0.2) | 1.3 (±0.2) | 1.2 (±0.2) | 0.8 (±0.2) | 1.1 (±0.2) | 0.629 | 0.651 | 0.076 |
| Vocalizations (Quantity) | 10.1 (±2.0) | 14.7 (±2.0) | 17.0 (±2.1) | 11.2 (±2.0) | 13.3 (±2.0) | 0.117 | 0.422 | - |
| Resistance to movement (Seconds) * | 53.3 (±0.9) | 52.1 (±0.9) | 50.4 (±0.9) | 50.9 (±0.9) | 50.1 (±0.9) | 0.089 | 0.018 | - |
| Sociability (n = 30) | ||||||||
| Negative interactions (Quantity) | 2.1 (±1.4) | 3.0 (±1.4) | 1.1 (±1.4) | 4.0 (±1.4) | 1.5 (±1.4) | 0.649 | 0.266 | - |
| Discriminative learning behavior (n = 35) | ||||||||
| Number of attempts on phase 1 (Quantity) | 2.6 (±0.3) | 3.1 (±0.2) | 2.7 (±0.2) | 2.6 (±0.3) | 2.8 (±0.2) | 0.778 | 0.617 | - |
| Number of attempts on phase 2 (Quantity) | 7.8 (±0.3) | 7.2 (±0.3) | 7.4 (±0.3) | 6.7 (±0.3) | 6.8 (±0.3) | 0.113 | 0.375 | - |
| Number of punishments (Quantity) | 0.5 (±0.1) | 0.4 (±0.1) | 0.4 (±0.1) | 0.5 (±0.1) | 0.4 (±0.1) | 0.920 | 0.698 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zigovski, G.; Bez, I.C.C.; Catoia, M.R.R.; Bickel, A.G.; Daros, R.R.; Monteiro, K.M.; Carvalho, S.T.; Carvalho, P.L.d.O.; Costa, L.B. Supplementation of a Homeopathic Complex in the Diet of Castrated Male and Female Nursery Piglets and Its Effects on Behavior. Animals 2025, 15, 1877. https://doi.org/10.3390/ani15131877
Zigovski G, Bez ICC, Catoia MRR, Bickel AG, Daros RR, Monteiro KM, Carvalho ST, Carvalho PLdO, Costa LB. Supplementation of a Homeopathic Complex in the Diet of Castrated Male and Female Nursery Piglets and Its Effects on Behavior. Animals. 2025; 15(13):1877. https://doi.org/10.3390/ani15131877
Chicago/Turabian StyleZigovski, Gustavo, Isabela Cristina Colaço Bez, Mariana Regina Rosa Catoia, Amanda Gabriela Bickel, Ruan R. Daros, Kelly Mazutti Monteiro, Silvana Teixeira Carvalho, Paulo Levi de Oliveira Carvalho, and Leandro Batista Costa. 2025. "Supplementation of a Homeopathic Complex in the Diet of Castrated Male and Female Nursery Piglets and Its Effects on Behavior" Animals 15, no. 13: 1877. https://doi.org/10.3390/ani15131877
APA StyleZigovski, G., Bez, I. C. C., Catoia, M. R. R., Bickel, A. G., Daros, R. R., Monteiro, K. M., Carvalho, S. T., Carvalho, P. L. d. O., & Costa, L. B. (2025). Supplementation of a Homeopathic Complex in the Diet of Castrated Male and Female Nursery Piglets and Its Effects on Behavior. Animals, 15(13), 1877. https://doi.org/10.3390/ani15131877








