Simple Summary
Reducing the feed-to-gain ratio remains a critical objective for cost reduction and efficiency enhancement in the livestock industry. This study investigated the effects of dietary supplementation with cyclic adenosine monophosphate nanoliposomes (Nano-cAMP) on growth performance, intestinal development, appetite-related hormone expression, and gut microbiota composition in broilers. Results demonstrated that Nano-cAMP supplementation significantly improved growth efficiency by reducing the feed-to-gain ratio. The treatment upregulated expression of cholecystokinin (CCK, an appetite-suppressing hormone) and secretin (a digestion-promoting hormone) genes, enhanced jejunal villus height, and increased the relative abundance of probiotic Lactobacillus species alongside cellulose-degrading bacterial populations in the intestinal microbiota. These findings indicate that Nano-cAMP supplementation effectively enhances growth performance and promotes intestinal health in broilers through multifaceted regulatory mechanisms.
Abstract
This study aimed to investigate the effects of Nano-cAMP on growth performance, gut development, and microbiota composition in broilers. A total of 108 21-day-old yellow-feathered female chicks were randomly divided into three groups with six replicates per group and six chicks per replicate according to the principle of consistent body weight. Experimental treatments included the following: (1) CON group (basal diet), (2) cAMP group (basal diet + 0.02 g/kg cAMP), and (3) Nano-cAMP group (basal diet + 0.37 g/kg Nano-cAMP liposomes). After a 21-day experimental period, results revealed the following: Compared with the CON group, the Nano-cAMP group exhibited a significantly reduced feed-to-gain ratio (p < 0.05). The cAMP group exhibited a significant increase in duodenal index (p < 0.05), whereas the Nano-cAMP group demonstrated greater jejunal villus height (p < 0.05). Both treatment groups showed significant upregulation of cholecystokinin (CCK) and secretin gene expression (p < 0.05). Analysis of alpha-diversity indices (Chao1, Shannon, Simpson) revealed no significant differences in jejunal and cecal microbiota composition between experimental groups (p > 0.05). Notably, the relative abundance of Firmicutes significantly increased (p < 0.05) in the cAMP and Nano-cAMP groups, whereas Proteobacteria, Gemmatimonadota, and Chloroflexi significantly decreased (p< 0.05). The combined relative abundance of three Lactobacillus genera and Bifidobacterium was obviously elevated. Linear discriminant analysis identified Bifidobacterium, Ruminococcus torques group, and uncultured_Thermoanaerobacterales_bacterium as dominant genera in the intestinal tract of Nano-cAMP group. In conclusion, dietary addition of Nano-cAMP promotes jejunal development, modulates appetite hormones mRNA expression, enhances absorption capacity, increases the relative abundance of intestinal probiotics such as Bifidobacterium and cellulose-degrading bacteria such as Ruminococcus torques group, optimizes gut microbiota composition, and ultimately reduces the feed-to-gain ratio in broilers.
1. Introduction
Cyclic adenosine monophosphate (cAMP), first discovered in 1958 by Earl W. Sutherland, plays a pivotal role in cellular signaling. Through molecular-level investigations into hormone action mechanisms, Sutherland established that hormones exert their regulatory effects on cells via cAMP, designating hormones as the “first messenger” and cAMP as the “second messenger” [1]. As a ubiquitous intracellular second messenger, cAMP is generally considered non-toxic and serves as a key mediator of signal transduction across diverse organisms. In microbial systems, cAMP participates in regulating gene expression associated with metabolic and growth processes. This signaling molecule not only modulates carbohydrate metabolism but also influences the integrity and function of microbial cell membranes, thereby affecting microorganism growth and reproduction [2]. Additionally, animal intestinal microbiota play crucial roles in food digestion and nutrient absorption. A balanced gut microbiome serves as the foundation for enhanced livestock productivity. Notably, cAMP demonstrates acid–base and thermal stability, with fermentation yields exceeding 7 g/L in current production systems [3]. However, the strong hydrophilicity of cAMP significantly limits its ability to traverse the lipid bilayer of cell membranes, resulting in poor bioavailability through oral administration. Early experimental evidence revealed that subcutaneous cAMP administration stimulates hypothalamic activity and enhances appetite in animal models [4]. Furthermore, parenteral cAMP administration has been shown to improve growth performance, increase lean muscle mass, enhance immune and digestive functions, and promote lactation in livestock [5]. Recent investigations have further established cAMP involvement in reproductive physiology [6], lipid metabolism regulation, and adipose tissue deposition [7], underscoring its multifaceted biological significance in animal systems. Despite these promising effects, practical applications in animal husbandry remain constrained by the impracticality of injection protocols and poor absorption via conventional feeding methods. To address these bioavailability challenges, researchers have explored the use of dibutyryl cyclic adenosine monophosphate (dbcAMP)-a lipophilic derivative with enhanced absorption properties-as a dietary supplement in swine nutrition. Experimental trials demonstrated that 20 mg/kg dietary supplementation effectively reduced adipose deposition in growing-finishing pigs [7]. Nevertheless, the predominant reliance on costly chemical synthesis methods for dbcAMP production continues to hinder its commercial-scale implementation.
Given liposomes’ well-documented advantages in targeted delivery efficiency and enhanced bioavailability [8,9], cyclic adenosine monophosphate (cAMP) encapsulated within nanoliposomes holds significant potential for improving growth performance and production efficiency in livestock. However, current research on cAMP nanoparticle-based formulations remains scarce. This study pioneers the dietary incorporation of Nano-cAMP liposomes to systematically evaluate their effects on broiler growth performance, intestinal morphology, and microbial composition. The findings aim to establish a scientific foundation for practical applications of nano-encapsulated cAMP in modern animal production systems.
2. Materials and Methods
All experimental procedures involving animals in this study were reviewed and approved by the Chongqing Academy of Animal Science Animal Ethics Committee (No. XKY-20240526, Chongqing, China).
2.1. Experimental Materials
Cyclic adenosine monophosphate (cAMP, analytical grade, ≥98% purity) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China, Nano-cAMP nanoliposomes were prepared using lecithin, cholesterol, citric acid monohydrate (C6H8O7·H2O), sodium citrate dihydrate (Na3C6H5O7·2H2O), chitosan (MW 200,000), and lactose. The encapsulation efficiency of cAMP in the nano-cAMP nanoliposomes was 84.73%. The preparation method of nano-cAMP nanoliposomes followed established protocols [10,11], with the detailed composition provided in Table 1.

Table 1.
Raw material composition and content of Nano-cAMP.
2.2. Experiment Design
The experimental chickens were housed in cages measuring 54 cm × 50 cm × 50 cm (Long × Wide × High). The environment was maintained at 25 °C with 24 h continuous lighting. Vaccinations were administered following a standard immunization protocol, with food and water provided allodially throughout the trial period. Daily sanitation of the animal housing facility was conducted to minimize disease risks. Mortality and morbidity rates were recorded across different experimental groups during the rearing period.
A total of 108 21-day-old yellow-feathered female chicks were randomly assigned to three treatment groups (6 replicates per group, 6 hens per replicate) for a 21-day experimental period. The treatment groups consisted of: (1) Control group (CON): basal diet; (2) cAMP group: basal diet supplemented with 20 mg/kg cAMP; (3) Nano-cAMP group: basal diet supplemented with 0.37 g/kg Nano-cAMP liposomes (equivalent to 20 mg/kg cAMP). Diets were formulated according to NY/T 33-2004 [12] with calculated metabolizable energy values. Table 2 presents the detailed composition and nutritional profile of the experimental diets.

Table 2.
Composition and nutrient levels of basal diets (feed basis).
2.3. Sample Collection
On day 21 of the experiment, one chicken approximating the group mean weight was randomly selected from each enclosure and humanely euthanized via electrical stunning followed by exsanguination through carotid incision. Eighteen chicks were processed following this protocol. Post-euthanasia, the abdominal cavity was aseptically dissected to carefully excise jejunal mesenteric adipose tissue, with particular attention to preserving intestinal mucosal integrity. Identical 1 cm mid-jejunal segments were aseptically excised from standardized anatomical positions, rinsed with sterile physiological saline, and immersion-fixed in 4% paraformaldehyde for histomorphometric analysis. Luminal contents from mid-jejunal and mid-cecal regions were separately collected, flash-frozen in liquid nitrogen, and stored at −80 °C for subsequent microbial community analysis. Standardized 1 cm mid-duodenal sections were dissected, physiological saline-perfused to clear luminal contents, snap-frozen in liquid nitrogen, and maintained at −80 °C for qPCR quantification of gastrointestinal hormone mRNA. Residual gastrointestinal contents and adipose tissue were removed before systematically labeling proventriculus (glandular stomach), gizzard (muscular stomach), duodenum, jejunum, ileum, and cecum segments according to defined anatomical landmarks.
2.4. Detection Indicators and Methods
2.4.1. Growth Performance
On days 1 and 21 of the experimental period, following a 12 h fasting period, all chicks were individually weighed by group. Daily feed consumption per cage was recorded throughout the trial. Subsequently, performance parameters including average daily gain (ADG), average daily feed intake (ADFI), and the feed-to-gain ratio were calculated. Average daily gain (g/d) = Total weight gain/(Number of chicks × Experimental days), Average daily feed intake (g/d) = Total feed intake/(Number of chicks per cage × Experimental days), Feed/gain ratio (g/g) = Total feed consumption/Total weight gain.
2.4.2. Gastrointestinal Index
The relative weight indices of the gastrointestinal organs, including the glandular stomach, muscular stomach, duodenum, jejunum, ileum, and cecum, were determined. Digestive organ indices were calculated according to a previously described method [13], using the following formula: Organ index (%) = (organ weight/body weight before slaughter) × 100.
2.4.3. Histological Morphology of Jejunum
The jejunum samples were fixed in 4% paraformaldehyde (Aladdin), sequentially dehydrated, cleared, and paraffin-embedded. Three tissue sections (5 μm) per sample were prepared (Leica microtome, RM2235, Wetzlar, Germany) and stained with hematoxylin (Sigma, Louis, MO, USA) and eosin (Sigma, Missouri, USA). Morphological examination (Leica optical microscope, DM500, 40×; Wetzlar, Germany) was performed equipped with a microscopic imaging system (Leica, DM1000, Wetzlar, Germany), with quantitative measurements of 10 villus heights and 10 crypt depths obtained from randomly selected fields per section (Image-Pro plus 6.0). Three sections were analyzed per sample. The villus height-to-crypt depth (V/C) ratio was subsequently calculated for statistical analysis. To minimize measurement bias, the procedure was performed by a researcher who was blinded to the group assignments.
2.4.4. Expression of Duodenal Hormone mRNA
Total RNA was extracted from duodenal tissues using the Animal Tissue Total RNA Extraction Kit (TSP413, Qingke Biotechnology Co., Ltd., Beijing, China). cDNA was synthesized with the SynScript® III RT SuperMix for qPCR (TSK314S, Qingke Biotechnology Co., Ltd.). Quantitative real-time PCR was performed using the ArtiCanCEO SYBR qPCR Mix (TSE401, Qingke Biotechnology Co., Ltd.) on a QuantStudio StepOne Plus Real-Time PCR System. The mRNA expression levels of cholecystokinin (CCK), ghrelin, secretin, and gastric inhibitory polypeptide (GIP) in the duodenum were analyzed according to the manufacturer’s recommended protocols. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the reference gene, and relative gene expression was calculated using the 2−△△Ct method. Primer sequences are listed in Table 3.

Table 3.
Primer sequences.
2.4.5. Microbiota of Jejunum and Cecum
Jejunal and cecal contents were isolated, snap-frozen in liquid nitrogen, and stored at −80 °C. Total DNA from the samples was extracted with the TGuide S96 magnetic bead-based soil/fecal genomic DNA extraction kit (Tiangen Biotech Co., Ltd., Beijing, China). The 16S rRNA V3-V4 hypervariable regions were amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), followed by paired-end sequencing on an Illumina platform (Beijing Baimaike Cloud Technology Co., Ltd., Beijing, China). Operational taxonomic units (OTUs) were clustered at 97% nucleotide similarity using USEARCH (V10.0). Alpha diversity metrics and taxonomic composition (phylum/genus levels) were analyzed with QIIME 2. Microbial community differences were further evaluated via linear discriminant analysis effect size (LEfSe) on the Huttenhower Lab server (http://huttenhower.sph.harvard.edu/lefse/, accessed on 23 December 2024).
2.4.6. Statistical Analysis of Data
Statistical analysis was performed using SAS 9.4 software. One-way analysis of variance (ANOVA) was conducted, followed by Duncan’s multiple range test for post hoc comparisons when significant differences were detected. The significance thresholds were defined as follows: p < 0.01 indicated statistically significant differences, while 0.01 ≤ p < 0.05 denoted marginally significant differences.
3. Results
3.1. Effects on Growth Performance
The effects of dietary interventions on broiler growth performance are summarized in Table 4. Compared with the CON group, the Nano-cAMP supplementation significantly reduced the feed-to-gain ratio (p < 0.05). No significant differences were observed in other growth parameters among the three experimental groups (p > 0.05).

Table 4.
Effect of Nano-cAMP on growth performance of broilers.
3.2. Effects on Indices of Major Digestive Tract
The effects of dietary interventions on digestive organ indices are presented in Table 5. The cAMP group exhibited a significantly elevated duodenal index compared to both the Nano-cAMP and CON groups (p < 0.05). No statistically significant difference was detected between the Nano-cAMP and CON groups regarding duodenal index (p > 0.05). Furthermore, all other digestive organ parameters showed homogeneity across experimental groups (p > 0.05).

Table 5.
Effects of Nano-cAMP on indices of major digestive tract of broilers.
3.3. Effects on Jejunal Intestinal Tissue Morphology
The effects of dietary treatments on jejunal morphology are presented in Table 6. Villus height was significantly greater in the Nano-cAMP group compared with the CON group (p < 0.05). Consistent with the tabulated data, a marked increase in villus height was observed in the Nano-cAMP group (Figure 1). Although the cAMP group showed a 22.67% increase in villus height relative to the CON group, this difference did not reach statistical significance (p > 0.05). No significant difference in villus height was observed between the Nano-cAMP and cAMP groups (p > 0.05). Furthermore, dietary interventions had no significant effect on crypt depth or the villus height-to-crypt depth ratio in any experimental groups (p > 0.05).

Table 6.
Effects of Nano-cAMP on intestinal tissue morphology of the jejunum.
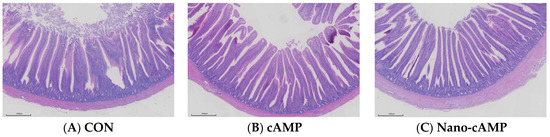
Figure 1.
Representative histological micrographs of the jejunum. (A) CON: fed with basal diet; (B) cAMP: fed with basal diet + cAMP 0.02 g/kg; (C) Nano-cAMP: fed with basal diet + 0.37 g/kg Nano-cAMP liposomes. Bar = 500 μm.
3.4. Effects on Duodenal Intestinal Hormone mRNA Expression
As shown in Table 7, dietary treatments significantly influenced mRNA expression levels of CCK and secretin in the duodenum (p < 0.01). Compared with the CON group, both cAMP and Nano-cAMP groups demonstrated significantly elevated mRNA expression of CCK and secretin (p < 0.05). However, no significant difference was observed in CCK or secretin expression between the cAMP and Nano-cAMP groups (p > 0.05). Furthermore, mRNA levels of ghrelin and gastric inhibitory polypeptide (GIP) remained comparable across all experimental groups.

Table 7.
Effect of Nano-cAMP on mRNA expression of duodenal intestinal hormones.
3.5. Effects on the Jejunal and Cecal Microbiota
As revealed by the Venn diagram (Figure 2A), a total of 1930 operational taxonomic units (OTUs) were identified. The CON, cAMP, and Nano-cAMP groups exhibited 497, 414, and 906 unique OTUs, respectively, with 39 OTUs shared among all three groups. Dietary treatments did not significantly alter the alpha diversity indices (Chao1, Shannon, and Simpson) of the jejunal microbial communities in broilers (p > 0.05; Figure 2B–D).
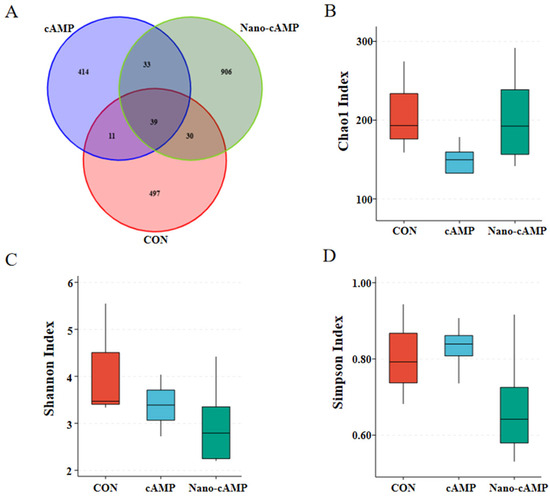
Figure 2.
Effect of Nano-cAMP on the alpha diversity of jejunum microbiota. (A) Venn diagram of the OTUs distribution; (B) Chao1 Index of alpha diversity of jejunum microbiota; (C) Shannon Index; (D) Simpson Index. CON: fed with basal diet; cAMP: fed with basal diet + cAMP 0.02 g/kg; Nano-cAMP: fed with basal diet + 0.37 g/kg Nano-cAMP liposomes.
As shown in Figure 3A, a total of 4046 OTUs were analyzed by Venn diagram. The CON, cAMP, and Nano-cAMP groups contained 1290, 1070, and 951 unique OTUs, respectively, while 380 OTUs were shared among all three groups. The alpha diversity indices (Chao1, Shannon, and Simpson) of the cecal microbial community in broilers exhibited no significant differences among the dietary treatments (p > 0.05; Figure 3B–D).
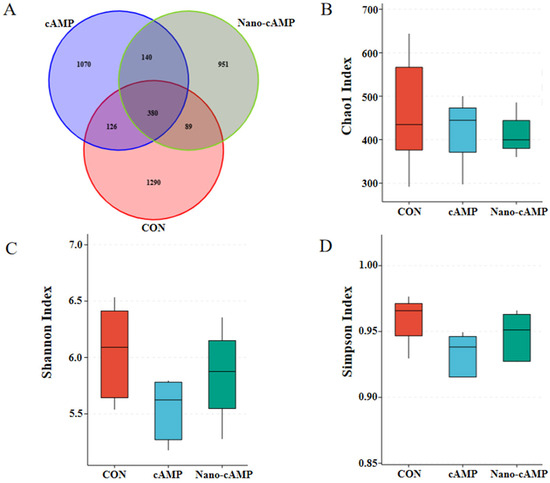
Figure 3.
Effect of Nano-cAMP on the alpha diversity of cecum microbiota. (A) Venn diagram of the OTUs distribution; (B) Chao1 Index of alpha diversity of cecum microbiota; (C) Shannon Index; (D) Simpson Index. CON: fed with basal diet; cAMP: fed with basal diet + cAMP 0.02 g/kg; Nano-cAMP: fed with basal diet + 0.37 g/kg Nano-cAMP liposomes.
As illustrated in Table 8, the three groups shared four dominant bacterial phyla at the phylum level—Firmicutes, Proteobacteria, Bacteroidota, and Actinobacteriota—collectively accounting for over 93% of total relative abundance. Compared to the CON group, both cAMP and Nano-cAMP groups exhibited significant increases in Firmicutes abundance (p < 0.05). Proteobacteria levels demonstrated differential reductions, with a more pronounced decrease in the cAMP group (p < 0.01) compared to the Nano-cAMP group (p < 0.05) relative to the CON group. Notably, significant declines (p < 0.05) were observed in Gemmatimonadota and Chloroflexi abundances across treatment groups. While Nano-cAMP administration enhanced Actinobacteriota representation, it concurrently reduced Bacteroidota levels along with six additional phyla. However, no significant compositional differences emerged between cAMP and Nano-cAMP groups at the phylum level.

Table 8.
Composition of the jejunum microbiota at the phylum level.
As shown in Table 9, no significant differences in cecal microbiota composition were detected at the phylum level across groups (p > 0.05). Firmicutes, Bacteroidota, and Actinobacteriota constituted the predominant phyla in all groups, collectively accounting for over 98% of the total relative abundance. Compared to the CON group, both Nano-cAMP and cAMP groups displayed marked reductions in the relative abundance of Firmicutes and Acidobacteriota. A concurrent decrease in Proteobacteria abundance was also observed in two treatment groups. In contrast, Bacteroidota and Actinobacteriota exhibited increased relative abundances in the cAMP and Nano-cAMP groups relative to the control.

Table 9.
Composition of the cecum microbiota at the phylum level.
As shown in Figure 4A, the combined relative abundance of Ligilactobacillus, Lactobacillus, Limosilactobacillus, and Bifidobacterium exceeded 60% at the genus level across all groups. Compared to the CON group, the cAMP and Nano-cAMP groups demonstrated a marked increase in the cumulative abundance of these four lactic acid-producing genera, with Ligilactobacillus and Bifidobacterium exhibiting particularly pronounced elevations. Conversely, taxa including unclassified Cyanobacteriales, Marivita, Phaeobacter, Winogradskyella, and the hgcI_clade (affiliated with the Actinobacteria phylum) were substantially reduced in both treatment groups relative to the control. Notably, these five genera showed near-complete depletion in the Nano-cAMP group. Figure 4B further revealed genus-level shifts: the cAMP and Nano-cAMP groups displayed modest increases in the relative abundance of Bifidobacterium, Ligilactobacillus, Faecalibacterium, and the Ruminococcus_torques_group compared to the CON group. In contrast, unclassified_Clostridia_UCG_014 exhibited a marginal reduction in both treatment groups.
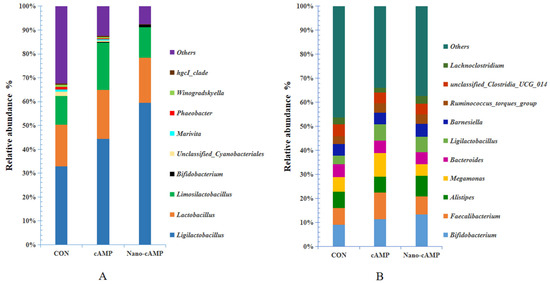
Figure 4.
Composition of the microbiota at genus level: (A) jejunal microbiota; (B) cecal microbiota. CON: fed with basal diet; cAMP: fed with basal diet + cAMP 0.02 g/kg; Nano-cAMP: fed with basal diet + 0.37 g/kg Nano-cAMP liposomes.
Figure 5A demonstrates the jejunal dominant genera identified by linear discriminant analysis. In the CON group, the predominant taxa were: Gemmatimonadota (unclassified Gemmatimonadaceae genus), Proteobacteria (unclassified Gammaproteobacteria genus), and Proteobacteria (unclassified Burkholderiales genus). The cAMP group was dominated by Firmicutes (Bacilli class; Lactobacillaceae family). The Nano-cAMP group exhibited multiple dominant genera: Actinobacteriota (Bifidobacterium genus), Firmicutes (Enterococcus genus), Firmicutes (Clostridia class; Peptostreptococcales-Tissierellales order; Peptostreptococcaceae family; Romboutsia genus), and Firmicutes (Clostridia class; Lachnospirales order; Lachnospiraceae family; Ruminococcus_torques_group genus). Figure 5B illustrates cecal microbial composition. The CON group showed dominance of Firmicutes (Clostridia class; Peptostreptococcales-Tissierellales order; Anaerovoracaceae family; Eubacterium_brachy_group genus), Firmicutes (Colidextribacter genus), and Firmicutes (Flavonifractor genus). Catenibacillus genus under the Firmicutes phylum predominated in the cAMP group. The Nano-cAMP group was characterized by uncultured Thermoanaerobacterales bacterium genus (Firmicutes phylum; uncultured Thermoanaerobacterales family).
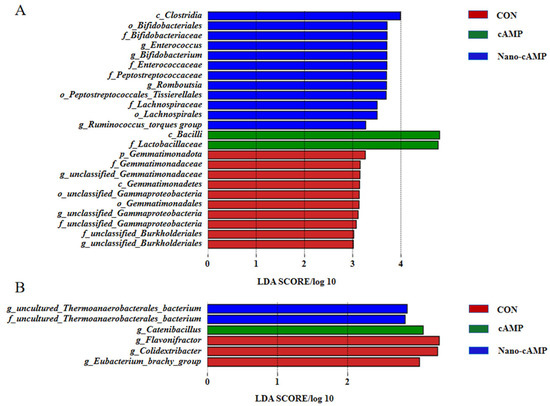
Figure 5.
Histogram of linear discriminant analysis distribution of microbiota. (A) Jejunal microbiota; (B) Cecal microbiota. CON: fed with basal diet; cAMP: fed with basal diet + cAMP 0.02 g/kg; Nano-cAMP: fed with basal diet + 0.37 g/kg Nano-cAMP liposomes.
Brief summary: Both cAMP and Nano-cAMP enhanced the relative abundance of probiotic genera, such as Ligilactobacillus and Bifidobacterium, as well as cellulose-degrading taxa Faecalibacterium and Ruminococcus_torques_group. Notably, Nano-cAMP additionally increased the relative abundance of an uncultured bacterium affiliated with the Thermoanaerobacterales family.
4. Discussion
4.1. Effects of Nano-cAMP Liposomes on Growth Performance of Broilers
Liposomes enhance absorption efficiency primarily through biomimetic delivery and transmembrane optimization mechanisms, and have demonstrated efficacy in improving vitamin and drug absorption [14,15]. Given the hydrophilic nature of cAMP, its limited ability to penetrate cell membranes [16] results in reduced bioavailability when administered orally. In a study by Li et al. [17], weaned piglets (average weight 3.31 kg) receiving 1.5 mg/day of calcium dibutyryl cyclic adenosine monophosphate (dbcAMP-Ca, a cAMP analog) for 10 days showed improved daily weight gain increased by 109.17% (p < 0.05). Interestingly, our findings revealed that neither directly adding cAMP or nano-cAMP liposomes significantly affected daily gain in broilers. However, the nano-cAMP formulation significantly reduced the feed-to-gain ratio. In healthy animals, improved feed efficiency reflects enhanced digestive capacity and intestinal microbial activity [18]. This suggests that dietary nano-cAMP liposomes may improve nutrient utilization, though the actual intestinal absorption of encapsulated cAMP requires further investigation.
Emerging evidence indicates that intracellular cAMP regulates growth-related gene expression via protein kinase A activation. Some researchers have demonstrated that dietary interventions combining prebiotics with cAMP elevation show potential for improving feed conversion efficiency. For instance, studies reported an 8.19–9.18% reduction in the feed/gain ratio of pigs (p < 0.05), a 9.22–9.68% increase in the relative breast muscle weight of broilers (p < 0.05), and a 3.01% increase in their relative thigh muscle weight (p < 0.01) [19,20,21]. Notably, Yue’s studies [22,23] on growing-finishing pigs demonstrated that elevated cAMP levels in high-efficiency groups downregulate lipogenesis genes while upregulating skeletal muscle development genes, confirming its regulatory role in growth processes. Although adenosine-based feed additives have shown growth-promoting effects [24], our study specifically highlights cAMP’s unique capacity to enhance growth performance. These findings collectively suggest that nano-cAMP formulations may offer novel strategies for improving dietary efficiency in poultry production.
4.2. Effects of Nano-cAMP Liposomes on Major Digestive Organ Indices and Jejunal Tissue Morphology
Digestion comprises two distinct processes: mechanical breakdown and enzymatic hydrolysis. As vital sites for nutrient processing and enzymatic absorption, digestive organ indices reflect developmental status, where higher indices correlate with enhanced digestive capacity [25]. This study demonstrated that dietary cAMP supplementation significantly elevated the duodenal index, while jejunal villus height showed no statistically significant increase. Notably, while nano-cAMP liposomes did not significantly alter duodenal index, they induced marked elevation in jejunal villus height. This differential effect may be attributed to the sustained-release properties and tissue-targeting capabilities inherent to liposomal formulations of cyclic adenosine monophosphate [26]. Both cAMP formulations promoted intestinal morphological development, thereby improving nutrient assimilation efficiency and consequently reducing the feed-to-gain ratio.
The jejunum, serving as the primary site of intestinal absorption, exhibits histological features representative of small intestinal development. Increased crypt depth typically indicates accelerated epithelial cell turnover, while elevated villus height and villus height/crypt depth ratio suggest enhanced mucosal differentiation with improved absorption capacity [27,28]. Although previous studies have established cAMP’s role in gastrointestinal smooth muscle relaxation [29], the current investigation provides novel evidence regarding exogenous cAMP’s impact on intestinal tissue morphogenesis, a previously unreported phenomenon in the literature.
4.3. Effects of Nano-cAMP Liposomes on Duodenal Enteric Hormone mRNA Expression
Cyclic adenosine monophosphate (cAMP), as an intracellular second messenger, mediates carbohydrate, protein, and lipid metabolism while coordinating hormone biosynthesis and physiological regulation. Our data revealed that dietary supplementation with both cAMP and nano-encapsulated cAMP significantly upregulated mRNA expression of CCK and secretin. Notably, ghrelin expression was elevated in both treatment groups, though without statistical significance. Previous studies indicate that CCK and ghrelin are key appetite-suppressing hormones in poultry, with elevated levels directly correlating with reduced feed intake [30,31]. This aligns with the observed downward trend in average daily feed consumption across treatment groups, albeit lacking statistical significance, thereby illustrating the multifactorial nature of appetite regulation involving digestive and growth-related pathways. Although a significant reduction in feed intake can negatively impact animal growth, it may improve the feed conversion ratio and benefit animal production. However, in this study, feed intake was not significantly reduced. Secretin enhances pancreatic juice secretion, and its upregulated expression in this study suggests improved digestive capacity in broilers. Mechanistically, cAMP-mediated regulation of enteric hormones involves two pathways: (1) activation of protein kinase A (PKA) through intracellular signaling cascades, modulating hormone CCK release [32]; (2) anatomical specificity, as CCK, secretin, and gastric inhibitory polypeptide are predominantly expressed in the duodenum [33], the primary site of enteric hormone production within the small intestine.
4.4. Effects of Nano-cAMP Liposomes on Intestinal Microbiota of Broilers
The gastrointestinal tract harbors a complex microbial ecosystem that critically regulates host physiology, including immunity, metabolism, and growth [34]. Gut microbial activities profoundly influence physiological homeostasis, exhibiting dual roles in health promotion and disease pathogenesis. Alpha diversity indices revealed microbial community characteristics: Chao1 estimates species richness (higher values = more taxa), whereas Shannon and Simpson indices reflect community diversity, influenced by both species richness and evenness [35,36].
Our findings indicate that neither conventional cAMP nor nano-cAMP significantly altered jejunal or cecal alpha diversity. However, both treatments enhanced the relative abundance of dominant taxa. Previous studies demonstrated that high-performing broilers exhibit jejunal microbiota dominated by Firmicutes, with elevated Lactobacillus and Bifidobacterium abundances and reduced pathogenic Escherichia populations [37]. Such probiotic dominance suppresses intestinal inflammation and enhances growth performance. Similarly, Qiu et al. [38] reported that ileal/cecal microbiota in healthy broilers showed increased Lactobacillus and Bifidobacterium with concurrent decreases in Escherichia coli and Clostridium perfringens, correlating with improved intestinal health and productivity.
Notably, both cAMP formulations increased jejunal Firmicutes abundance, particularly Ligilactobacillus and Bifidobacterium. Direct cAMP supplementation additionally elevated Limosilactobacillus abundance, while nano-cAMP specifically enhanced Ruminococcus_torques_group and Romboutsia. Furthermore, Romboutsia can metabolize complex carbohydrates into beneficial metabolites like short-chain fatty acids, oligosaccharides, and other prebiotic substances. [39]. Both treatments reduced jejunal Gemmatimonadota and Proteobacteria abundances. Although cecal Firmicutes slightly decreased, key beneficial genera (Ligilactobacillus, Faecalibacterium, Ruminococcus_torques_group, and Bifidobacterium [phylum Actinobacteria]) exhibited increased abundances. These microbiota shifts—probiotic enrichment coupled with pathogen suppression—positively correlate with enhanced immune competence and production efficiency [40,41,42], consistent with the observed reduction in feed-to-gain ratio.
The relative abundance of unclassified_Thercoanaerobacterales_bacterium in the cecum was significantly elevated by nano-cAMP liposome supplementation. Key microbial taxa including Faecalibacterium [43], Ruminococcus_torques_group [44], and unclassified_Thercoanaerobacterales_bacterium [45] function as primary degraders of lignocellulosic and other non-starch polysaccharides (NSPs). Their enzymatic hydrolysis releases metabolizable energy substrates for the host while concurrently reducing the feed-to-gain ratio. Notably, although direct cAMP administration markedly increased Lactobacillaceae (a beneficial family), it simultaneously elevated the pathogenic genus Catenibacillus (associated with gut diseases such as ulcer, constipation) [46]. Unclassified Bacterial taxa within Gemmatimonadota and Proteobacteria phyla, Chloroflexi (linked to white feces syndrome in shrimp) [47], Eubacterium_brachy_group (associated with human Glioblastoma) [48], Colidextribacter (Mice colitis) [49], and Flavonifractor (enriched in human irritable bowel syndrome) [50], are predominantly classified as opportunistic pathogens. These organisms may induce intestinal inflammation, impair nutrient absorption, and potentially increase the feed-to-gain ratio in control groups. Furthermore, the intestinal homeostatic equilibrium mediated by dominant probiotics (e.g., lactic acid bacteria) maintained clinical health status in experimental animals, with no overt disease manifestations observed during the trial period.
5. Conclusions
In conclusion, dietary supplementation with 0.37 g/kg nano-cAMP liposomes enhances broiler performance through multifaceted mechanisms: (1) upregulating duodenal CCK and secretin gene expression; (2) increasing jejunal villus height to amplify nutrient absorption capacity; (3) enriching beneficial microbiota and cellulolytic taxa, thereby optimizing intestinal ecosystem functionality. These synergistic effects collectively improve absorption efficiency and reduce the feed-to-gain ratio.
Author Contributions
Conceptualization, L.C. and G.S.; methodology, G.S. and S.J.; software, L.W. and S.J.; validation, L.W.; formal analysis, L.C.; investigation, L.W. and S.H.; resources, L.C.; data curation, L.W.; writing—original draft preparation, L.C.; writing—review and editing, F.Y. and J.H.; visualization, S.H.; supervision, L.C., F.Y. and J.H.; project administration, L.C., F.Y. and J.H.; funding acquisition, L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Special Project for Performance Incentive and Guidance of Scientific Research Institutions in Chongqing, No. 23503J.
Institutional Review Board Statement
The animal study protocol was approved by the Chongqing Academy of Animal Science Animal Ethics Committee (No:XKY-20240526, Chongqing, China) for studies involving animals.
Informed Consent Statement
Informed consent was obtained from the owner of the animals involved in the study.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
The authors would like to thank the Southwest Chinese Veterinary Medicine Evaluation Center of Chongqing Academy of Animal Sciences technicians who participated in the research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qi, L.; Friml, J. Tale of cAMP as a second messenger in auxin signaling and beyond. New Phytol. 2023, 240, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, D.; Zhu, J.; Liu, J.; Zhu, W. The Regulation of Bacterial Biofilm Formation by cAMP-CRP: A Mini-Review. Front. Microbiol. 2020, 11, 802. [Google Scholar] [CrossRef]
- Li, Z.; Tan, H.; Lu, N.; Zhang, Z.; Chang, J. Enhanced ATP and antioxidant levels for cAMP biosynthesis by Arthrobacter sp. CCTCC 2013431 with polyphosphate addition. Biotechnol. Lett. 2021, 43, 2223–2231. [Google Scholar] [CrossRef]
- Allan, Z. Control of food intake through regulation of cAMP. Curr. Top. Dev. Biol. 2005, 67, 207–224. [Google Scholar] [CrossRef]
- Wang, S.; Chen, A. The physiological functions of cyclic adenosine monophosphate and its application in animal production. China Feed 2003, 10, 3–5. [Google Scholar] [CrossRef]
- Chen, W.; Xia, W.G.; Ruan, D.; Wang, S.; Abouelezz, K.F.M.; Wang, S.L.; Zhang, Y.N.; Zheng, C.T. Dietary calcium deficiency suppresses follicle selection in laying ducks through mechanism involving cyclic adenosine monophosphate-mediated signaling pathway. Animal 2020, 14, 2100–2108. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Li, W.; Xiao, Y.N.; Yang, X.; Gao, K.; Jiang, Z. Dietary dibutyryl cAMP supplementation regulates the fat deposition in adipose tissues of finishing pigs via cAMP/PKA pathway. Anim. Biotechnol. 2023, 34, 921–934. [Google Scholar] [CrossRef]
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Parvin, Z.; Behzad, B. Liposome and immune system interplay: Challenges and potentials. J. Control. Release 2019, 305, 194–209. [Google Scholar] [CrossRef]
- Shahida, A.S.; Nur, A.B.; Ahmed, T.; Abdel-Moneim, E.A.M.; Abdelrazeq, M.S.; Chen, T.; Mohammad, S.K.; Yuan, L.; Elham, A.; Roberto, C.M.; et al. Bioactive-loaded nanodelivery systems for the feed and drugs of livestock; purposes, techniques and applications. Adv. Colloid Interface Sci. 2022, 308, 102772. [Google Scholar] [CrossRef]
- Gomes, I.; Sharma, S.K. Uptake of Liposomally Entrapped Adenosine-3′-5′-Cyclic Monophosphate in Mouse Brain. Neurochem. Res. 2004, 29, 441–446. [Google Scholar] [CrossRef]
- Fu, M.Q.; Wang, Y.S. Cyclic Adenosine Monophosphate Liposome Injection and Preparation Method. CN102327224A, 25 January 2012. [Google Scholar]
- NY/T 33-2004; Ministry of Agriculture of the People’s Republic of China. Feeding Standard of Chicken. China Agriculture Press: Beijing, China, 2004.
- Lin, J.; Comi, M.; Vera, P.; Alessandro, A.; Qiu, K.; Wang, J.; Wu, S.; Qi, G.; Zhang, H. Effects of Saccharomyces cerevisiae hydrolysate on growth performance, immunity function, and intestinal health in broilers. Poult. Sci. 2023, 102, 102237. [Google Scholar] [CrossRef]
- Sreerag, G.; Preetha, B. Evaluation and Clinical Comparison Studies on Liposomal and Non-Liposomal Ascorbic Acid (Vitamin C) and their Enhanced Bioavailability. J. Liposome Res. 2020, 31, 356–364. [Google Scholar] [CrossRef]
- Ahmed, K.; Hussein, S.; Ali, A.; Sameh, A.; Qiu, L.; Chen, J. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Gancedo, J.M. Biological roles of cAMP: Variations on a theme in the different kingdoms of life. Biol. Rev. 2013, 88, 645–668. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chang, L.; Zhang, G.; Song, Z.H.; Wan, D.; Xie, C.Y.; Wang, H.; Fan, Z.Y. Oral administration of dibutyryl adenosine cyclophosphate improved growth performance in weaning piglets by enhancing lipid fatty acids metabolism. Anim. Nutr. 2018, 4, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, G.E.; Metzler-Zebeli, B.U.; Lawlor, P.G. Impact of Intestinal Microbiota on Growth and Feed Efficiency in Pigs: A Review. Microorganisms 2020, 8, 1886. [Google Scholar] [CrossRef]
- Banerjee, P.; Carmelo, V.A.O.; Kadarmideen, H.N. Genome-wide epistatic interaction networks affecting feed efficiency in duroc and landrace pigs. Front. Genet. 2020, 11, 121. [Google Scholar] [CrossRef]
- Bedford, A.; Yu, H.; Hernandez, M.; Squires, E.J.; Leeson, S.; Gong, J. Effects of fatty acid glyceride product SILOhealth 104 on the growth performance and carcass composition of broiler chickens. Poult. Sci. 2018, 97, 1315–1323. [Google Scholar] [CrossRef]
- Elsharkawy, M.; Chen, Y.; Liu, R.; Xiaodong, T.; Wei, L.; Ibrahim, E.; Dongqin, Z.; Maiqing, Z.; Jie, W.; Guiping, Z. Paternal dietary methionine supplementation improves carcass traits and meat quality of chicken progeny. Animals 2021, 11, 325. [Google Scholar] [CrossRef]
- Yue, Y.X.; Xiao, L.Q.; Ming, Y.H.; Ruiyi, L.; Ye, H.; Zhangxu, W.; Huanhuan, Z.; Yunxia, Z.; Yu, L.; Shuhong, Z.; et al. Transcriptome Analysis of Adipose Tissue Indicates That the cAMP Signaling Pathway Affects the Feed Efficiency of Pigs. Genes 2018, 9, 336. [Google Scholar] [CrossRef]
- Lu, J.; Ye, H.; Hui, W.; Yuanxin, M.; Xinyun, L.; Jianhua, C.; John, M.B.; Tim, P.; Shuhong, Z. Transcriptome analysis of mRNA and miRNA in skeletal muscle indicates an important network for differential Residual Feed Intake in pigs. Sci. Rep. 2015, 5, 11953. [Google Scholar] [CrossRef]
- Chuang, W.Y.; Hsieh, Y.C.; Lee, T.T. The Effects of Fungal Feed Additives in Animals: A Review. Animals 2020, 10, 805. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Wu, S.G.; Zhang, H.J.; Guanghai, Q.; Jing, W. Dynamic alterations in early intestinal development, microbiota and metabolome induced by in ovo feeding of L-arginine in a layer chick model. J. Anim. Sci. Biotechnol. 2020, 011, 896–911. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Guo, J.; Huang, Q. Liposomes for Tumor Targeted Therapy: A Review. Int. J. Mol. Sci. 2023, 24, 2643. [Google Scholar] [CrossRef]
- Adewole, D.I.; Oladokun, S.; Santin, E. Effect of organic acids-essential oils blend and oat fiber combination on broiler chicken growth performance, blood parameters, and intestinal health. Anim. Nutr. 2021, 4, 1039–1051. [Google Scholar] [CrossRef]
- Monika, B.T.; Ewa, Z.; Anna, S.; Maria, S.; Joanna, B. Modulation of Intestinal Histology by Probiotics, Prebiotics and Synbiotics Delivered In Ovo in Distinct Chicken Genotypes. Animals 2021, 11, 3293. [Google Scholar] [CrossRef] [PubMed]
- Guerra, D.G.; Bok, R.; Ramon, A.L.; Joseph, H.K. Protein kinase A facilitates relaxation of mouse ileum via phosphorylation of neuronal nitric oxide synthase. Br. J. Pharmacol. 2020, 177, 2765–2778. [Google Scholar] [CrossRef]
- Ahmed, K.; Takaoki, S.; Hiroshi, K.; Kazuhisa, H. Effects of fasting and re-feeding on the expression of CCK, PYY, hypothalamic neuropeptides, and IGF-related genes in layer and broiler chicks. Comp. Biochem. Physiol. 2021, 257, 110940. [Google Scholar] [CrossRef]
- Anastasiia, R.V.; Ilya, R.A.; Mikhail, A.K.; Ivan, S.Y.; Michael, N.R.; Elena, I.S.; Oleg, A.G.; Fedor, A.K. A bird’s-eye overview of molecular mechanisms regulating feed intake in chickens-with mammalian comparisons. Anim. Nutr. 2024, 17, 61–74. [Google Scholar] [CrossRef]
- Bany, B.R.; Reimann, F.; Gribble, F.M. The intestine as an endocrine organ and the role of gut hormones in metabolic regulation. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 784–796. [Google Scholar] [CrossRef]
- Zhu, S.G.; Xu, C.F. Biochemistry, 4th ed.; Higher Education Press: Beijing, China, 2017; pp. 1–596. [Google Scholar]
- Wu, S.; Shen, Y.; Zhang, S.; Yunqi, X.; Shourong, S. Salmonella Interacts with Autophagy to Offense or Defense. Front. Microbiol. 2020, 11, 721. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Lee, J.; Tan, K. Infection with pathogenic Blastocystis ST7 is associated with decreased bacterial diversity and altered gut microbiome profiles in diarrheal patients. Parasites Vectors 2022, 15, 312. [Google Scholar] [CrossRef]
- Cao, H.; Chen, D.; Guo, L.; Rong, J.; Yunteng, X.; Wei, M.; Chen, W.; Pengfei, L.; Hui, W. Effects of Bacillus subtilis on growth performance and intestinal flora of Penaeus vannamei. Aquac. Rep. 2022, 23, 101070. [Google Scholar] [CrossRef]
- Zhang, X.; Akhtar, M.; Chen, Y.; Ziyu, M.; Yuyun, L.; Deshi, S.; Ranran, C.; Lei, C.; Yafang, H.; Abdallah, A.N.; et al. Chicken jejunal microbiota improves growth performance by mitigating intestinal inflammation. Microbiome 2022, 10, 107. [Google Scholar] [CrossRef]
- Qiu, K.; Li, C.L.; Wang, J. Effects of Dietary Supplementation with Bacillus subtilis, as an Alternative to Antibiotics, on Growth Performance, Serum Immunity, and Intestinal Health in Broiler Chickens. Front. Nutr. 2021, 8, 786878. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.; Li, F.; Hu, L.; Xiao, T.; Zhao, Y.; Yang, M. The Effects of Dietary Fermented Soybean Residue on the Growth, Antioxidant Capacity, Digestive Enzyme Activities, and Microbial Compositions of the Intestine in Furong Crucian Carp (Furong Carp♀×Red Crucian Carp♂). Fishes 2024, 9, 138. [Google Scholar] [CrossRef]
- Ivan, R. Composition and Function of Chicken Gut Microbiota. Animals 2020, 10, 103. [Google Scholar] [CrossRef]
- Yu, X.; Niu, S.; Tie, K.; Zhang, Q.; Deng, H.; Gao, C.; Yu, T.; Lei, L.; Feng, X. Characteristics of the intestinal flora of specific pathogen free chickens with age. Microb. Pathog. 2019, 132, 325–334. [Google Scholar] [CrossRef]
- Paneru, D.; Tellez, I.G.; Bottje, W.G.; Emmanuel, A.; Ahmed, A.A.A.W.; Md, S.; Jayant, L. Modulation of Immune Response and Cecal Microbiota by Dietary Fenugreek Seeds in Broilers. Vet. Sci. 2024, 11, 57. [Google Scholar] [CrossRef]
- Francesca, D.F.; Edoardo, P.; Danilo, E. Newly Explored Faecalibacterium Diversity Is Connected to Age, Lifestyle, Geography and Disease. Curr. Biol. 2020, 30, 4932–4943. [Google Scholar] [CrossRef]
- Shinuo, L.; Qingfeng, W.; Jinqiu, M.; Haotian, C.; Tianhao, Y.; Yue, W.; Lihong, Z.; Qiugang, M.; Shimeng, H. Lactobacillus crispatus-Mediated Gut-Reproductive Tract Axis-Alleviated Microbial Dysbiosis and Oviductal Inflammation in a Laying Hen Model. Microorganisms 2024, 12, 1559. [Google Scholar] [CrossRef]
- Przemysław, L.; Sebastian, B. Effect of hyperthermophilic pretreatment on methane and hydrogen production from garden waste under mesophilic and thermophilic CONditions. Bioresour. Technol. 2021, 335, 125264. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Y.; Xu, Z.; Yuming, Z.; Lei, W.; Chunguang, L.; Ailing, C.; Shanyu, J.; Weimin, S.; Guohui, Y.; et al. Dysregulation of gut health in zebrafish by differentially charged nanoplastic exposure: An integrated analysis of histopathology, immunology, and microbial informatics. Environ. Sci. Nano 2023, 10, 933–947. [Google Scholar] [CrossRef]
- Chaudhary, D.K.; Kim, S.E.; Park, H.J.; Kyoung, H.K. Unveiling the Bacterial Community across the Stomach, Hepatopancreas, Anterior Intestine, and Posterior Intestine of Pacific Whiteleg Shrimp. J. Microbiol. Biotechnol. 2024, 34, 1260–1269. [Google Scholar] [CrossRef]
- Wang, S.; Yin, F.; Guo, Z.; Rui, L.; Wei, S.; Yuchao, W.; Yichen, G.; Chao, S.; Daqing, S. Association between gut microbiota and glioblastoma: A Mendelian randomization study. Front. Genet. 2024, 14, 1308263. [Google Scholar] [CrossRef]
- Ren, X.; Xu, J.; Xu, Y.; Qin, W.; Kunlun, H.; Xiaoyun, H. Artemether Attenuates Gut Barrier Dysfunction and Intestinal Flora Imbalance in High-Fat and High-Fructose Diet-Fed Mice. Nutrients 2023, 15, 4860. [Google Scholar] [CrossRef]
- Hu, X.; Ouyang, S.; Xie, Y.; Gong, Z.; Du, J. Characterizing the gut microbiota in patients with chronic kidney disease. Postgrad. Med. 2020, 132, 495–505. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).