Effects of Dietary Ratio of Insoluble Fiber to Soluble Fiber on Reproductive Performance, Biochemical Parameters, and Fecal Microbial Composition of Gestating Sows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Swine and Diet
2.2. Recording and Sampling
2.3. Chemical Analyses
2.4. Microbial Analyses
2.5. Statistical Analysis
3. Result
3.1. Reproductive Performance of Sows
3.2. Fecal Score of Sows
3.3. Plasma SCFAs
3.4. Serum Biochemical Index
3.5. Colostrum Composition
3.6. Plasma Antioxidant Capacity
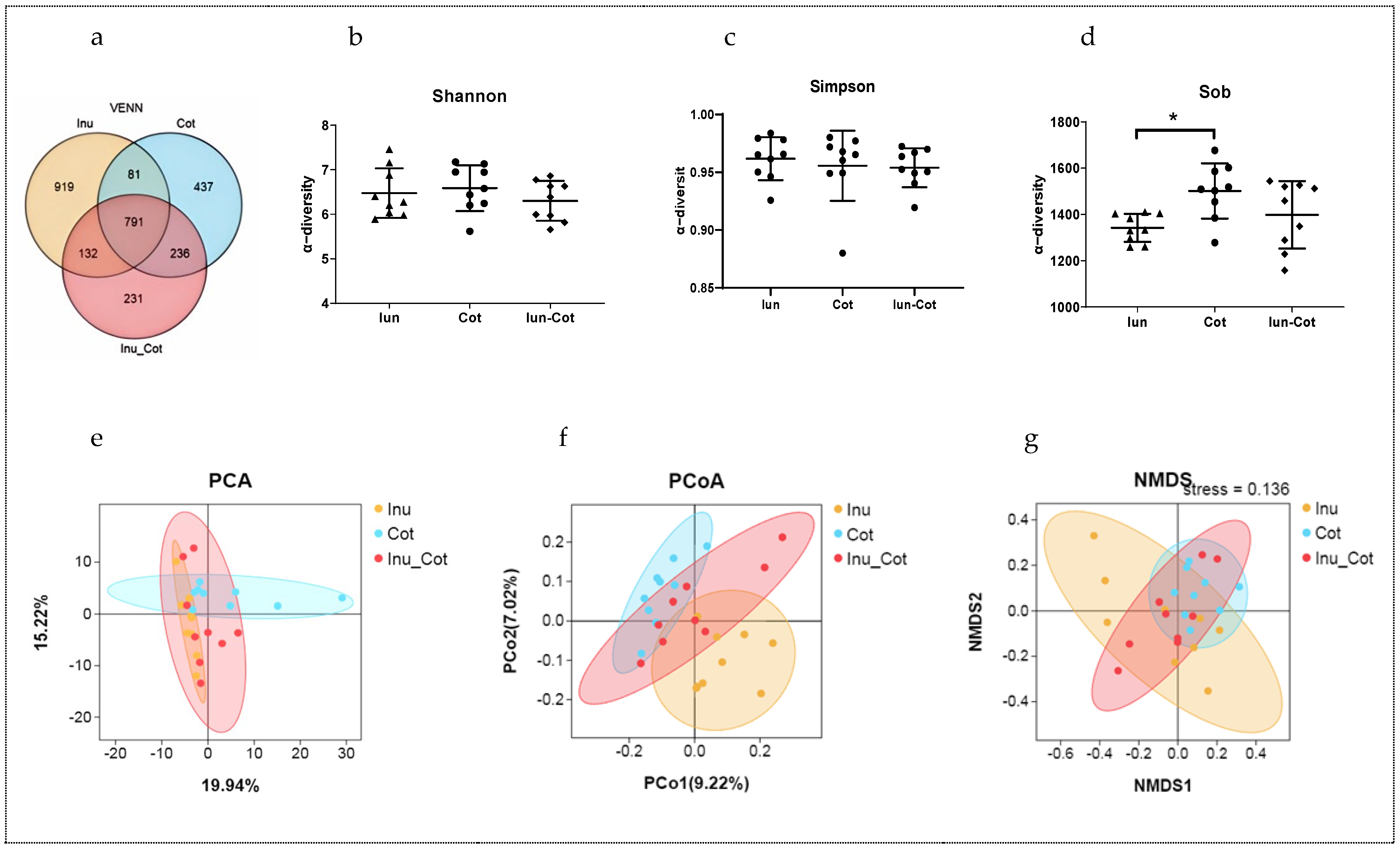
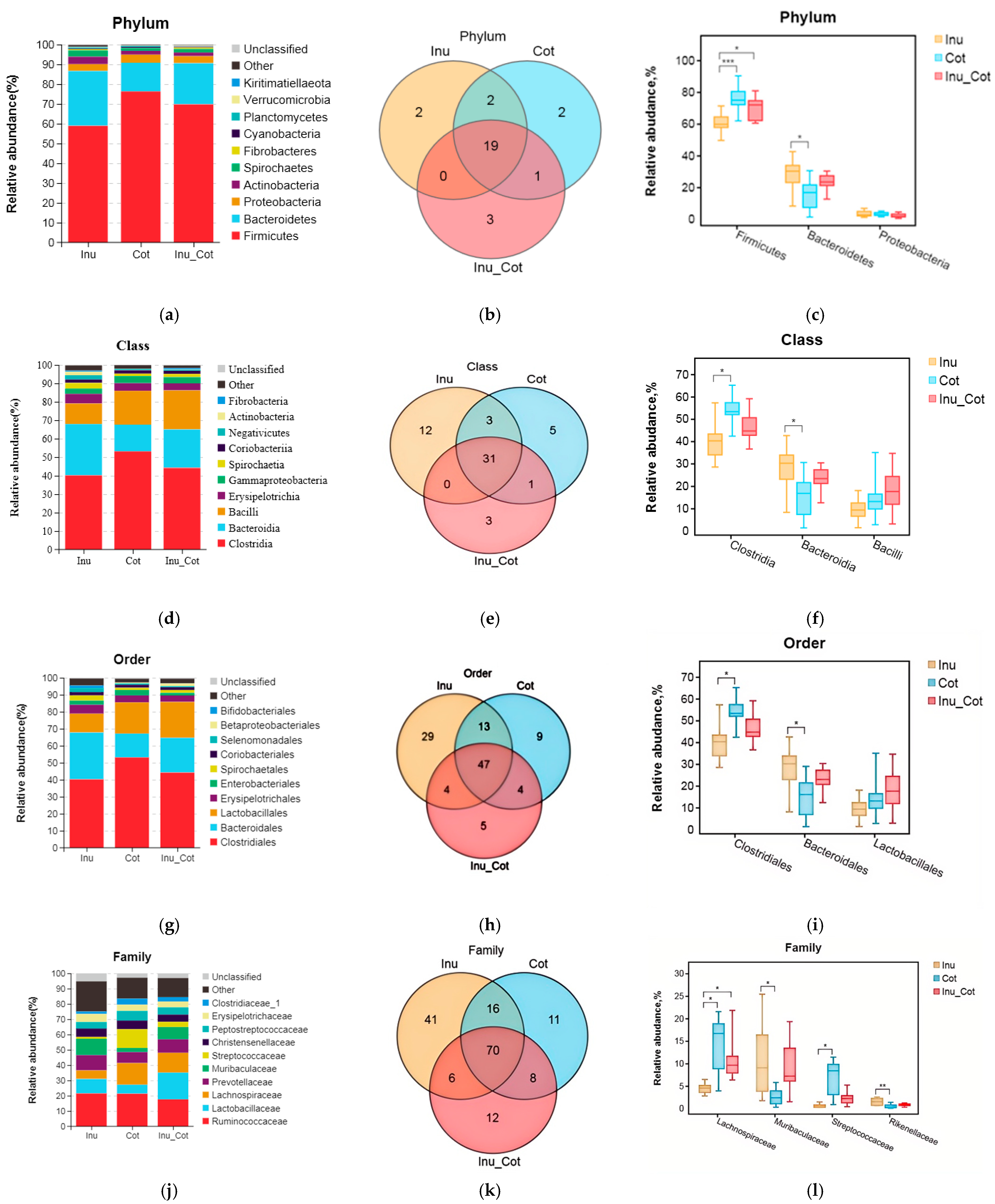
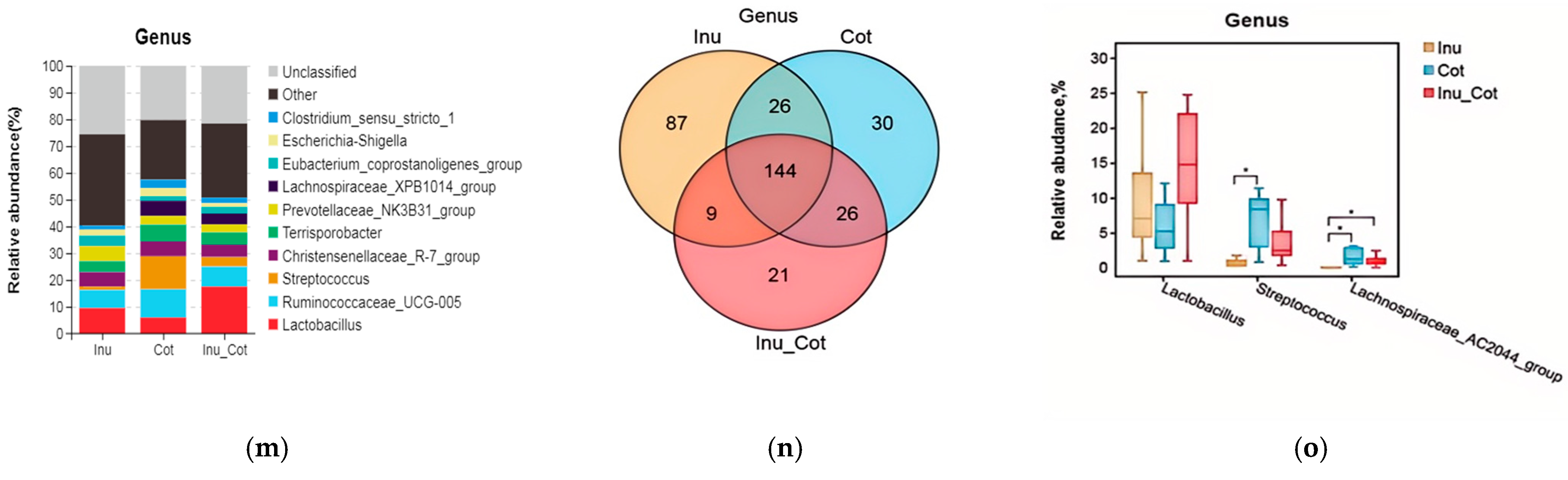
3.7. Fecal Microbiota
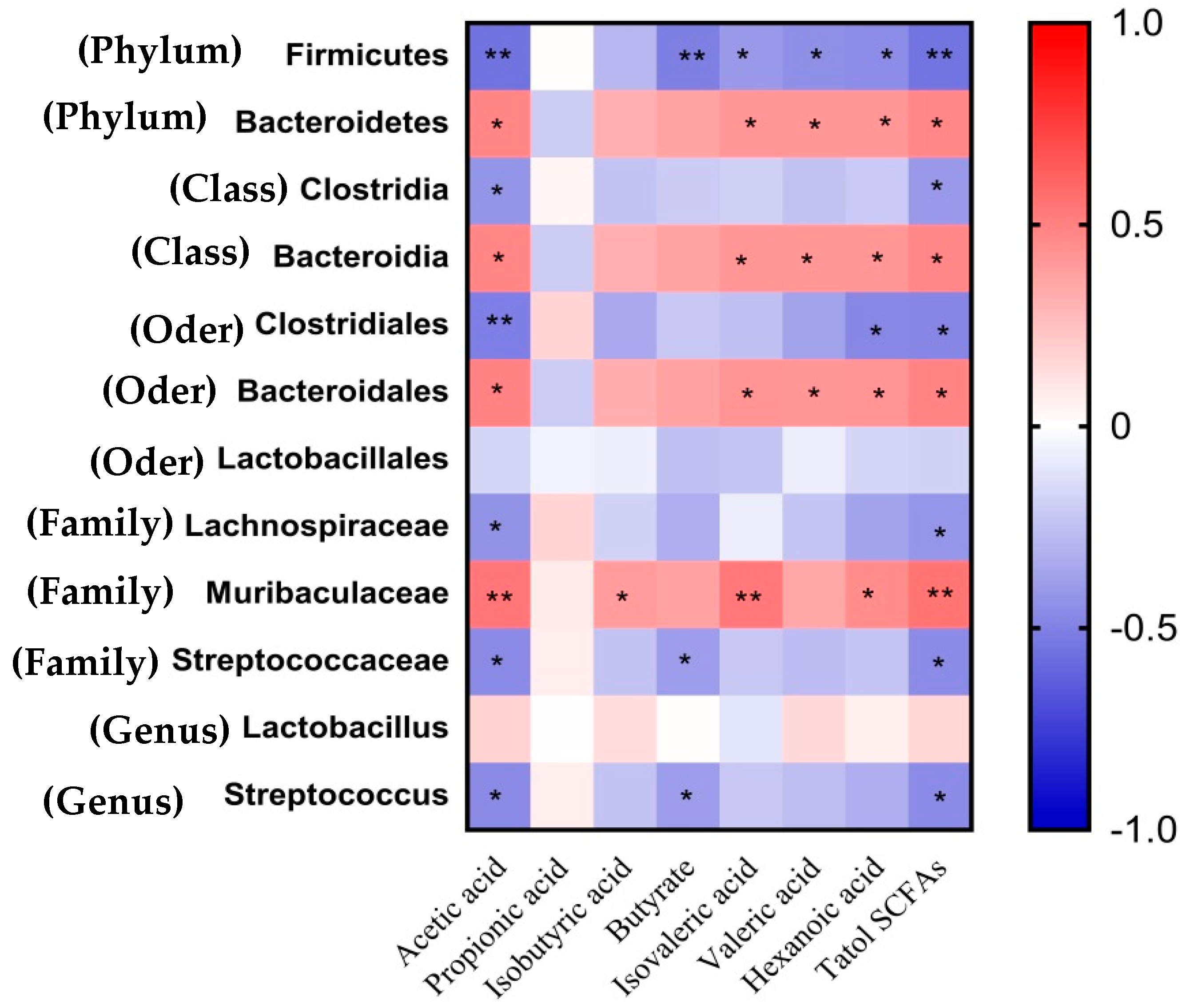
3.8. Associations of Differential Fecal Microbes with the Plasma SCFA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wu, J.; Xiong, Y.; Zhong, M.; Li, Y.; Wan, H.; Wu, D.; Liu, Q. Effects of purified fibre-mixture supplementation of gestation diet on gut microbiota, immunity and reproductive performance of sows. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1144–1154. [Google Scholar] [CrossRef]
- Weaver, A.C.; Kelly, J.M.; Kind, K.L.; Gatford, K.L.; Kennaway, D.J.; Herde, P.J.; van Wettere, W.H.E.J. Oocyte maturation and embryo survival in nulliparous female pigs (gilts) is improved by feeding a lupin-based high-fibre diet. Reprod. Fertil. Dev. 2013, 25, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Patil, G.R. Soluble dietary fiber. Compr. Rev. Food Sci. Food Saf. 2010, 9, 178–196. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Huang, W.; Guo, H.-L.; Deng, X.; Zhu, T.-T.; Xiong, J.-F.; Xu, Y.-H.; Xu, Y. Short-Chain Fatty Acids Inhibit Oxidative Stress and Inflammation in Mesangial Cells Induced by High Glucose and Lipopolysaccharide. Exp. Clin. Endocrinol. Diabetes 2017, 125, 98–105. [Google Scholar] [CrossRef]
- Renteria-Flores, J.A.; Johnston, L.J.; Shurson, G.C.; Moser, R.L.; Webel, S.K. Effect of soluble and insoluble dietary fiber on embryo survival and sow performance. J. Anim. Sci. 2008, 86, 2576–2584. [Google Scholar] [CrossRef]
- Sun, H.Q.; Tan, C.Q.; Wei, H.K.; Zou, Y.; Long, G.; Ao, J.T.; Xue, H.X.; Jiang, S.W.; Peng, J. Effects of different amounts of konjac flour inclusion in gestation diets on physio-chemical properties of diets, postprandial satiety in pregnant sows, lactation feed intake of sows and piglet performance. Anim. Reprod. Sci. 2015, 152, 55–64. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Zhang, L.; Liu, N.; Li, Z.; Zhang, F.; Liu, X.; Ma, X. Dietary inulin regulated gut microbiota and improved neonatal health in a pregnant sow model. Front. Nutr. 2021, 8, 716723. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, J.; He, J.; Liu, H.; Huang, Y.; Huang, L.; Wang, K.; Zhao, X.; Feng, B.; Che, L.; et al. Dietary fiber during gestation improves lactational feed intake of sows by modulating gut microbiota. J. Anim. Sci. Biotechnol. 2023, 14, 65. [Google Scholar] [CrossRef]
- Feyera, T.; Højgaard, C.K.; Vinther, J.; Bruun, T.S.; Theil, P.K. Dietary supplement rich in fiber fed to late gestating sows during transition reduces rate of stillborn piglets. J. Anim. Sci. 2017, 95, 5430–5438. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, N.; Li, D.; Li, H.; Fang, Z.F.; Lin, Y.; Xu, S.G.; Feng, B.; Zhuo, Y.; Wu, D.; et al. Effects of dietary soluble or insoluble fiber intake in late gestation on litter performance, milk composition, immune function, and redox status of sows around parturition. J. Anim. Sci. 2020, 98, 1–7. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Swine, 11th Revised ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- AOAC Official Method 2001. 11, 978.10, 991.43. Official Methods of Analysis of AOAC INTERNATIONAL, 18th ed.; AOAC INTERNATIONAL: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Li, H.; Liu, Z.; Lyu, H.; Gu, X.; Song, Z.; He, X.; Fan, Z. Effects of dietary inulin during late gestation on sow physiology, farrowing duration and piglet performance. Anim. Reprod. Sci. 2020, 219, 106531. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Liu, H.; Yang, Y.; He, J.; Cao, M.; Yang, M.; Zhong, W.; Lin, Y.; Zhuo, Y.; et al. Effects of the ratio of insoluble fiber to soluble fiber in gestation diets on sow performance and offspring intestinal development. Animals 2019, 9, 422. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.Q.; Sun, H.Q.; Wei, H.K.; Tan, J.J.; Long, G.; Jiang, S.W.; Peng, J. Effects of soluble fiber inclusion in gestation diets with varying fermentation characteristics on lactational feed intake of sows over two successive parities. Animal 2018, 12, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Shi, X.; Lv, G.; Hua, L.; Zhou, P.; Che, L.; Fang, Z.; Lin, Y.; Xu, S.; Li, J.; et al. Beneficial effects of dietary soluble fiber supplementation in replacement gilts: Pubertal onset and subsequent performance. Anim. Reprod. Sci. 2017, 186, 11–20. [Google Scholar] [CrossRef]
- Vestergaard, E.-M.; Danielsen, V. Dietary fibre for sows: Effects of large amounts of soluble and insoluble fibres in the pregnancy period on the performance of sows during three reproductive cycles. Anim. Sci. 1998, 67, 355–362. [Google Scholar] [CrossRef]
- Burkhalter, T.M.; Merchen, N.R.; Bauer, L.L.; Murray, S.M.; Patil, A.R.; Fahey, G.C., Jr.; Brent, J.L., Jr. The ratio of insoluble to soluble fiber components in soybean hulls affects ileal and total-tract nutrient digestibilities and fecal characteristics of dogs. J. Nutr. 2001, 131, 1978–1985. [Google Scholar] [CrossRef]
- Oliviero, C.; Kokkonen, T.; Heinonen, M.; Sankari, S.; Peltoniemi, O. Feeding sows with high fibre diet around farrowing and early lactation: Impact on intestinal activity, energy balance related parameters and litter performance. Res. Vet. Sci. 2009, 86, 314–319. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, T.; Han, L.; Zhao, L.; Niu, Y.; Chen, H. L-Glutamine Supplementation Alleviates Constipation during Late Gestation of Mini Sows by Modifying the Microbiota Composition in Feces. BioMed Res. Int. 2017, 2017, 4862861. [Google Scholar] [CrossRef] [PubMed]
- Pearodwong, P.; Muns, R.; Tummaruk, P. Prevalence of constipation and its influence on post-parturient disorders in tropical sows. Trop. Anim. Health Prod. 2016, 48, 525–531. [Google Scholar] [CrossRef]
- Tabeling, R.; Schwier, S.; Kamphues, J. Effects of different feeding and housing conditions on dry matter content and consistency of faeces in sows. J. Anim. Physiol. Anim. Nutr. 2003, 87, 116–121. [Google Scholar] [CrossRef]
- Tan, C.Q.; Wei, H.K.; Sun, H.Q.; Long, G.; Ao, J.T.; Jiang, S.W.; Peng, J. Effects of supplementing sow diets during two gestations with konjac flour and Saccharomyces boulardii on constipation in peripartal period, lactation feed intake and piglet performance. Anim. Feed. Sci. Technol. 2015, 210, 254–262. [Google Scholar] [CrossRef]
- Lan, J.; Wang, K.; Chen, G.; Cao, G.; Yang, C. Effects of inulin and isomalto-oligosaccharide on diphenoxylate-induced constipation, gastrointestinal motility-related hormones, short-chain fatty acids, and the intestinal flora in rats. Food Funct. 2020, 11, 9216–9225. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Lange, K.; Havinga, R.; van Dijk, T.H.; Gerding, A.; van Eunen, K.; Muller, M.; Groen, A.K.; Hooiveld, G.J.; Bakker, B.M.; et al. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G900–G910. [Google Scholar] [CrossRef]
- Tan, C.; Wei, H.; Ao, J.; Long, G.; Peng, J.; Björkroth, J. Inclusion of konjac flour in the gestation diet changes the gut microbiota, alleviates oxidative stress, and improves insulin sensitivity in sows. Appl. Environ. Microbiol. 2016, 82, 5899–5909. [Google Scholar] [CrossRef] [PubMed]
- Moturi, J.; Hosseindoust, A.; Tajudeen, H.; Mun, J.Y.; Ha, S.H.; Kim, J.S. Influence of dietary fiber intake and soluble to insoluble fiber ratio on reproductive performance of sows during late gestation under hot climatic conditions. Sci. Rep. 2020, 12, 19749. [Google Scholar] [CrossRef]
- Li, Y.; Liu, H.; Zhang, L.; Yang, Y.; Lin, Y.; Zhuo, Y.; Fang, Z.F.; Che, L.Q.; Feng, B.; Xu, S.Y.; et al. Maternal dietary fiber composition during gestation induces changes in offspring antioxidative capacity, inflammatory response, and gut microbiota in a sow model. Int. J. Mol. Sci. 2019, 21, 31. [Google Scholar] [CrossRef]
- Wang, J.P.; Yoo, J.S.; Kim, H.J.; Lee, J.H.; Kim, I.H. Nutrient digestibility, blood profiles and fecal microbiota are influenced by chitooligosaccharide supplementation of growing pigs. Livest. Sci. 2009, 125, 298–303. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, S.; Liu, H.; Mahfuz, S.; Piao, X. Impact of sugar beet pulp and wheat bran on serum biochemical profile, inflammatory responses and gut microbiota in sows during late gestation and lactation. J. Anim. Sci. Biotechnol. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Zervas, S.; Zijlstra, R.T. Effects of dietary protein and fermentable fiber on nitrogen excretion patterns and plasma urea in grower pigs. J. Anim. Sci. 2002, 80, 3247–3256. [Google Scholar] [CrossRef]
- Moore, R.J.; Kornegay, E.T.; Grayson, R.L.; Lindemann, M.D. Growth, nutrient utilization and intestinal morphology of pigs fed high-fiber diets. J. Anim. Sci. 1988, 66, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Luo, Z.; Wang, J.; Wu, K.; Wang, W.; Liu, Z.; Wen, J.P.; Wang, Z.B.; Duns, G.J.; Ma, X.K.; et al. Effects of different ratios of soluble to insoluble dietary fiber on growth performance and intestinal health of piglets. Anim. Nutr. 2024, 18, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chen, D.; Tian, G.; Zheng, P.; Mao, X.; Yu, J.; He, J.; Huang, Z.; Luo, Y.; Luo, J.; et al. Effects of soluble and insoluble dietary fiber supplementation on growth performance, nutrient digestibility, intestinal microbe and barrier function in weaning piglet. Anim. Feed. Sci. Technol. 2020, 260, 114335. [Google Scholar] [CrossRef]
- Mueller, A.; Koebnick, C.; Binder, H.; Hoffmann, I.; Schild, R.L.; Beckmann, M.W.; Dittrich, R. Placental defence is considered sufficient to control lipid peroxidation in pregnancy. Med. Hypotheses 2005, 64, 553–557. [Google Scholar] [CrossRef]
- Ren, W.; Yin, Y.; Liu, G.; Yu, X.; Li, Y.; Yang, G.; Li, T.; Wu, G. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 2012, 42, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, F.; Duan, L.-R.; Sheng, J.-J.; Xie, Y.-H.; Yang, Q.; Chen, Y.; Dong, Q.-Q.; Zhang, B.-L.; Wang, S.-W. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci. Rep. 2016, 6, 23693. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Zhou, P.; Liu, H.; Li, S.; Zhao, Y.; Deng, K.; Cao, D.D.; Che, L.Q.; Fang, Z.F.; Xu, S.Y.; et al. Effects of inulin supplementation in low-or high-fat diets on reproductive performance of sows and antioxidant defence capacity in sows and offspring. Reprod. Domest. Anim. 2016, 51, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jin, Z.; Zhao, R.; Ren, K.; Deng, C.; Yu, S. Resveratrol attenuates inflammation and oxidative stress induced by myocardial ischemia-reperfusion injury: Role of Nrf2/ARE pathway. Int. J. Clin. Exp. Med. 2015, 8, 10420. [Google Scholar]
- Hakansson, A.; Molin, G. Gut microbiota and inflammation. Nutrients 2011, 3, 637–682. [Google Scholar] [CrossRef]
- Sasaki, D.; Sasaki, K.; Kondo, A. Glycosidic linkage structures influence dietary fiber ferment ability and propionate production by human colonic microbiota in vitro. Biotechnol. J. 2020, 15, e1900523. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Walter, S.; Belheouane, M.; Bonfiglio, F.; Heinsen, F.-A.; Andreasson, A.; Agreus, L.; Engstrand, L.; Baines, J.F.; Rafter, J.; et al. Stool frequency is associated with gut microbiota composition. Gut 2017, 66, 559–560. [Google Scholar] [CrossRef]
- Tsatsaronis, J.A.; Walker, M.J.; Sanderson-Smith, M.L.; Chitnis, C.E. Host responses to group a streptococcus: Cell death and inflammation. PLoS Pathog. 2014, 10, e1004266. [Google Scholar] [CrossRef]

| Ingredient | 8% Inulin | 8% Cotton Fiber | 4% Inulin + 4% Cotton Fiber |
|---|---|---|---|
| Corn | 69.85 | 69.85 | 69.85 |
| Soybean meal | 18.00 | 18.00 | 18.00 |
| Inulin | 8.00 | - | 4.00 |
| Cotton fiber powder | - | 8.00 | 4.00 |
| L-Lysine-HCl | 0.10 | 0.10 | 0.10 |
| Dicalcium phosphate | 1.20 | 1.20 | 1.20 |
| Limestone | 1.20 | 1.20 | 1.20 |
| Salt | 0.40 | 0.40 | 0.40 |
| Choline chloride (50%) | 0.25 | 0.25 | 0.25 |
| Premix a | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated nutritional level b | |||
| Digestible energy, kcal/kg | 3077 | 3077 | 3077 |
| Net energy, kcal/kg | 2250 | 2250 | 2250 |
| Crude protein, % | 13.60 | 13.60 | 13.60 |
| Crude fiber, % | 9.75 | 9.75 | 9.75 |
| Soluble fiber, % | 8.68 | 2.28 | 5.48 |
| Insoluble fiber, % | 9.75 | 16.15 | 12.95 |
| Lysine, % | 0.79 | 0.79 | 0.79 |
| Methionine + Cysteine, % | 0.50 | 0.50 | 0.50 |
| Threonine, % | 0.53 | 0.53 | 0.53 |
| Calcium, % | 0.78 | 0.78 | 0.78 |
| Phosphorus, % | 0.50 | 0.50 | 0.50 |
| STTD Phosphorus, % | 0.32 | 0.32 | 0.32 |
| Analyzed nutritional level | |||
| Crude protein, % | 13.71 | 13.52 | 13.65 |
| Crude fiber, % | 9.45 | 9.50 | 9.66 |
| Soluble fiber, % | 8.50 | 2.45 | 5.46 |
| Insoluble fiber, % | 9.65 | 16.20 | 12.95 |
| Insoluble fiber:Soluble fiber | 1.14 | 6.61 | 2.37 |
| Item | Inulin | Cotton Fiber | Inulin + Cotton Fiber | SEM | p-Value |
|---|---|---|---|---|---|
| Total born, n | 13.33 | 12.30 | 12.44 | 0.460 | 0.080 |
| Born alive, n | 13.11 a | 11.50 b | 11.89 b | 0.477 | 0.045 |
| Stillborn, n | 0.33 | 0.30 | 0.44 | 0.143 | 0.940 |
| Mummified, n | 0.38 | 0.10 | 0.22 | 0.170 | 0.237 |
| Litter weight of born alive, kg | 18.84 | 17.18 | 17.55 | 0.708 | 0.769 |
| Average live birth weight, kg | 1.44 | 1.41 | 1.42 | 0.091 | 0.963 |
| Weight of placenta, kg | 4.46 | 3.98 | 3.72 | 0.223 | 0.407 |
| Farrowing duration, min | 223 | 189 | 224 | 15.590 | 0.589 |
| Birth intervals, min | 17.00 | 15.98 | 17.05 | 1.680 | 0.651 |
| Back fat, cm | |||||
| Day 30 | 2.21 | 2.23 | 2.34 | 0.093 | 0.786 |
| Day 90 | 2.20 | 2.25 | 2.30 | 0.074 | 0.456 |
| Day 110 | 2.17 | 2.33 | 2.29 | 0.082 | 0.414 |
| Item | Inulin | Cotton Fiber | Inulin + Cotton Fiber | SEM | p-Value |
|---|---|---|---|---|---|
| Acetic acid, μg/L | 2934 a | 1229 c | 1893 b | 126 | 0.001 |
| Propionic acid, μg/L | 383 | 417 | 430 | 14 | 0.413 |
| Isobutyric acid, μg/L | 33.19 | 15.26 | 21.70 | 3.83 | 0.142 |
| Butyric acid, μg/L | 276.00 a | 73.03 b | 132.75 b | 23.25 | 0.001 |
| Isovaleric acid, μg/L | 41.16 | 23.28 | 27.79 | 4.14 | 0.180 |
| Valeric acid, μg/L | 14.28 | 9.74 | 11.89 | 1.05 | 0.197 |
| Hexanoic acid, μg/L | 64.54 a | 37.79 b | 43.69 b | 3.69 | 0.001 |
| Total-SCFAs, μg/L | 3746.17 a | 1805.1 c | 2560.82 b | 144 | 0.001 |
| Item | Inulin | Cotton Fiber | Inulin + Cotton Fiber | SEM | p-Value |
|---|---|---|---|---|---|
| 90 day | |||||
| Total protein, g/L | 74.78 | 67.41 | 74.12 | 2.411 | 0.315 |
| Creatinine, μmol/L | 235.02 | 206.78 | 226.32 | 5.24 | 0.072 |
| Albumin, g/L | 32.60 b | 37.93 a | 39.23 a | 1.005 | 0.013 |
| Urea, mmol/L | 12.03 b | 17.04 a | 17.43 a | 1.081 | 0.001 |
| Uric acid, μmol/L | 19.32 b | 22.35 a | 25.73 a | 1.084 | 0.001 |
| Glucose, mmol/L | 3.66 | 3.66 | 3.95 | 0.086 | 0.306 |
| Triacylglycerol, mmol/L | 1.47 b | 1.62 b | 1.95 a | 0.069 | 0.011 |
| Cholesterol, mmol/L | 1.44 | 1.54 | 1.55 | 0.045 | 0.593 |
| HDL—Cholesterol, mmol/L | 3.79 | 4.43 | 4.19 | 0.161 | 0.278 |
| LDL—Cholesterol, mmol/L | 0.68 | 0.70 | 0.69 | 0.026 | 0.953 |
| Calcium, mmol/L | 2.45 b | 2.54 a | 2.73 a | 0.028 | 0.001 |
| Phosphorus, mmol/L | 1.68 b | 1.85 a | 1.83 a | 0.023 | 0.001 |
| 110 day | |||||
| Total protein, g/L | 70.89 | 73.26 | 71.86 | 1.469 | 0.435 |
| Creatinine, μmol/L | 236.25 a | 205.88 b | 213.35 b | 4.795 | 0.02 |
| Albumin, g/L | 41.60 | 42.06 | 36.82 | 1.074 | 0.097 |
| Urea, mmol/L | 17.53 | 18.07 | 18.43 | 0.431 | 0.722 |
| Uric acid, μmol/L | 30.90 c | 36.55 b | 44.39 a | 1.427 | 0.001 |
| Glucose, mmol/L | 4.03 | 4.00 | 4.16 | 0.094 | 0.776 |
| Triacylglycerol, mmol/L | 2.46 a,b | 2.01 b | 2.97 a | 0.155 | 0.036 |
| Cholesterol, mmol/L | 1.41 | 1.44 | 1.41 | 0.041 | 0.929 |
| HDL—Cholesterol, mmol/L | 4.84 a,b | 4.47 b | 5.34 a | 0.146 | 0.05 |
| LDL—Cholesterol, mmol/L | 0.62 | 0.64 | 0.61 | 0.025 | 0.857 |
| Calcium, mmol/L | 2.81 b | 2.97 a | 3.07 a | 0.033 | 0.003 |
| Phosphorus, mmol/L | 1.62 b | 1.92 a | 1.84 a | 0.041 | 0.003 |
| Item | Inulin | Cotton Fiber | Inulin + Cotton Fiber | SEM | p-Value |
|---|---|---|---|---|---|
| Milk protein, % | 15.04 | 15.81 | 17.13 | 0.571 | 0.344 |
| Milk fat, % | 5.72 | 5.34 | 6.46 | 0.236 | 0.140 |
| Total solid, % | 27.46 | 27.75 | 30.50 | 0.690 | 0.147 |
| Non-fat solid, % | 19.24 | 19.83 | 20.60 | 0.487 | 0.552 |
| Lactose, % | 4.01 | 3.76 | 3.51 | 0.117 | 0.229 |
| IgA, μg/mL | 14.92 | 12.66 | 14.02 | 0.98 | 0.653 |
| IgM, μg/mL | 25.30 | 24.21 | 25.04 | 0.42 | 0.539 |
| IgG, μg/mL | 192.36 | 189.00 | 180.76 | 8.38 | 0.850 |
| Item | Inulin | Cotton Fiber | Inulin + Cotton Fiber | SEM | p-Value |
|---|---|---|---|---|---|
| 90 day | |||||
| T-AOC, mmol/L | 0.32 | 0.31 | 0.32 | 0.007 | 0.643 |
| T-SOD, U/mL | 396 | 408 | 406 | 8.320 | 0.817 |
| CAT, U/mL | 2.68 a | 1.66 b | 1.90 b | 0.165 | 0.025 |
| MDA, nmol/mL | 2.57 b | 3.59 a | 1.70 c | 0.180 | 0.001 |
| 110 day | |||||
| T-AOC, mmol/L | 0.26 a | 0.25 a,b | 0.23 b | 0.005 | 0.005 |
| T-SOD, U/mL | 434 | 394 | 346 | 14.960 | 0.059 |
| CAT, U/mL | 1.93 | 1.65 | 2.06 | 0.113 | 0.153 |
| MDA, nmol/mL | 1.26 | 1.32 | 1.20 | 0.065 | 0.769 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Wu, Q.; Gao, K.; Yang, X.; Xiao, H.; Jiang, Z.; Wang, L. Effects of Dietary Ratio of Insoluble Fiber to Soluble Fiber on Reproductive Performance, Biochemical Parameters, and Fecal Microbial Composition of Gestating Sows. Animals 2025, 15, 1850. https://doi.org/10.3390/ani15131850
Wen X, Wu Q, Gao K, Yang X, Xiao H, Jiang Z, Wang L. Effects of Dietary Ratio of Insoluble Fiber to Soluble Fiber on Reproductive Performance, Biochemical Parameters, and Fecal Microbial Composition of Gestating Sows. Animals. 2025; 15(13):1850. https://doi.org/10.3390/ani15131850
Chicago/Turabian StyleWen, Xiaolu, Qiwen Wu, Kaiguo Gao, Xuefen Yang, Hao Xiao, Zongyong Jiang, and Li Wang. 2025. "Effects of Dietary Ratio of Insoluble Fiber to Soluble Fiber on Reproductive Performance, Biochemical Parameters, and Fecal Microbial Composition of Gestating Sows" Animals 15, no. 13: 1850. https://doi.org/10.3390/ani15131850
APA StyleWen, X., Wu, Q., Gao, K., Yang, X., Xiao, H., Jiang, Z., & Wang, L. (2025). Effects of Dietary Ratio of Insoluble Fiber to Soluble Fiber on Reproductive Performance, Biochemical Parameters, and Fecal Microbial Composition of Gestating Sows. Animals, 15(13), 1850. https://doi.org/10.3390/ani15131850








