Diagnostic Approach and Pathological Characterization of Metastatic Intrahepatic Cholangiocarcinoma in a Captive Puma (Puma concolor)
Simple Summary
Abstract
1. Introduction
2. Case Presentation
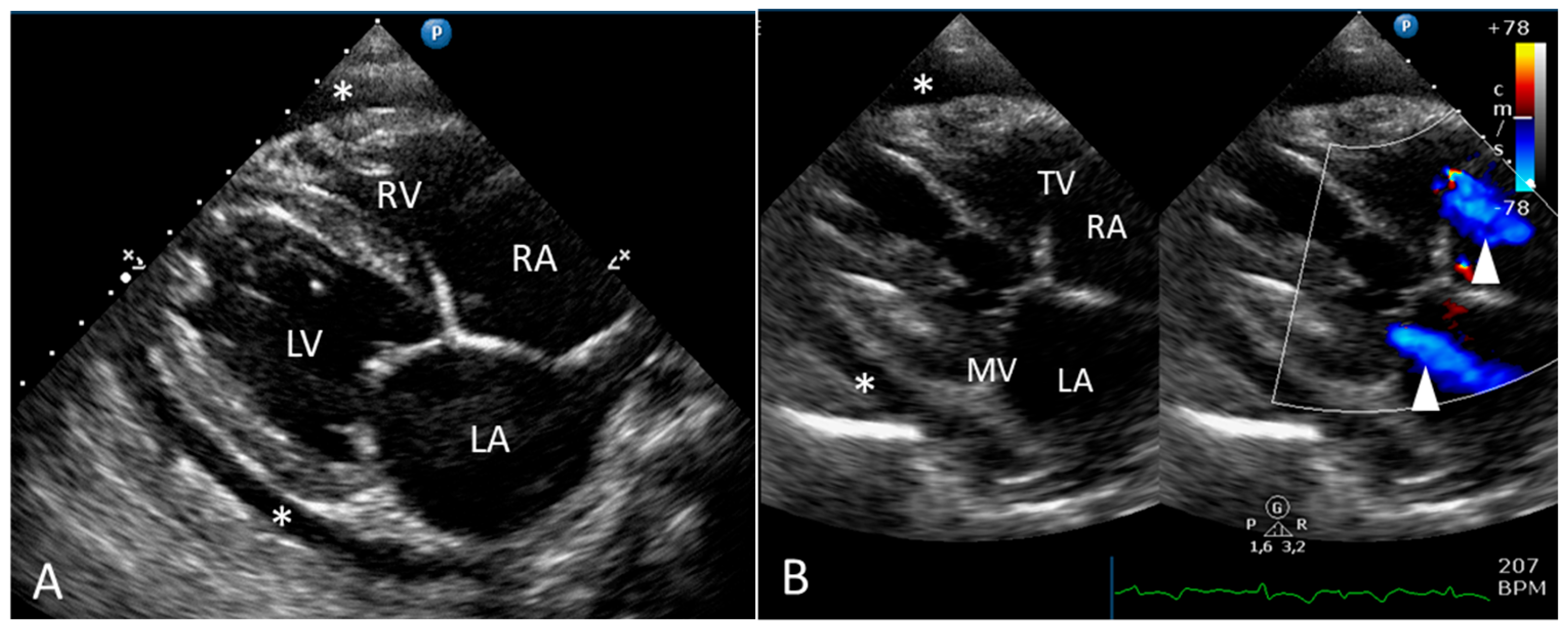
2.1. Clinical Report
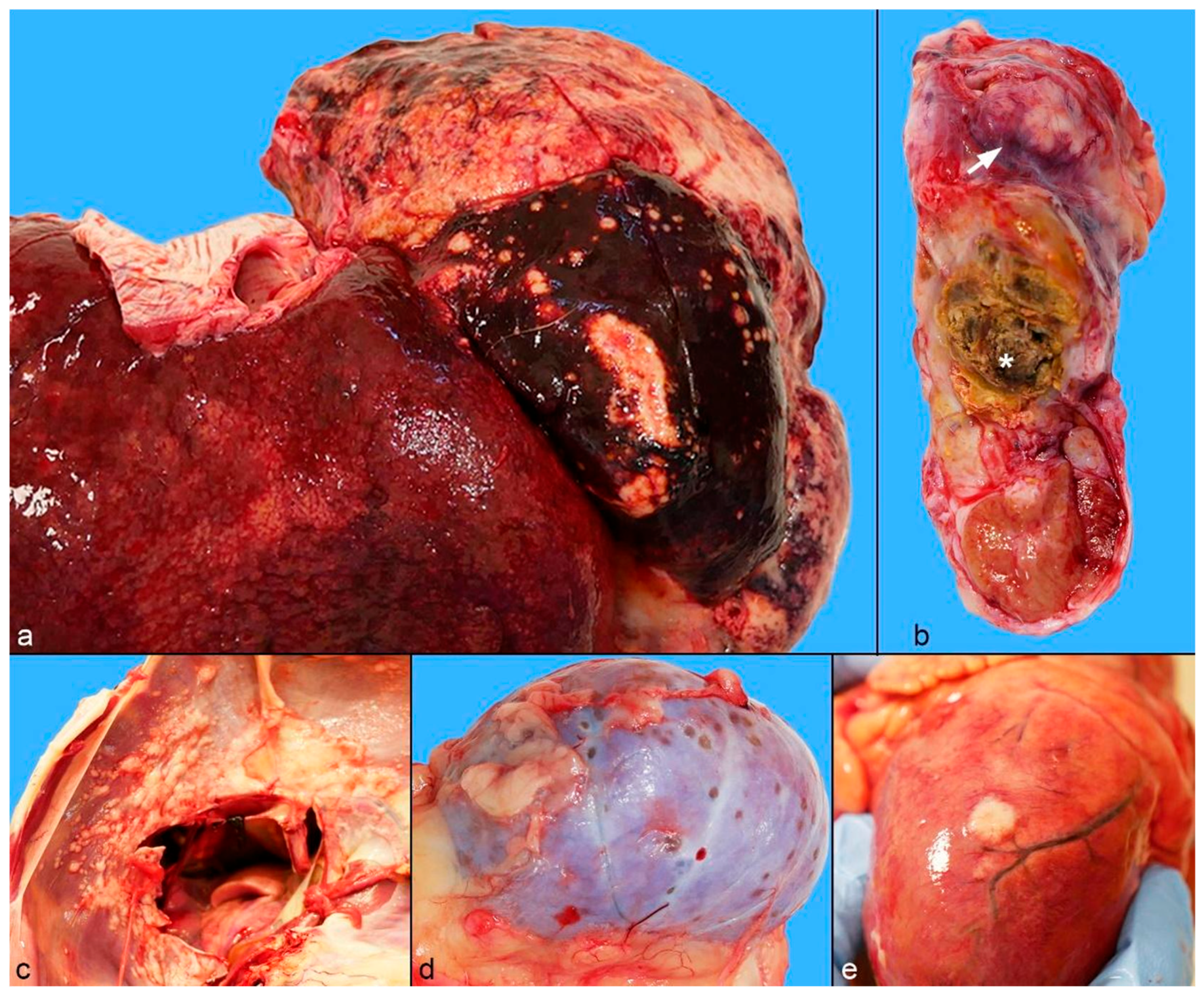
2.2. Gross Pathology
2.3. Cytological, Histological, and Immunohistochemical Findings
2.4. Microbiological Findings
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Junginger, J.; Hansmann, F.; Herder, V.; Lehmbecker, A.; Peters, M.; Beyerbach, M.; Wohlsein, P.; Baumgärtner, W. Pathology in Captive Wild Felids at German Zoological Gardens. PLoS ONE 2015, 10, e0130573. [Google Scholar] [CrossRef] [PubMed]
- Thalwitzer, S.; Wachter, B.; Robert, N.; Wibbelt, G.; Muller, T.; Lonzer, J.; Meli, M.L.; Bay, G.; Hofer, H.; Lutz, H. Seroprevalences to Viral Pathogens in Free-Ranging and Captive Cheetahs (Acinonyx Jubatus) on Namibian Farmland. Clin. Vaccine Immunol. 2010, 17, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Lepri, E.; Sforna, M.; Chiara, B.; Giovanni, V. Cholangiocarcinoma of intrahepatic bile ducts with disseminated metastases in an african lion (Panthera leo). J. Zoo Wildl. Med. 2013, 44, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, A.; Garner, M.M. A Retrospective Study of Neoplasia in Nondomestic Felids in Human Care, with a Comparative Literature Review. J. Zoo Wildl. Med. 2021, 52, 413–426. [Google Scholar] [CrossRef]
- D’Aquino, I.; Piegari, G.; Casciaro, S.M.; Prisco, F.; Rosato, G.; Silvestre, P.; Degli Uberti, B.; Capasso, M.; Laricchiuta, P.; Paciello, O.; et al. An Overview of Neoplasia in Captive Wild Felids in Southern Italy Zoos. Front. Vet. Sci. 2022, 9, 899481. [Google Scholar] [CrossRef]
- Cho, H.-S.; Oh, Y. Cholangiocarcinoma with Multiple Organ Metastasis in a Captive Puma (Puma concolor). Pak. J. Zool. 2021, 54, 487–490. [Google Scholar] [CrossRef]
- Daigle, C.L.; Brown, J.L.; Carlstead, K.; Pukazhenthi, B.; Freeman, E.W.; Snider, R.J. Multi-Institutional Survey of Social, Management, Husbandry and Environmental Factors for the SSP African Lion Panthera Leo Population: Examining the Effects of a Breeding Moratorium in Relation to Reproductive Success. Int. Zoo Yearb. 2015, 49, 198–213. [Google Scholar] [CrossRef]
- Coon, C.A.C.; Nichols, B.C.; McDonald, Z.; Stoner, D.C. Effects of Land-Use Change and Prey Abundance on the Body Condition of an Obligate Carnivore at the Wildland-Urban Interface. Landsc. Urban. Plan. 2019, 192, 103648. [Google Scholar] [CrossRef]
- Carrino, M.; Tassoni, L.; Campalto, M.; Cavicchio, L.; Mion, M.; Corrò, M.; Natale, A.; Beato, M.S. Molecular Investigation of Recent Canine Parvovirus-2 (CPV-2) in Italy Revealed Distinct Clustering. Viruses 2022, 14, 917. [Google Scholar] [CrossRef]
- Decaro, N.; Elia, G.; Martella, V.; Desario, C.; Campolo, M.; Di Trani, L.; Tarsitano, E.; Tempesta, M.; Buonavoglia, C. A Real-Time PCR Assay for Rapid Detection and Quantitation of Canine Parvovirus Type 2 in the Feces of Dogs. Vet. Microbiol. 2005, 105, 19–28. [Google Scholar] [CrossRef]
- Owston, M.A.; Ramsay, E.C.; Rotstein, D.S. Neoplasia in Felids at the Knoxville Zoological Gardens, 1979–2003. J. Zoo Wildl. Med. 2008, 39, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.H.M.A.; Flores, A.S.; Fedullo, J.D.L.; Teixeira, R.H.F.; Mirandola, R.M.S.; Ito, F.H.; Pessoa, R.B.; Itikawa, P.H. Biochemical Parameters of Wild Felids (Panthera Leo and Panthera Tigris Altaica) Kept in Captivity. Semin. Agrar. 2017, 38, 791–800. [Google Scholar] [CrossRef]
- Dias, F.G.G.; de Brito, C.C.C.; Paulino Júnior, D.; Branco, C.H.; de Brito, V.J.S.C.; Tinasi, A.L.S.N.; Alves, M.Z.; Badoco, F.R.; Rodrigues, M.A. Parâmetros Hematológicos de Gatos Selvagens Das Espécies Puma concolor, Panthera Onca e Panthera Leo Mantidos Em Cativeiro. Res. Soc. Dev. 2022, 11, e35711629288. [Google Scholar] [CrossRef]
- Mazzotta, E.; Foiani, G.; De Benedictis, G.M.; Fiore, E.; Natale, A.; Spagnolo, E.; Vascellari, M.; Cento, G.; Corrò, M. Salmonella Enteritidis Fatal Septicemia with Meningoencephalitis in a Tiger (Panthera Tigris) Cub. Animals 2022, 12, 2490. [Google Scholar] [CrossRef]
- Bevins, S.N.; Carver, S.; Boydston, E.E.; Lyren, L.M.; Alldredge, M.; Logan, K.A.; Riley, S.P.D.; Fisher, R.N.; Vickers, T.W.; Boyce, W.; et al. Three Pathogens in Sympatric Populations of Pumas, Bobcats, and Domestic Cats: Implications for Infectious Disease Transmission. PLoS ONE 2012, 7, e31403. [Google Scholar] [CrossRef]
- Terio, K.A.; Mitchell, E.; Walzer, C.; Schmidt-Küntzel, A.; Marker, L.; Citino, S. Diseases Impacting Captive and Free-Ranging Cheetahs. In Cheetahs: Biology and Conservation: Biodiversity of the World: Conservation from Genes to Landscapes; Academic Press: Cambridge, MA, USA, 2018; pp. 349–364. ISBN 9780128041208. [Google Scholar]
- Martella, V.; Campolo, M.; Lorusso, E.; Cavicchio, P.; Camero, M.; Bellacicco, A.L.; Decaro, N.; Elia, G.; Greco, G.; Corrente, M.; et al. Norovirus in Captive Lion Cub (Panthera Leo). Emerg. Infect. Dis. 2007, 13, 1071–1073. [Google Scholar] [CrossRef]
- Coleman, K.K.; Bemis, I.G. Avian Influenza Virus Infections in Felines: A Systematic Review of Two Decades of Literature. Open Forum Infect. Dis. 2025, 12, ofaf261. [Google Scholar] [CrossRef]
- Redrobe, S.P. Avian Influenza H5N1: A Review of the Current Situation and Relevance to Zoos. Int. Zoo Yearb. 2007, 41, 96–109. [Google Scholar] [CrossRef]
- Beirne, P. COVID-19 as an Anthroponosis: Toward a Nonspeciesist Criminology of Human-to-Animal Pathogen Transmission. Int. J. Crime Justice Soc. Democr. 2021, 10, 139–152. [Google Scholar] [CrossRef]
- Fernández-Bellon, H.; Rodon, J.; Fernández-Bastit, L.; Almagro, V.; Padilla-Solé, P.; Lorca-Oró, C.; Valle, R.; Roca, N.; Grazioli, S.; Trogu, T.; et al. Monitoring Natural SARS-CoV-2 Infection in Lions (Panthera Leo) at the Barcelona Zoo: Viral Dynamics and Host Responses. Viruses 2021, 13, 1683. [Google Scholar] [CrossRef]
- Schiffmann, C.; Unterhitzenberger, G.; Ortmann, S. Extrahepatic Biliary Tract Pathologies in Mammalian Species of Zoo Animals and Wildlife: A Review. J. Basic Appl. Zool. 2020, 81, 20. [Google Scholar] [CrossRef]
- Grillini, M.; Frangipane di Regalbono, A.; Tessarin, C.; Beraldo, P.; Cassini, R.; Marchiori, E.; Simonato, G. Evidence of Dirofilaria Immitis in Felids in North-Eastern Italy. Pathogens 2022, 11, 1216. [Google Scholar] [CrossRef] [PubMed]
- Ready, Z.C.; LoBato, D.; LaDouceur, E.; Garner, M.M.; Cushing, A.C. Melanocytic neoplasia in panthera species: Clinical presentations, pathologic findings and responses to treatment. J. Zoo Wildl. Med. 2023, 53, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.M.; Newkirk, K.M.; McRee, A.E.; Whittemore, J.C.; Ramsay, E.C. Hepatic Lesions in 90 Captive Nondomestic Felids Presented for Autopsy. Vet. Pathol. 2015, 52, 369–376. [Google Scholar] [CrossRef]
- Kloft, H.M.; Ramsay, E.C.; Sula, M.M. Neoplasia in Captive Panthera Species. J. Comp. Pathol. 2019, 166, 35–44. [Google Scholar] [CrossRef]
- Tidière, M.; Gaillard, J.-M.; Berger, V.; Müller, D.W.H.; Bingaman Lackey, L.; Gimenez, O.; Clauss, M.; Lemaître, J.-F. Comparative Analyses of Longevity and Senescence Reveal Variable Survival Benefits of Living in Zoos across Mammals. Sci. Rep. 2016, 6, 36361. [Google Scholar] [CrossRef]
- Van Sprundel, R.G.H.M.; van den Ingh, T.S.G.A.M.; Guscetti, F.; Kershaw, O.; van Wolferen, M.E.; Rothuizen, J.; Spee, B. Classification of Primary Hepatic Tumours in the Cat. Vet. J. 2014, 202, 255–266. [Google Scholar] [CrossRef]
- McAloose, D.; Newton, A.L. Wildlife Cancer: A Conservation Perspective. Nat. Rev. Cancer 2009, 9, 517–526. [Google Scholar] [CrossRef]
- Cullen, J.M. Tumors of the Liver and Gallbladder. In Tumors in Domestic Animals; Wiley: Hoboken, NJ, USA, 2016; pp. 602–631. [Google Scholar]
- Balkman, C. Hepatobiliary Neoplasia in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 617–625. [Google Scholar] [CrossRef]
- Patnaik, A.K. A Morphologic and Immunocytochemical Study of Hepatic Neoplasms in Cats. Vet. Pathol. 1992, 29, 405–415. [Google Scholar] [CrossRef]
- Post, G.; Patnaik, A.K. Nonhematopoietic Hepatic Neoplasms in Cats: 21 Cases (1983–1988). J. Am. Vet. Med. Assoc. 1992, 201, 1080–1082. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Yanai, T.; Yonemaru, K.; Hirata, A.; Masegi, T. Gallbladder Adenocarcinomas in Two Captive African Lions (Panthera Leo). J. Zoo Wildl. Med. 2003, 34, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.L.; Andrews, L.K.; Holzworth, J. Tumors and Tumor-like Lesions. In Diseases of the Cat; W.B. Saunders Company: Philadelphia, PA, USA, 1987; pp. 406–596. ISBN 0-7216-4863-4. [Google Scholar]
- Ramos-Vara, J.A.; Miller, M.A.; Johnson, G.C. Immunohistochemical Characterization of Canine Hyperplastic Hepatic Lesions and Hepatocellular and Biliary Neoplasms with Monoclonal Antibody Hepatocyte Paraffin 1 and a Monoclonal Antibody to Cytokeratin 7. Vet. Pathol. 2001, 38, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Espinosa de Los Monteros, A.; Fernández, A.; Millán, M.Y.; Rodríguez, F.; Herráez, P.; Martín de Las Mulas, J. Coordinate Expression of Cytokeratins 7 and 20 in Feline and Canine Carcinomas. Vet. Pathol. 1999, 36, 179–190. [Google Scholar] [CrossRef]
- Foster, G.W.; Cunningham, M.W. Hematology and Serum Chemistry Values for Free-Ranging Florida Panther Neonates with a Comparison to Adult Panther Values. J. Wildl. Dis. 2009, 45, 857–862. [Google Scholar] [CrossRef]
- Hawkey, C.M.; Hart, M.G. Haematological Reference Values for Adult Pumas, Lions, Tigers, Leopards, Jaguars and Cheetahs. Res. Vet. Sci. 1986, 41, 268–269. [Google Scholar] [CrossRef]
- Proverbio, D.; Perego, R.; Baggiani, L.; Ravasio, G.; Giambellini, D.; Spada, E. Hematological and Biochemical Reference Values in Healthy Captive Tigers (Panthera Tigris). Animals 2021, 11, 3440. [Google Scholar] [CrossRef]
- Thrall, M.A.; Weiser, G.; Allison, R.W.; Campbell, T.W. Veterinary Hematology, Clinical Chemistry, and Cytology, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2022; ISBN 978-1-119-28652-3. [Google Scholar]
- Harvey, J.W. The Feline Blood Film: 2. Leukocyte and Platelet Morphology. J. Feline Med. Surg. 2017, 19, 747–757. [Google Scholar] [CrossRef]
- Tasker, S.; Addie, D.D.; Egberink, H.; Hofmann-Lehmann, R.; Hosie, M.J.; Truyen, U.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Feline Infectious Peritonitis: European Advisory Board on Cat Diseases Guidelines. Viruses 2023, 15, 1847. [Google Scholar] [CrossRef]
- Stout, A.E.; André, N.M.; Whittaker, G.R. Feline coronavirus and feline infectious peritonitis in nondomestic felid species. J. Zoo Wildl. Med. 2021, 52, 14–27. [Google Scholar] [CrossRef]
- Stephenson, N.; Swift, P.; Moeller, R.B.; Worth, S.J.; Foley, J. Feline Infectious Peritonitis in a Mountain Lion (Puma concolor), California, USA. J. Wildl. Dis. 2013, 49, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.W. Risk Factors for Biliary Tract Carcinogenesis. Ann. Oncol. 1999, 10, S308–S312. [Google Scholar] [CrossRef]
- Sripa, B.; Mairiang, E.; Thinkhamrop, B.; Laha, T.; Kaewkes, S.; Sithithaworn, P.; Tessana, S.; Loukas, A.; Brindley, P.J.; Bethony, J.M. Advanced Periductal Fibrosis from Infection with the Carcinogenic Human Liver Fluke Opisthorchis Viverrini Correlates with Elevated Levels of Interleukin-6. Hepatology 2009, 50, 1273–1281. [Google Scholar] [CrossRef]
- Loeuillard, E.; Fischbach, S.R.; Gores, G.J.; Ilyas, S.I. Animal Models of Cholangiocarcinoma. Biochim. Biophys. Acta-Mol. Basis Dis. 2019, 1865, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.P. Pathological Changes in the Intrahepatic Bile Ducts of Cats (Felis Catus) Infested with Clonorchis Sinensis. J. Pathol. Bacteriol. 1965, 89, 357–364. [Google Scholar] [CrossRef]
- Maronpot, R.R.; Giles, H.D.; Dykes, D.J.; Irwin, R.D. Furan-Induced Hepatic Cholangiocarcinomas in Fischer 344 Rats. Toxicol. Pathol. 1991, 19, 561–570. [Google Scholar] [CrossRef]
- Hirao, K.; Matsumura, K.; Imagawa, A.; Enomoto, Y.; Hosogi, Y. Primary Neoplasms in Dog Liver Induced by Diethylnitrosamine. Cancer Res. 1974, 34, 1870–1882. [Google Scholar]


| Hematology | Value |
|---|---|
| RBCs | 9.96 M/μL |
| Hgb | 14.8 g/dL |
| Hct | 41.8% |
| MCV | 42.0 fL |
| MCH | 14.9 pg |
| MCHC | 35.4 g/dL |
| RDW | 21.2% |
| RBCs Morphology | Anisocytosis, Echinocytosis |
| WBCs | 14.94 (103/μL) |
| NEUT | 13.13 (103/μL) |
| LYMPH | 0.72 (103/μL) |
| MONO | 1.02 (103/μL) |
| EO | 0.06 (103/μL) |
| BASO | 0.01 (103/μL) |
| WBCs Morphology | Neutrophil Toxicity, Döhle bodies |
| PLT | 329 (103/μL) |
| MPV | 9.7 fL |
| Serum Biochemistry | Value |
| Total Proteins | 71 g/L |
| Albumin | 34 g/L |
| Globulin | 37 g/L |
| Urea Nitrogen | 25.2 mmol/L |
| Creatinine | 284 μmol/L |
| Glucose | 3.1 mmol/L |
| Cholesterol | 4.25 mmol/L |
| Triglycerides | 0.50 mmol/L |
| Total Bilirubin | 4.84 μmol/L |
| Direct Bilirubin | 2.55 μmol/L |
| Unconjugated Bilirubin | 2.29 μmol/L |
| AST | 50 U/L |
| ALT | 34 U/L |
| ALP | 19 U/L |
| GGT | 8 U/L |
| Cholinesterase | 4385 U/L |
| CK | 229 U/L |
| Calcium | 2.58 mmol/L |
| Phosphorus | 1.65 mmol/L |
| Magnesium | 1.27 mmol/L |
| Sodium | 156 mmol/L |
| Potassium | 4.53 mmol/L |
| Chlorine | 115 mmol/L |
| Iron | 55 μg/dL |
| Antibody | Antigen Retrieval | Antibody Incubation | Detection System |
|---|---|---|---|
| Pan cytokeratin | CC1 a,b; 95 °C; 32 min | 1:50; 24 min; RT | Discovery OmniMap anti Mouse HRP b 16 min; Discovery Chromomap DAB b |
| CK7 | CC1; 95 °C; 64 min | 1:20; 1 h; RT | Discovery Anti Mouse HQ b 36 °C 8 min; Discovery anti HQ HRP b 8 min; Discovery Chromomap DAB |
| CK20 | CC1; 95 °C; 40 min | 1:20; 1 h; 37 °C | Discovery Anti Mouse HQ 36 °C 8 min; Discovery anti HQ HRP 8 min; Discovery Chromomap DAB |
| von Willebrand factor (vWF) | Protease 2; 36 °C; 8 min | 1:200; 32 min; RT | Discovery OmniMap anti Rabbit HRPb 16 min; Discovery Chromomap DAB b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzotta, E.; Zanardello, C.; De Zottis, G.; Barberio, A.; Campalto, M.; Martignago, F.; De Benedictis, G.M.; Guglielmini, C.; Zanusso, F.; Foiani, G. Diagnostic Approach and Pathological Characterization of Metastatic Intrahepatic Cholangiocarcinoma in a Captive Puma (Puma concolor). Animals 2025, 15, 1821. https://doi.org/10.3390/ani15121821
Mazzotta E, Zanardello C, De Zottis G, Barberio A, Campalto M, Martignago F, De Benedictis GM, Guglielmini C, Zanusso F, Foiani G. Diagnostic Approach and Pathological Characterization of Metastatic Intrahepatic Cholangiocarcinoma in a Captive Puma (Puma concolor). Animals. 2025; 15(12):1821. https://doi.org/10.3390/ani15121821
Chicago/Turabian StyleMazzotta, Elisa, Claudia Zanardello, Giovanni De Zottis, Antonio Barberio, Mery Campalto, Federico Martignago, Giulia Maria De Benedictis, Carlo Guglielmini, Francesca Zanusso, and Greta Foiani. 2025. "Diagnostic Approach and Pathological Characterization of Metastatic Intrahepatic Cholangiocarcinoma in a Captive Puma (Puma concolor)" Animals 15, no. 12: 1821. https://doi.org/10.3390/ani15121821
APA StyleMazzotta, E., Zanardello, C., De Zottis, G., Barberio, A., Campalto, M., Martignago, F., De Benedictis, G. M., Guglielmini, C., Zanusso, F., & Foiani, G. (2025). Diagnostic Approach and Pathological Characterization of Metastatic Intrahepatic Cholangiocarcinoma in a Captive Puma (Puma concolor). Animals, 15(12), 1821. https://doi.org/10.3390/ani15121821









