Preclinical Evaluation of Fenbendazole for Controlling Gyrodactylus kobayashii (Monogenea, Gyrodactylidae) in Goldfish: Dose Optimization and Safety Assessment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish, Parasites and Agents
2.2. In Vivo Anthelmintic Assay
2.3. Acute Toxicity
2.4. Oral Treatment with Fenbendazole
2.4.1. Phase 1: Dose Screening (5-Day Treatment)
2.4.2. Phase 2: Time Course Evaluation (Optimal Dose)
2.5. Safety Evaluation of Oral Fenbendazole
2.6. Statistical Analysis
3. Results
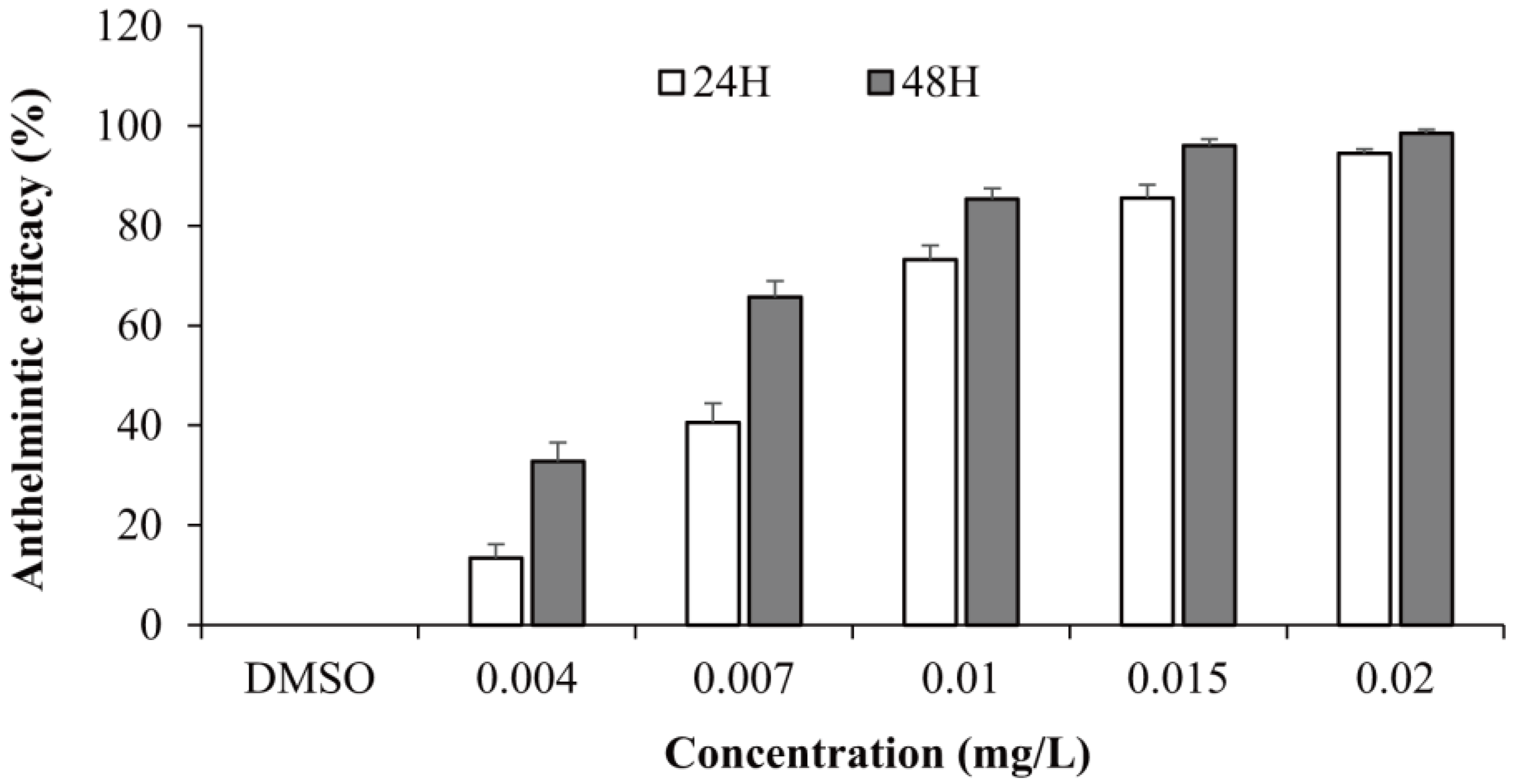
3.1. In Vivo Anthelmintic Activity
3.2. Acute Toxicity
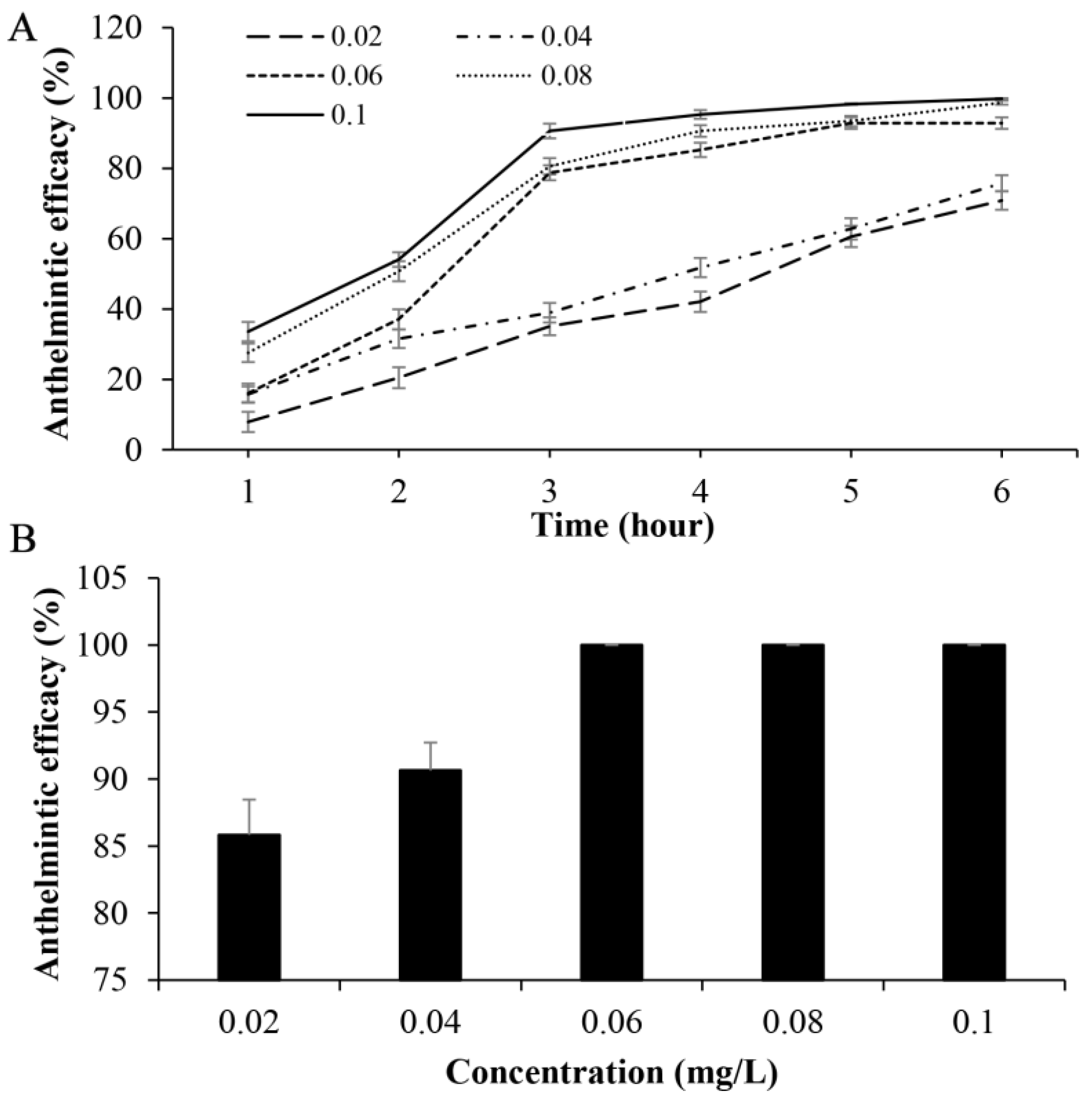
3.3. Oral Administration with Fenbendazole
3.4. Safety Evaluation of Oral Administration with Fenbendazole
3.4.1. Biochemical Analysis
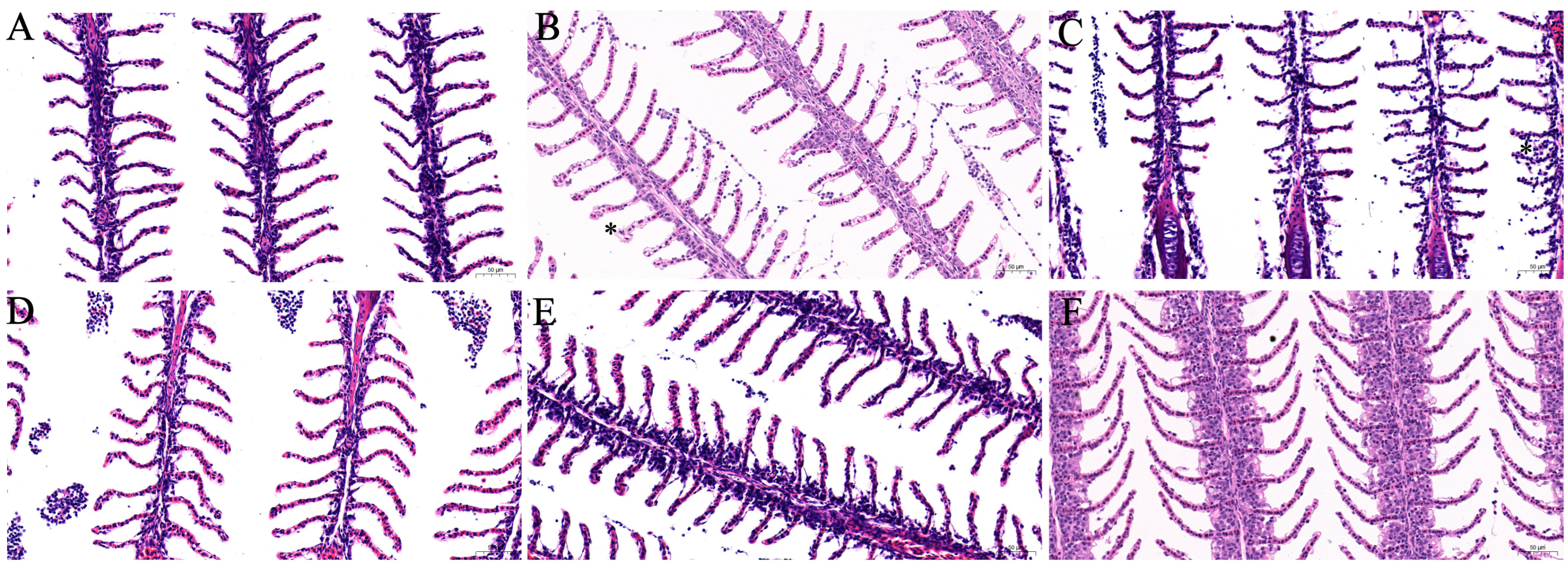
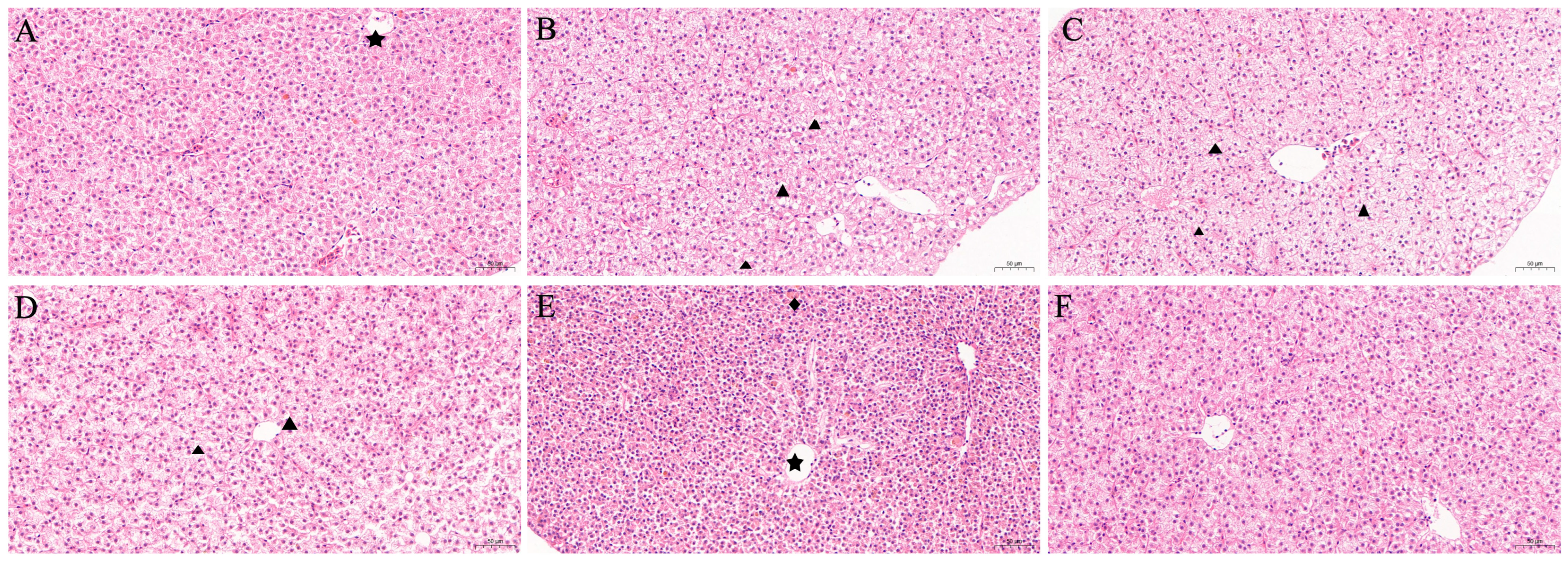
3.4.2. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakke, T.A.; Cable, J.; Harris, P.D. The biology of gyrodactylid monogeneans: The “Russian-doll killers”. Adv. Parasit. 2007, 64, 161–376. [Google Scholar] [CrossRef]
- Janulewicz, J.; Pietkiewicz, M.; Ziętara, M.S. Revision of the most primitive taxa of the family Gyrodactylidae (van Beneden et Hesse, 1864) (Platyhelminthes, Monopisthocotyla) based on ITS rDNA phylogeny. Genes 2024, 15, 1236. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.S.; Alvarez, J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar, C.; Herskin, M.S.; Michel, V.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) 2016/429): Infection with Gyrodactylus salaris (GS). EFSA J. 2023, 21, e08325. [Google Scholar] [CrossRef]
- Mo, T.A.; Norheim, K.; Jansen, P.A. The surveillance and control programme for Gyrodactylus salaris in Atlantic salmon and rainbow trout in Norway. Nord. Vet. Med. 2004, 3, 157–163. [Google Scholar]
- Schelkle, B.; Shinn, A.P.; Peeler, E.; Cable, J. Treatment of gyrodactylid infections in fish. Dis. Aquat. Org. 2009, 86, 65–75. [Google Scholar] [CrossRef]
- Ministry of agriculture and rural affairs of the People’s Republic of China. Fishery Drug Use Standard; China Agriculture Press: Beijing, China, 2016; Volume SC/T 1132-2016.
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Announcement No. 1435 of the Ministry of Agriculture and Rural Areas; Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2010. Available online: https://www.moa.gov.cn/gk/tzgg_1/gg/201008/t20100823_1622639.htm (accessed on 16 June 2025).
- Buchmann, K.; Roepstorff, A.; Waller, P.J. Experimental selection of mebendazole-resistant gill monogeneans from the European eel, Anguilla anguilla L. J. Fish. Dis. 1992, 15, 393–408. [Google Scholar] [CrossRef]
- Wen-Xiang, L.; Hao, W.; Bing-Wen, X.; Ming, L.; Pin, N.; Gui-Tang, W. Parasite drug resistance and its enlightenment for anthelmintic therapy of parasitic diseases in aquaculture. Acta Hydrobi. Sin. 2024, 48, 351–360. [Google Scholar]
- Sebastiao, F.D.; Rocha, M.J.S.; Brandao, F.R.; de Oliveira, M.I.B.; Souza, D.C.D.; Barbosa, B.C.D.; Monteiro, P.C.; Majolo, C.; Crescêncio, R.; Tavares-Dias, M.; et al. Evaluation of the clinical safety and efficacy of fenbendazole and levamisole in the control of Neoechinorhynchus buttnerae in Colossoma macropomum. Aquacult. Int. 2022, 30, 1341–1351. [Google Scholar] [CrossRef]
- Kolarova, J.; Zuskova, E.; Velisek, J. Efficacy of a therapeutic bath with selected antiparasitic drugs on a Dactylogyrus anchoratus infection in juvenile common carp (Cyprinus carpio). Vet. Med. 2022, 67, 620–627. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.Y.; Shin, D.W.; Kim, H.K. Investigation of safety and efficacy of febantel and fenbendazole in fish and exposure assessment. Appl. Biol. Chem. 2024, 67, 38. [Google Scholar] [CrossRef]
- Leach, J.; Suber, H.N.; Banks, E.; Kaskocsak, A.; Valencia, H.; Hames, B.; Rivera, R.; Colette, S.; Kendall, R.J. In vitro lethality of fenbendazole to the eyeworm Oxyspirura petrowi. Animals 2024, 14, 1659. [Google Scholar] [CrossRef]
- Jones, B.; van Vliet, A.H.M.; LaCourse, E.J.; Betson, M. Identification of key interactions of benzimidazole resistance-associated amino acid mutations in β-tubulins by molecular docking simulations. Sci. Rep. 2022, 12, 13725. [Google Scholar] [CrossRef] [PubMed]
- Abongwa, M.; Martin, R.J.; Robertson, A.P. A brief review on the mode of action of antinematodal drugs. Acta Vet. 2017, 67, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Chatterjee, A.; Kumar, M.; Sardar, P.; Varghese, T.; Srivastava, P.P.; Gupta, S. Efficacy of single and multiple doses of fenbendazole against gill parasites (Dactylogyrus sp.) of Labeo rohita (Hamilton, 1822) and its physio-metabolic effects on the fish. Aquac. Res. 2020, 51, 1190–1199. [Google Scholar] [CrossRef]
- Papich, M.G. Saunders Handbook of Veterinary Drugs-E-Book: Small and Large Animal; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Omori, Y.; Kon, T. Goldfish: An old and new model system to study vertebrate development, evolution and human disease. J. Biochem. 2019, 165, 209–218. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Q.H.; Dong, J.; Liu, Y.T.; Xu, N.; Yang, Y.B.; Ai, X.H. Anthelmintic efficacy of palmarosa oil and curcuma oil against the fish ectoparasite Gyrodactylus kobayashii (monogenean). Animals 2022, 12, 1685. [Google Scholar] [CrossRef]
- Zhou, S.; Li, W.X.; Wang, Y.Q.; Zou, H.; Wu, S.G.; Wang, G.T. Anthelmintic efficacies of three common disinfectants and extracts of four traditional Chinese medicinal plants against Gyrodactylus kobayashii (Monogenea) in goldfish (Carassius auratus). Aquaculture 2017, 466, 72–77. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vanhove, M.P.M.; Pariselle, A.; Kmentová, N. Monogenean parasitic flatworms. Curr. Biol. 2024, 34, R1122–R1124. [Google Scholar] [CrossRef]
- Mladineo, I.; Volpatti, D.; Beraldo, P.; Rigos, G.; Katharios, P.; Padros, F. Monogenean Sparicotyle chrysophrii: The major pathogen of the Mediterranean gilthead seabream aquaculture. Rev. Aquacult. 2024, 16, 287–308. [Google Scholar] [CrossRef]
- Rigos, G.; Padrós, F.; Golomazou, E.; Zarza, C. Antiparasitic approaches and strategies in European aquaculture, with emphasis on Mediterranean marine finfish farming: Present scenarios and future visions. Rev. Aquacult. 2024, 16, 622–643. [Google Scholar] [CrossRef]
- Suryawanshi, S.J.; Jain, S.; Sharma, R.K.; Chawla, R.; Arora, S.; Shukla, V.K.; Sharma, N. Current veterinary regulations and way ahead. Res. Vet. Sci. 2024, 166, 105101. [Google Scholar] [CrossRef] [PubMed]
- Reimschuessel, R.; Gieseker, C.; Poynton, S. In vitro effect of seven antiparasitics on Acolpenteron ureteroecetes (Dactylogyridae) from largemouth bass Micropterus salmoides (Centrarchidae). Dis. Aquat. Org. 2011, 94, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.; Horta, R.S.; Vieira, R.A.M.; Hume, K.R.; Dobson, J. Repurposing drugs in small animal oncology. Animals 2023, 13, 139. [Google Scholar] [CrossRef]
- Satyam, S.M.; El-Tanani, M.; Patni, M.A.; Rehman, A.; Wali, A.F.; Rangraze, I.R.; Babiker, R.; Rabbani, S.A.; El-Tanani, Y.; Rizzo, M. Repurposing anthelmintic drugs for COVID-19 treatment: A comprehensive meta-analysis of randomized clinical trials on ivermectin and mebendazole. Antibiotics 2025, 14, 459. [Google Scholar] [CrossRef]
- Hittalamani, V.M.; Sunilchandra, U.; Shridhar, N.; Kavitha Rani, B.; Niranjan, D.; Mohan, B. Drug repurposing with reference to veterinary. Pharma Innov. 2022, 11, 36–45. [Google Scholar]
- AbdelKhalek, A.; Seleem, M.N. Repurposing the veterinary antiprotozoal drug ronidazole for the treatment of Clostridioides difficile infection. Int. J. Antimicrob. Agents 2020, 56, 106188. [Google Scholar] [CrossRef]
- Andrews, K.T.; Fisher, G.; Skinner-Adams, T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 95–111. [Google Scholar] [CrossRef]
- Dong, J.; Xia, L.; Liu, Y.; Yang, Q.; Xu, N.; Ai, X.; Zhou, S. Discovery of potential anthelmintic agents against Gyrodactylus kobayashii through computer-aided drug design and in vivo evaluation. J. Fish. Dis. 2025, 48, e14102. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, X.; Zhang, Y.; Ling, F.; Liu, T.; Wang, G. Isoimperatorin: A promising anti Gyrodactylus kobayashii natural compound from Angelica dahurica. Aquaculture 2022, 560, 738552. [Google Scholar] [CrossRef]
- Tu, X.; Duan, C.; Wu, S.; Fu, S.; Ye, J. Identification of plumbagin as an effective chemotherapeutic agent for treatment of Gyrodactylus infections. Aquaculture 2021, 535, 736372. [Google Scholar] [CrossRef]
- Buchmann, K. Control of parasitic diseases in aquaculture. Parasitology 2022, 149, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Hutson, K.S.; Mooney, A.J.; Ernst, I.; Brazenor, A.K.; Scheel, M.; Atalah, J. A decision support tool for parasite management in fish aquaculture. Rev. Aquacult. 2022, 14, 1656–1670. [Google Scholar] [CrossRef]
- Zhou, S.; Xia, L.; Dong, J.; Liu, Y.; Yang, Q.; Xu, N.; Yang, Y.; Ai, X. Anthelmintic efficacy of febantel against a monogenean parasite, Gyrodactylus kobayashii. Vet. Parasitol. 2023, 324, 110058. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Nizami, W.A. In vitro effects of mebendazole on the carbohydrate metabolism of Avitellina lahorea (Cestoda). J. Helminthol. 1987, 61, 247–252. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, S.; Xia, L.; Dong, J.; Liu, Y.; Yang, Q.; Zhang, L.; Ai, X. Efficacy and safety assessment of triclabendazole for treating Gyrodactylus infections in goldfish. Aquaculture 2024, 584, 740640. [Google Scholar] [CrossRef]
- Tojo, J.; Santamarina, M.; Ubeira, F.; Estevez, J.; Sanmartin, M. Anthelmintic activity of benzimidazoles against Gyrodactylus sp. infecting rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 1992, 12, 185–189. [Google Scholar] [CrossRef]
- Kramer, J.A.; Sagartz, J.E.; Morris, D.L. The application of discovery toxicology and pathology towards the design of safer pharmaceutical lead candidates. Nat. Rev. Drug Discov. 2007, 6, 636–649. [Google Scholar] [CrossRef]
- Dunn, E.J.; Polk, A.; Scarrett, D.J.; Olivier, G.; Lall, S.; Goosen, M.F.A. Vaccines in aquaculture: The search for an efficient delivery system. Aquacult. Eng. 1990, 9, 23–32. [Google Scholar] [CrossRef]
- Zhou, S.; Li, W.X.; Zou, H.; Zhang, J.; Wu, S.G.; Li, M.; Wang, G.T. Expression analysis of immune genes in goldfish (Carassius auratus) infected with the monogenean parasite Gyrodactylus kobayashii. Fish. Shellfish. Immunol. 2018, 77, 40–45. [Google Scholar] [CrossRef]
- Abele, D.; Puntarulo, S. Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 138, 405–415. [Google Scholar] [CrossRef] [PubMed]

| Concentration (mg/L) | No. of Fish/Tank | No. Dead Fish (Mean ± SE) | |||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | ||
| 0 | 10 | 0 | 0 | 0 | 0 |
| 0.02% DMSO | 10 | 0 | 0 | 0 | 0 |
| 0.02 | 10 | 0 | 0 | 0 | 0 |
| 0.03 | 10 | 0 | 0 | 0 | 0.67 ± 0.33 |
| 0.04 | 10 | 0 | 1.33 ± 0.33 | 6.0 ± 0.58 | 7.33 ± 0.33 |
| 0.05 | 10 | 0 | 1.67 ± 0.33 | 7.67 ± 0.67 | 8.0 ± 0.58 |
| 0.06 | 10 | 0 | 3.33 ± 0.33 | 8.33 ± 0.33 | 10 |
| 0.08 | 10 | 1.33 ± 0.33 | 5.67 ± 0.33 | 10 | 10 |
| Treatment (mg/kg Body Weight) | Infection Abundance (Mean ± SE) | Anthelmintic Efficacy (%, Mean ± SE) | |
|---|---|---|---|
| 0th | 5th | ||
| drug-free control group | 58.87 ± 6.69 | 100.73 ± 12.11 | / |
| vehicle control group | 53.40 ± 4.54 | 97.60 ± 7.85 | / |
| 5 | 66.67 ± 5.25 | 42.40 ± 5.28 | 38.24 ± 4.57 |
| 10 | 59.80 ± 8.21 | 21.40 ± 4.71 | 64.08 ± 3.58 |
| 20 | 62.27 ± 8.01 | 4.47 ± 0.98 | 91.76 ± 2.17 |
| 40 | 63.47 ± 5.44 | 1.93 ± 0.44 | 96.41 ± 0.98 |
| 60 | 60.60 ± 7.46 | 0.93 ± 0.54 | 98.55 ± 0.73 |
| 80 | 59.00 ± 8.39 | 0.67 ± 0.30 | 98.83 ± 0.56 |
| Treatment (mg/kg Body Weight) | Infection Abundance (Mean ± SE) | Anthelmintic Efficacy (%, Mean ± SE) | |||||
|---|---|---|---|---|---|---|---|
| 0th | 3th | 5th | 7th | 3th | 5th | 7th | |
| 0 | 56.20 ± 4.96 | 74.07 ± 5.61 | 92.73 ± 6.91 | 122.13 ± 8.42 | / | / | / |
| DMSO | 60.07 ± 6.04 | 81.40 ± 7.25 | 97.67 ± 6.74 | 127.53 ± 11.79 | / | / | / |
| 10 | 62.47 ± 6.03 | 39.20 ± 4.14 | 19.47 ± 2.53 | 15.60 ± 2.44 | 36.21 ± 3.77 | 66.43 ± 4.09 | 74.31 ± 3.08 |
| 20 | 57.33 ± 6.17 | 8.93 ± 0.81 | 3.20 ± 0.54 | 1.87 ± 0.47 | 83.35 ± 1.61 | 93.83 ± 1.17 | 96.28 ± 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Li, J.; Liu, Y.; Yang, Q.; Xu, N.; Ai, X.; Zhou, S. Preclinical Evaluation of Fenbendazole for Controlling Gyrodactylus kobayashii (Monogenea, Gyrodactylidae) in Goldfish: Dose Optimization and Safety Assessment. Animals 2025, 15, 1811. https://doi.org/10.3390/ani15121811
Dong J, Li J, Liu Y, Yang Q, Xu N, Ai X, Zhou S. Preclinical Evaluation of Fenbendazole for Controlling Gyrodactylus kobayashii (Monogenea, Gyrodactylidae) in Goldfish: Dose Optimization and Safety Assessment. Animals. 2025; 15(12):1811. https://doi.org/10.3390/ani15121811
Chicago/Turabian StyleDong, Jing, Jiangtao Li, Yongtao Liu, Qiuhong Yang, Ning Xu, Xiaohui Ai, and Shun Zhou. 2025. "Preclinical Evaluation of Fenbendazole for Controlling Gyrodactylus kobayashii (Monogenea, Gyrodactylidae) in Goldfish: Dose Optimization and Safety Assessment" Animals 15, no. 12: 1811. https://doi.org/10.3390/ani15121811
APA StyleDong, J., Li, J., Liu, Y., Yang, Q., Xu, N., Ai, X., & Zhou, S. (2025). Preclinical Evaluation of Fenbendazole for Controlling Gyrodactylus kobayashii (Monogenea, Gyrodactylidae) in Goldfish: Dose Optimization and Safety Assessment. Animals, 15(12), 1811. https://doi.org/10.3390/ani15121811





