Embryonic Thermal Manipulation Affects Body Performance Parameters and Cecum Microbiome in Broiler Chickens in Response to Post-Hatch Chronic Heat Stress Challenge
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
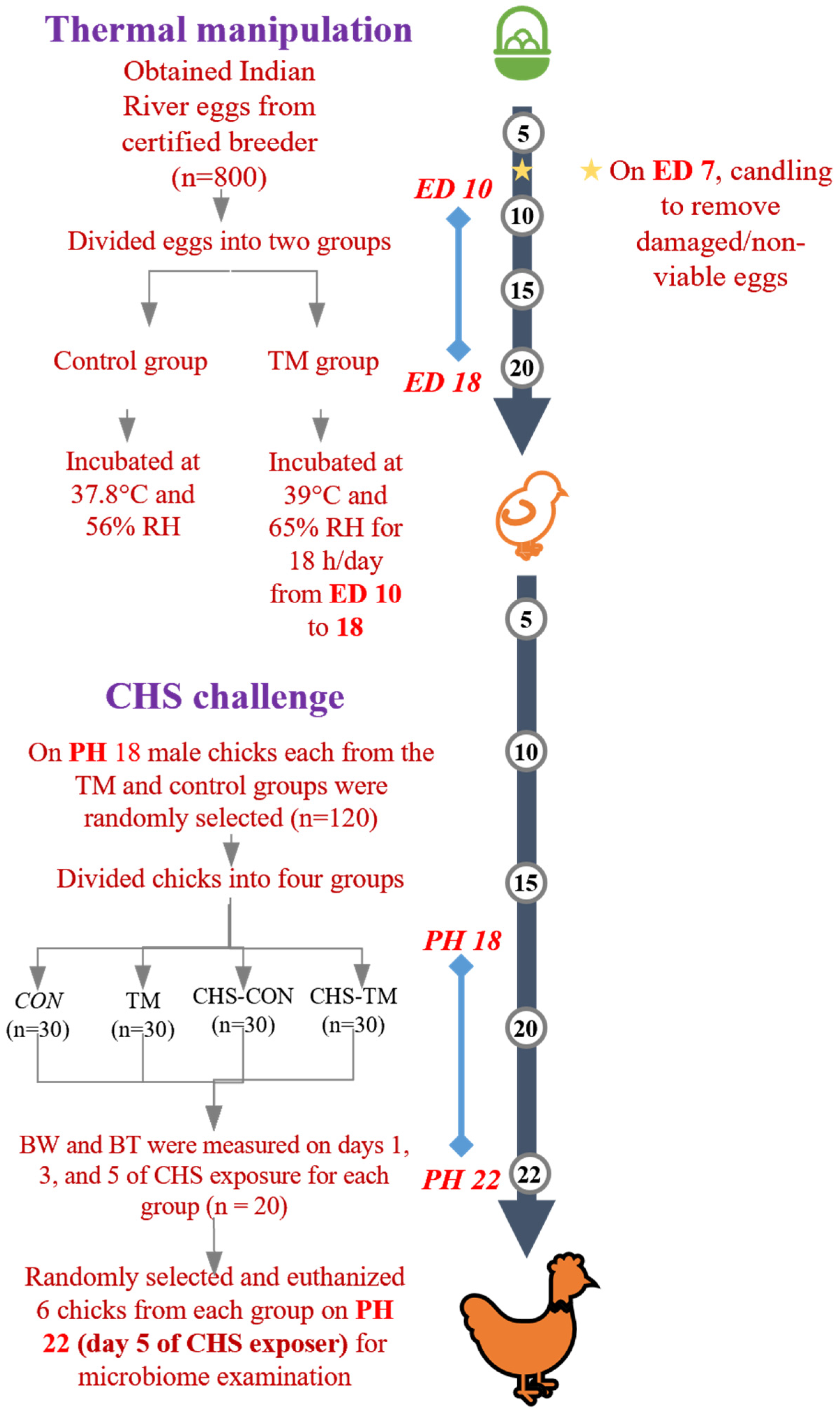
2.1. Study Population and Incubation
2.2. Hatching Management and Post-Hatching Rearing
2.3. CHS Challenge
2.4. Microbiological Analysis
2.4.1. DNA Isolation and Sequencing
2.4.2. Data Analysis by Bioinformatic Tools
2.5. Statistical Analysis
3. Results
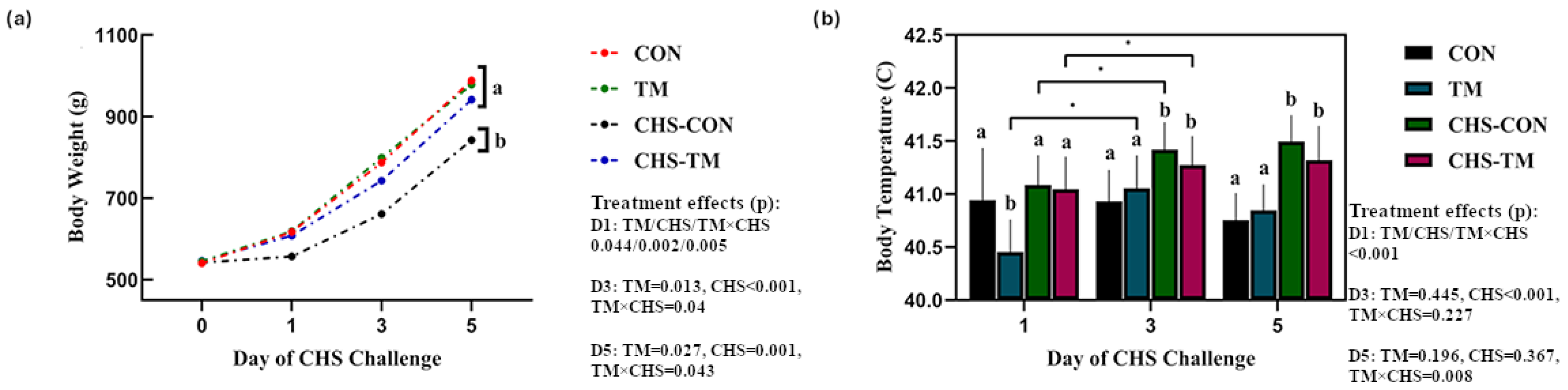
3.1. TM and CHS Challenge Effects on Body Weight (BW) and Body Temperature (BT)
3.2. Effects of TM and CHS on Alpha Diversity
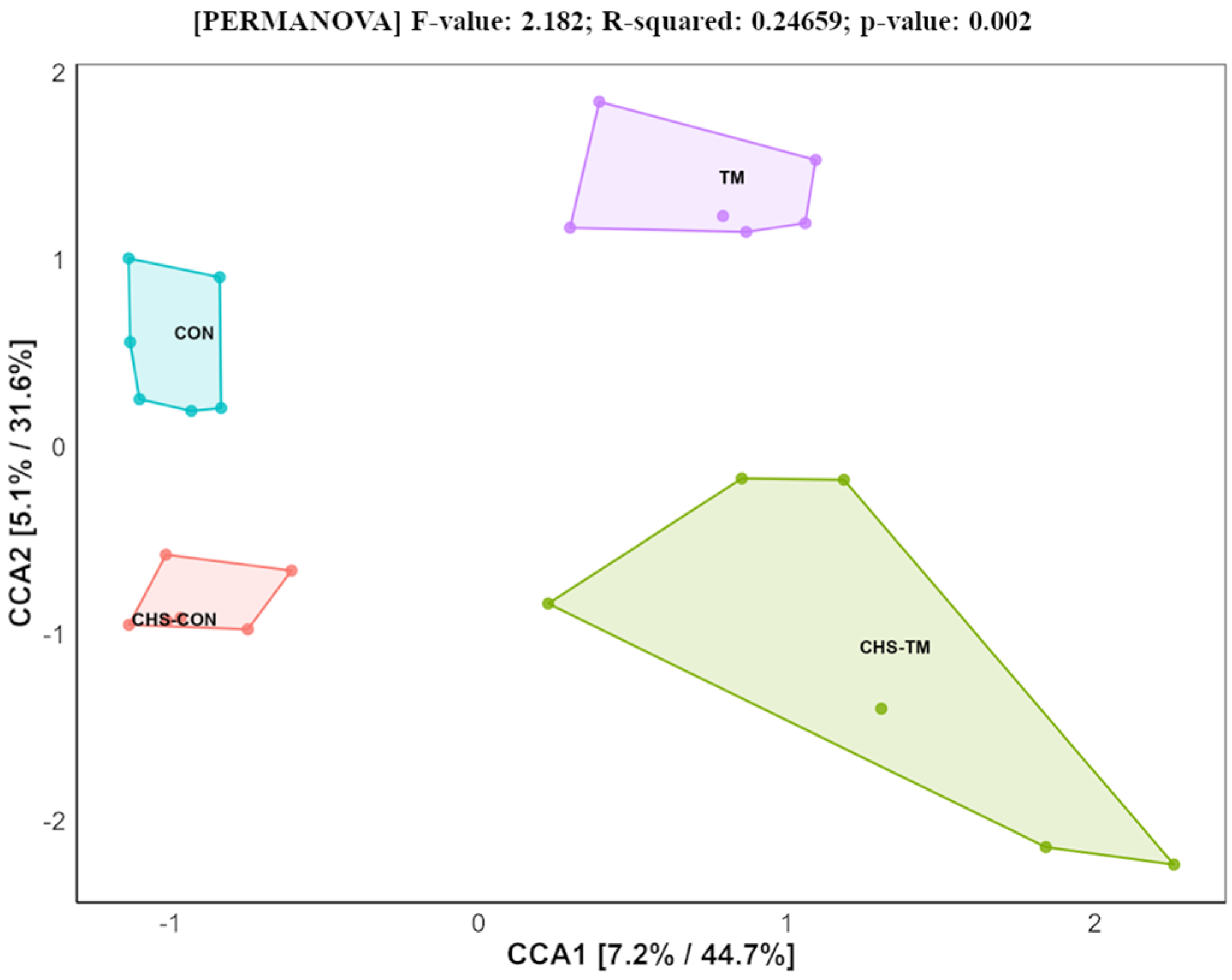
3.3. Effects of TM and CHS on Beta Diversity
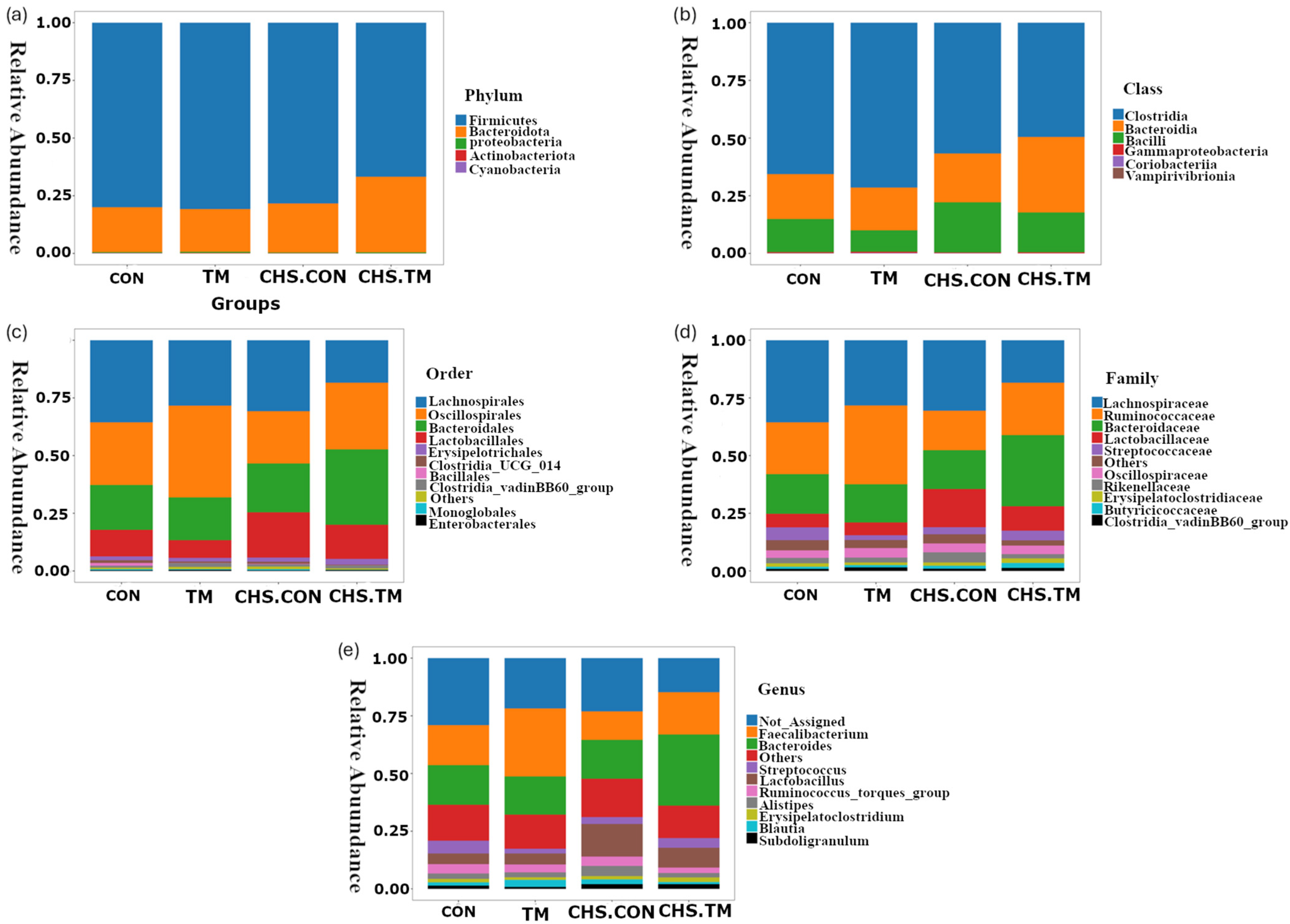
3.4. Effects of TM and CHS on Cecal Microbiota: Phylum-Level Composition
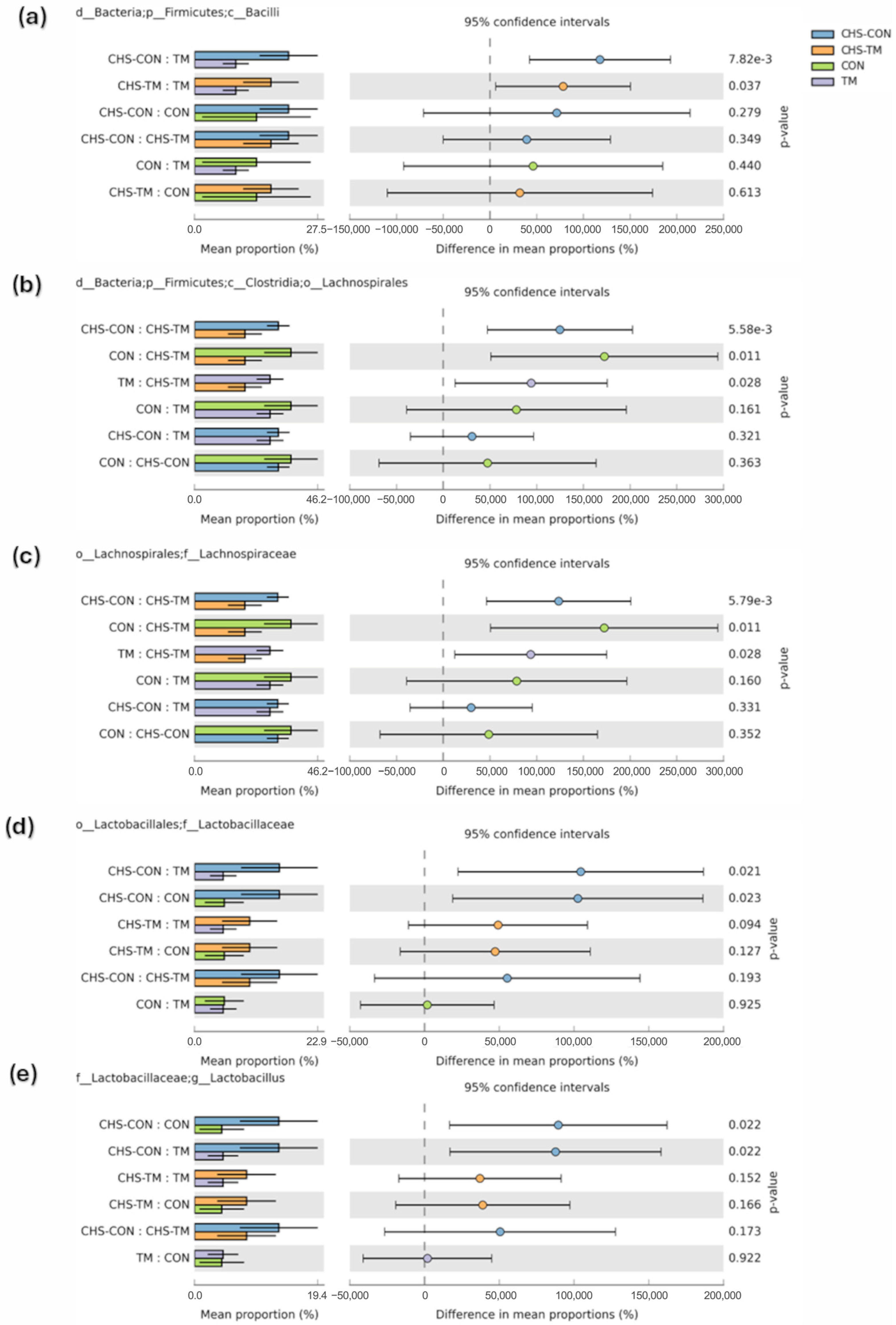
3.5. Effects of TM and CHS on Cecal Microbiota: Class-Level Composition
3.6. Effects of TM and CHS on Cecal Microbiota: Order-Level Composition
3.7. Effects of TM and CHS on Cecal Microbiota: Family-Level Composition
3.8. Effects of TM and CHS on Cecal Microbiota: Genus-Level Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TM | thermal manipulation |
| CON | control |
| CHS | chronic heat stress |
| CHS-CON | control with chronic heat stress challenge |
| CHS-TM | thermal manipulation with chronic heat stress challenge |
| RH | relative humidity |
| ED | embryonic day |
| PH | post-hatch |
| ASV | Amplicon Sequencing Variant |
| CCA | Canonical Correspondence Analysis |
| PERMANOVA | Permutational Multivariate Analysis of Variance |
References
- Wasti, S.; Sah, N.; Mishra, B. Impact of Heat Stress on Poultry Health and Performances, and Potential Mitigation Strategies. Animals 2020, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Sugiharto, S. Alleviation of heat stress in broiler chicken using turmeric (Curcuma longa)—A short review. J. Anim. Behav. Biometeorol. 2020, 8, 215–222. [Google Scholar] [CrossRef]
- Kpomasse, C.C.; Oke, O.E.; Houndonougbo, F.M.; Tona, K. Broiler production challenges in the tropics: A review. Vet. Med. Sci. 2021, 7, 831–842. [Google Scholar] [CrossRef]
- Apalowo, O.O.; Ekunseitan, D.A.; Fasina, Y.O. Impact of Heat Stress on Broiler Chicken Production. Poultry 2024, 3, 107–128. [Google Scholar] [CrossRef]
- Nanto-Hara, F.; Kikusato, M.; Ohwada, S.; Toyomizu, M. Heat Stress Directly Affects Intestinal Integrity in Broiler Chickens. J. Poult. Sci. 2020, 57, 284–290. [Google Scholar] [CrossRef]
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090. [Google Scholar] [CrossRef] [PubMed]
- Schreier, J.; Rychlik, I.; Karasova, D.; Crhanova, M.; Breves, G.; Rautenschlein, S.; Jung, A. Influence of heat stress on intestinal integrity and the caecal microbiota during Enterococcus cecorum infection in broilers. Vet. Res. 2022, 53, 110. [Google Scholar] [CrossRef]
- Ringseis, R.; Eder, K. Heat stress in pigs and broilers: Role of gut dysbiosis in the impairment of the gut-liver axis and restoration of these effects by probiotics, prebiotics and synbiotics. J. Anim. Sci. Biotechnol. 2022, 13, 126. [Google Scholar] [CrossRef]
- Liu, W.-C.; Huang, M.-Y.; Balasubramanian, B.; Jha, R. Heat stress affects jejunal immunity of yellow-feathered broilers and is potentially mediated by the microbiome. Front. Physiol. 2022, 13, 913696. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Redweik, G.A.J.; Mellata, M. Immunological Mechanisms of Probiotics in Chickens. In Gut Microbiota, Immunity, and Health in Production Animals; The Microbiomes of Humans, Animals, Plants, and the Environment; Springer: Cham, Switzerland, 2022; pp. 263–276. [Google Scholar]
- Patra, A.K.; Kar, I. Heat stress on microbiota composition, barrier integrity, and nutrient transport in gut, production performance, and its amelioration in farm animals. J. Anim. Sci. Technol. 2021, 63, 211–247. [Google Scholar] [CrossRef]
- Campos, P.M.; Schreier, L.L.; Proszkowiec-Weglarz, M.; Dridi, S. Cecal microbiota composition differs under normal and high ambient temperatures in genetically distinct chicken lines. Sci. Rep. 2023, 13, 16037. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liao, R.; Wu, N.; Zhu, G.; Yang, C. Heat stress mediates changes in fecal microbiome and functional pathways of laying hens. Appl. Microbiol. Biotechnol. 2019, 103, 461–472. [Google Scholar] [CrossRef]
- Vargas, N.; Marino, F. Heat stress, gastrointestinal permeability and interleukin-6 signaling—Implications for exercise performance and fatigue. Temperature 2016, 3, 240–251. [Google Scholar] [CrossRef]
- Cao, C.; Chowdhury, V.S.; Cline, M.A.; Gilbert, E.R. The Microbiota-Gut-Brain Axis During Heat Stress in Chickens: A Review. Front. Physiol. 2021, 12, 752265. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Alliftawi, A.R.S.; Saleh, K.M.M.; Jaradat, Z.W. Expression of digestive enzyme and intestinal transporter genes during chronic heat stress in the thermally manipulated broiler chicken. Poult. Sci. 2019, 98, 4113–4122. [Google Scholar] [CrossRef] [PubMed]
- Al-Zghoul, M.B.; El-Bahr, S.M. Thermal manipulation of the broilers embryos: Expression of muscle markers genes and weights of body and internal organs during embryonic and post-hatch days. BMC Vet. Res. 2019, 15, 166. [Google Scholar] [CrossRef]
- Piestun, Y.; Yahav, S.; Halevy, O. Thermal manipulation during embryogenesis affects myoblast proliferation and skeletal muscle growth in meat-type chickens. Poult. Sci. 2015, 94, 2528–2536. [Google Scholar] [CrossRef]
- Yahav, S.; Straschnow, A.; Plavnik, I.; Hurwitz, S. Effects of diurnally cycling versus constant temperatures on chicken growth and food intake. Br. Poult. Sci. 1996, 37, 43–54. [Google Scholar] [CrossRef]
- Kisliouk, T.; Ziv, M.; Meiri, N. Epigenetic control of translation regulation: Alterations in histone H3 lysine 9 post-translation modifications are correlated with the expression of the translation initiation factor 2B (Eif2b5) during thermal control establishment. Dev. Neurobiol. 2010, 70, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Yahav, S.; Collin, A.; Shinder, D.; Picard, M. Thermal manipulations during broiler chick embryogenesis: Effects of timing and temperature. Poult. Sci. 2004, 83, 1959–1963. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Jaradat, Z.W.; Ababneh, M.M.; Okour, M.Z.; Saleh, K.M.M.; Alkofahi, A.; Alboom, M.H. Effects of embryonic thermal manipulation on the immune response to post-hatch Escherichia coli challenge in broiler chicken. Vet. World 2023, 16, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Loyau, T.; Bedrani, L.; Berri, C.; Metayer-Coustard, S.; Praud, C.; Coustham, V.; Mignon-Grasteau, S.; Duclos, M.J.; Tesseraud, S.; Rideau, N.; et al. Cyclic variations in incubation conditions induce adaptive responses to later heat exposure in chickens: A review. Animal 2015, 9, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Iraqi, E.; Hady, A.A.; Elsayed, N.; Khalil, H.; El-Saadany, A.; El-Sabrout, K. Effect of thermal manipulation on embryonic development, hatching process, and chick quality under heat-stress conditions. Poult. Sci. 2024, 103, 103257. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Cong, W.; Zhang, K.; Jia, Y.; Wu, L. Chronic Heat Stress Affects Bile Acid Profile and Gut Microbiota in Broilers. Int. J. Mol. Sci. 2023, 24, 10238. [Google Scholar] [CrossRef]
- Shakouri, M.D.; Malekzadeh, M. Responses of broiler chickens to the nutrient recommendations of NRC (1994) and the Ross broiler management manual. Rev. Colomb. Cienc. Pecu. 2016, 29, 91–98. [Google Scholar] [CrossRef]
- Christoff, A.P.; Flores Cruz, G.N.; Sereia, A.F.R.; Yamanaka, L.E.; Silveira, P.P.; de Oliveira, L.F.V. End-to-end assessment of fecal bacteriome analysis: From sample processing to DNA sequencing and bioinformatics results. BioRxiv 2019, 646349. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio: Boston, MA, USA, 2021. [Google Scholar]
- Andersen, K.S.; Kirkegaard, R.H.; Karst, S.M.; Albertsen, M. ampvis2: An R package to analyse and visualise 16S rRNA amplicon data. bioRxiv 2018, 299537. [Google Scholar]
- Zhou, L.; Liu, Z.; Liu, F.; Peng, J.; Zhou, T. Nonlinear canonical correspondence analysis and its application. Sci. Rep. 2023, 13, 7518. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Mohammad Saleh, K.M. Effects of thermal manipulation of eggs on the response of jejunal mucosae to posthatch chronic heat stress in broiler chickens. Poult. Sci. 2020, 99, 2727–2735. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Sukker, H.; Ababneh, M.M. Effect of thermal manipulation of broilers embryos on the response to heat-induced oxidative stress. Poult. Sci. 2019, 98, 991–1001. [Google Scholar] [CrossRef]
- Piestun, Y.; Shinder, D.; Ruzal, M.; Halevy, O.; Brake, J.; Yahav, S. Thermal manipulations during broiler embryogenesis: Effect on the acquisition of thermotolerance. Poult. Sci. 2008, 87, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Loyau, T.; Métayer-Coustard, S.; Praud, C.; Berri, C.; Duclos, M.J.; Tesseraud, S.; Rideau, N.; Chartrin, P.; Hennequet-Antier, C.; Everaert, N.; et al. Embryo thermal manipulation has long-lasting effects on energy metabolism in chickens. In Energy and Protein Metabolism and Nutrition in Sustainable Animal Production: 4th International Symposium on Energy and Protein Metabolism and Nutrition Sacramento, California, USA 9–12 September 2013; Oltjen, J.W., Kebreab, E., Lapierre, H., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 265–266. [Google Scholar]
- Collin, A.; Picard, M.; Yahav, S. The effect of duration of thermal manipulation during broiler chick embryogenesis on body weight and body temperature of post-hatched chicks. Anim. Res. 2005, 54, 105–111. [Google Scholar] [CrossRef]
- Sumanu, V.O.; Naidoo, V.; Oosthuizen, M.C.; Chamunorwa, J.P. Evaluating the efficacy of probiotics and ascorbic acid as anti-stress agents against heat stress in broiler chickens. Front. Vet. Sci. 2024, 11, 1482134. [Google Scholar] [CrossRef]
- Ito, K.; Miyamoto, H.; Matsuura, M.; Ishii, C.; Nakanishi, Y.; Suda, W.; Satoh, T.; Honda, F.; Kurotani, A.; Tsuji, N. A thermoprotective probiotic function by thermostable lactic acid bacteria and its causal structure. J. Funct. Foods 2024, 113, 106001. [Google Scholar] [CrossRef]
- Aydin, S.S.; Hatipoglu, D. Probiotic strategies for mitigating heat stress effects on broiler chicken performance. Int. J. Biometeorol. 2024, 68, 2153–2171. [Google Scholar] [CrossRef]
- Hatipoglu, D.; Senturk, G.; Aydin, S.S.; Kirar, N.; Top, S.; Demircioglu, I. Rye-grass-derived probiotics alleviate heat stress effects on broiler growth, health, and gut microbiota. J. Therm. Biol. 2024, 119, 103771. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, M.N.; Das, N.C.; Ahmed, M.M.; Hoque, M.M. Potential thermotolerant lactobacilli isolated from chicken gastrointestinal tract for probiotic use in poultry feeds. Bangladesh J. Microbiol. 2019, 36, 63–68. [Google Scholar] [CrossRef]
- Loyau, T.; Metayer-Coustard, S.; Berri, C.; Crochet, S.; Cailleau-Audouin, E.; Sannier, M.; Chartrin, P.; Praud, C.; Hennequet-Antier, C.; Rideau, N. Thermal manipulation during embryogenesis has long-term effects on muscle and liver metabolism in fast-growing chickens. PLoS ONE 2014, 9, e105339. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Wang, K.; Liu, L.; Chen, K.; Shan, W.; Liu, L.; Kahiel, M.; Li, C. Embryo thermal manipulation enhances mitochondrial function in the skeletal muscle of heat-stressed broilers by regulating transient receptor potential V2 expression. Poult. Sci. 2024, 103, 104034. [Google Scholar] [CrossRef]
- Dalab, A.S.; Ali, A.M.; Althnaian, T.A.; Alkhodair, K.M.; Al-Ramadan, S.Y. Molecular and ultrastructural investigations of the effect of thermal manipulation during embryogenesis on pectoral and thigh muscles growth factors in broilers. J. Appl. Poult. Res. 2022, 31, 100188. [Google Scholar] [CrossRef]
- Piestun, Y.; Harel, M.; Barak, M.; Yahav, S.; Halevy, O. Thermal manipulations in late-term chick embryos have immediate and longer term effects on myoblast proliferation and skeletal muscle hypertrophy. J. Appl. Physiol. 2009, 106, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Al-Zghoul, M.B.; Al-Natour, M.; Dalab, A.; Alturki, O.; Althnaian, T.; Al-Ramadan, S.; Hannon, K.; El-Bahr, S. Thermal manipulation mid-term broiler chicken embryogenesis: Effect on muscle growth factors and muscle marker genes. Braz. J. Poult. Sci. 2016, 18, 607–618. [Google Scholar] [CrossRef]
- He, Y.; Maltecca, C.; Tiezzi, F. Potential use of gut microbiota composition as a biomarker of heat stress in monogastric species: A review. Animals 2021, 11, 1833. [Google Scholar] [CrossRef]
- Kers, J.G.; Saccenti, E. The power of microbiome studies: Some considerations on which alpha and beta metrics to use and how to report results. Front. Microbiol. 2022, 12, 796025. [Google Scholar] [CrossRef]
- Al Amaz, S.; Chaudhary, A.; Mahato, P.L.; Jha, R.; Mishra, B. Pre-hatch thermal manipulation of embryos and post-hatch baicalein supplementation mitigated heat stress in broiler chickens. J. Anim. Sci. Biotechnol. 2024, 15, 8. [Google Scholar] [CrossRef]
- Meteyake, H.T.; Bilalissi, A.; Aimee, Y.; Kouame, E.; N’Nanle, O.; Tona, J. Thermal Manipulation During Incubation: Effects on Embryo Development, Production Performance, Meat Quality, and Thermal Tolerance of Broiler Chickens. J. World’s Poult. Res. 2023, 13, 29–40. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Lin, B.; Melnikov, V.; Guo, S.; Cao, Z.; Ye, Z.; Ye, Z.; Ji, C.; Chen, J.; Wang, J.; Zhang, H.; et al. Concomitant gut dysbiosis and defective gut barrier serve as the bridges between hypercortisolism and chronic systemic inflammation in Cushing’s disease. Eur. J. Endocrinol. 2024, 191, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Guangorena-Gomez, J.O.; Lozano, O., II; Rivera-Medina, I.L.; Mendez-Hernandez, A.; Espinosa-Fematt, J.A.; Munoz-Yanez, C. Relationship Among Blastocystis, the Firmicutes/Bacteroidetes Ratio and Chronic Stress in Mexican University Students. Curr. Microbiol. 2022, 79, 72. [Google Scholar] [CrossRef]
- Shi, D.; Bai, L.; Qu, Q.; Zhou, S.; Yang, M.; Guo, S.; Li, Q.; Liu, C. Impact of gut microbiota structure in heat-stressed broilers. Poult. Sci. 2019, 98, 2405–2413. [Google Scholar] [CrossRef]
- Tong, Q.; Cui, L.Y.; Hu, Z.F.; Du, X.P.; Abid, H.M.; Wang, H.B. Environmental and host factors shaping the gut microbiota diversity of brown frog Rana dybowskii. Sci. Total Environ. 2020, 741, 140142. [Google Scholar] [CrossRef]
- de Jong, I.C.; Schokker, D.; Gunnink, H.; van Wijhe, M.; Rebel, J.M.J. Early life environment affects behavior, welfare, gut microbiome composition, and diversity in broiler chickens. Front. Vet. Sci. 2022, 9, 977359. [Google Scholar] [CrossRef] [PubMed]
- David, S.A.; Vitorino Carvalho, A.; Gimonnet, C.; Brionne, A.; Hennequet-Antier, C.; Piegu, B.; Crochet, S.; Courousse, N.; Bordeau, T.; Bigot, Y.; et al. Thermal Manipulation During Embryogenesis Impacts H3K4me3 and H3K27me3 Histone Marks in Chicken Hypothalamus. Front. Genet. 2019, 10, 1207. [Google Scholar] [CrossRef]
- Dunislawska, A.; Pietrzak, E.; Wishna Kadawarage, R.; Beldowska, A.; Siwek, M. Pre-hatching and post-hatching environmental factors related to epigenetic mechanisms in poultry. J. Anim. Sci. 2021, 100, skab370. [Google Scholar] [CrossRef] [PubMed]
- Sikorska, M.; Siwek, M.; Slawinska, A.; Dunislawska, A. miRNA Profiling in the Chicken Liver under the Influence of Early Microbiota Stimulation with Probiotic, Prebiotic, and Synbiotic. Genes 2021, 12, 685. [Google Scholar] [CrossRef]
- Dunislawska, A.; Slawinska, A.; Stadnicka, K.; Bednarczyk, M.; Gulewicz, P.; Jozefiak, D.; Siwek, M. Synbiotics for broiler chickens—In vitro design and evaluation of the influence on host and selected microbiota populations following in ovo delivery. PLoS ONE 2017, 12, e0168587. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.; Wilson, J.; Guthrie, A.; Cookson, K.; Vancraeynest, D.; Schaeffer, J.; Moody, R.; Clark, S. New issues and science in broiler chicken intestinal health: Intestinal microbial composition, shifts, and impacts. World’s Poult. Sci. J. 2015, 71, 259–270. [Google Scholar] [CrossRef]
- Du, W.; Deng, J.; Yang, Z.; Zeng, L.; Yang, X. Metagenomic analysis reveals linkages between cecal microbiota and feed efficiency in Xiayan chickens. Poult. Sci. 2020, 99, 7066–7075. [Google Scholar] [CrossRef]
- Yin, Z.; Ji, S.; Yang, J.; Guo, W.; Li, Y.; Ren, Z.; Yang, X. Cecal Microbial Succession and Its Apparent Association with Nutrient Metabolism in Broiler Chickens. mSphere 2023, 8, e0061422. [Google Scholar] [CrossRef]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and Environmental Factors Affecting the Intestinal Microbiota in Chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- McNeil, N.I. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 1984, 39, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H. Short chain fatty acids in the human colon. Gut 1981, 22, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Sun, X. Research progress on intestinal microecological regulation of aquatic animals. Feed Res. 2009, 9, 68–70. [Google Scholar]
- Polansky, O.; Sekelova, Z.; Faldynova, M.; Sebkova, A.; Sisak, F.; Rychlik, I. Important metabolic pathways and biological processes expressed by chicken cecal microbiota. Appl. Environ. Microbiol. 2016, 82, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Wang, X.; Diao, H.; Zhang, M.; Zhou, Y.; Feng, J. Changes in the cecal microbiota of laying hens during heat stress is mainly associated with reduced feed intake. Poult. Sci. 2019, 98, 5257–5264. [Google Scholar] [CrossRef]
- Zulkifli, I.; Abdulllah, N.; Azrin, N.M.; Ho, Y.W. Growth performance and immune response of two commercial broiler strains fed diets containing Lactobacillus cultures and oxytetracycline under heat stress conditions. Br. Poult. Sci. 2000, 41, 593–597. [Google Scholar] [CrossRef]
- Faseleh Jahromi, M.; Wesam Altaher, Y.; Shokryazdan, P.; Ebrahimi, R.; Ebrahimi, M.; Idrus, Z.; Tufarelli, V.; Liang, J.B. Dietary supplementation of a mixture of Lactobacillus strains enhances performance of broiler chickens raised under heat stress conditions. Int. J. Biometeorol. 2016, 60, 1099–1110. [Google Scholar] [CrossRef]
- Zhang, H.; Pertiwi, H.; Hou, Y.; Majdeddin, M.; Michiels, J. Protective effects of Lactobacillus on heat stress-induced intestinal injury in finisher broilers by regulating gut microbiota and stimulating epithelial development. Sci. Total Environ. 2024, 918, 170410. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Zhu, C.; Xiong, Y.; Yan, K.; He, S. Effects of Bacillus subtilis and Lactobacillus on growth performance, serum biochemistry, nutrient apparent digestibility, and cecum flora in heat-stressed broilers. Int. J. Biometeorol. 2024, 68, 2705–2713. [Google Scholar] [CrossRef]
- Tolonen, A.C. Engineering of Lachnospiraceae Fermentation for Health and the Bioeconomy. Ph.D. Thesis, Universite Paris Saclay, Paris, France, 2024. [Google Scholar]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Bailey, R. Intestinal Microbiota and the Pathogenesis of Dysbacteriosis in Broiler Chickens. Ph.D. Thesis, University of East Anglia, Norwich, UK, 2010. [Google Scholar]
| Ingredient (% of Diet) | Starter | Grower |
|---|---|---|
| Corn | 56.80 | 64.76 |
| Soybean Meal (CP 44%) | 35.40 | 27.85 |
| Fish Meal | 1.00 | 1.00 |
| Soybean Oil | 2.31 | 1.39 |
| Oyster Shell | 1.34 | 1.84 |
| Dicalcium Phosphate | 1.53 | 1.66 |
| Common Salt | 0.396 | 0.326 |
| Vitamin Premix a | 0.50 | 0.50 |
| Mineral Premix b | 0.50 | 0.50 |
| DL-Methionine | 0.151 | 0.055 |
| L-Lysine HCl | 0.073 | 0.119 |
| Total | 100 | 100 |
| Nutrient Composition | ||
| Nutrient | Starter | Grower |
| AMEn (Kcal/Kg) | 2950 | 2960 |
| Crude Protein (%) | 21.203 | 18.499 |
| Arg (%) | 1.365 | 1.157 |
| Lys (%) | 1.208 | 1.052 |
| Met (%) | 0.490 | 0.360 |
| Met + Cys (%) | 0.832 | 0.666 |
| Ca (%) | 0.997 | 1.200 |
| Available P (%) | 0.453 | 0.467 |
| Na (%) | 0.180 | 0.150 |
| Index | CON | TM | CHS-CON | CHS-TM | TM Effect (p) | CHS Effect (p) | TM × CHS (p) |
|---|---|---|---|---|---|---|---|
| Chao1 | 149.67 ± 31.7 | 166.89 ± 24.12 | 155.67 ± 39.39 | 138.5 ± 34.92 | 0.997 | 0.419 | 0.219 |
| Shannon | 3.73 ± 0.23 | 3.74 ± 0.31 | 3.8 ± 0.50 | 3.4 ± 0.31 | 0.214 | 0.333 | 0.125 |
| Pair | F-Value | p-Value |
|---|---|---|
| TM vs. CON | 2.033 | 0.041 |
| TM vs. CHS-CON | 3.4913 | 0.012 |
| TM vs. CHS-TM | 1.5514 | 0.08 |
| CON vs. CHS-CON | 1.0491 | 0.398 |
| CON vs. CHS-TM | 1.3863 | 0.143 |
| CHS-CON vs. CHS-TM | 1.6511 | 0.087 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahadha, R.; Hundam, S.; Al-Zghoul, M.B.; Alanagreh, L.; Ababneh, M.; Mayyas, M.; Alghizzawi, D.; Mustafa, M.A.; Gerrard, D.E.; Dalloul, R.A. Embryonic Thermal Manipulation Affects Body Performance Parameters and Cecum Microbiome in Broiler Chickens in Response to Post-Hatch Chronic Heat Stress Challenge. Animals 2025, 15, 1677. https://doi.org/10.3390/ani15121677
Dahadha R, Hundam S, Al-Zghoul MB, Alanagreh L, Ababneh M, Mayyas M, Alghizzawi D, Mustafa MA, Gerrard DE, Dalloul RA. Embryonic Thermal Manipulation Affects Body Performance Parameters and Cecum Microbiome in Broiler Chickens in Response to Post-Hatch Chronic Heat Stress Challenge. Animals. 2025; 15(12):1677. https://doi.org/10.3390/ani15121677
Chicago/Turabian StyleDahadha, Rahmeh, Seif Hundam, Mohammad Borhan Al-Zghoul, Lo’ai Alanagreh, Mustafa Ababneh, Mohammad Mayyas, Daoud Alghizzawi, Minas A. Mustafa, David E. Gerrard, and Rami A. Dalloul. 2025. "Embryonic Thermal Manipulation Affects Body Performance Parameters and Cecum Microbiome in Broiler Chickens in Response to Post-Hatch Chronic Heat Stress Challenge" Animals 15, no. 12: 1677. https://doi.org/10.3390/ani15121677
APA StyleDahadha, R., Hundam, S., Al-Zghoul, M. B., Alanagreh, L., Ababneh, M., Mayyas, M., Alghizzawi, D., Mustafa, M. A., Gerrard, D. E., & Dalloul, R. A. (2025). Embryonic Thermal Manipulation Affects Body Performance Parameters and Cecum Microbiome in Broiler Chickens in Response to Post-Hatch Chronic Heat Stress Challenge. Animals, 15(12), 1677. https://doi.org/10.3390/ani15121677






