Stock Dynamics of Female Red King Crab in a Small Bay of the Barents Sea in Relation to Environmental Factors
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Red King Crab Stock Indices
2.2. Environmental Data
2.3. Data Analysis
3. Results
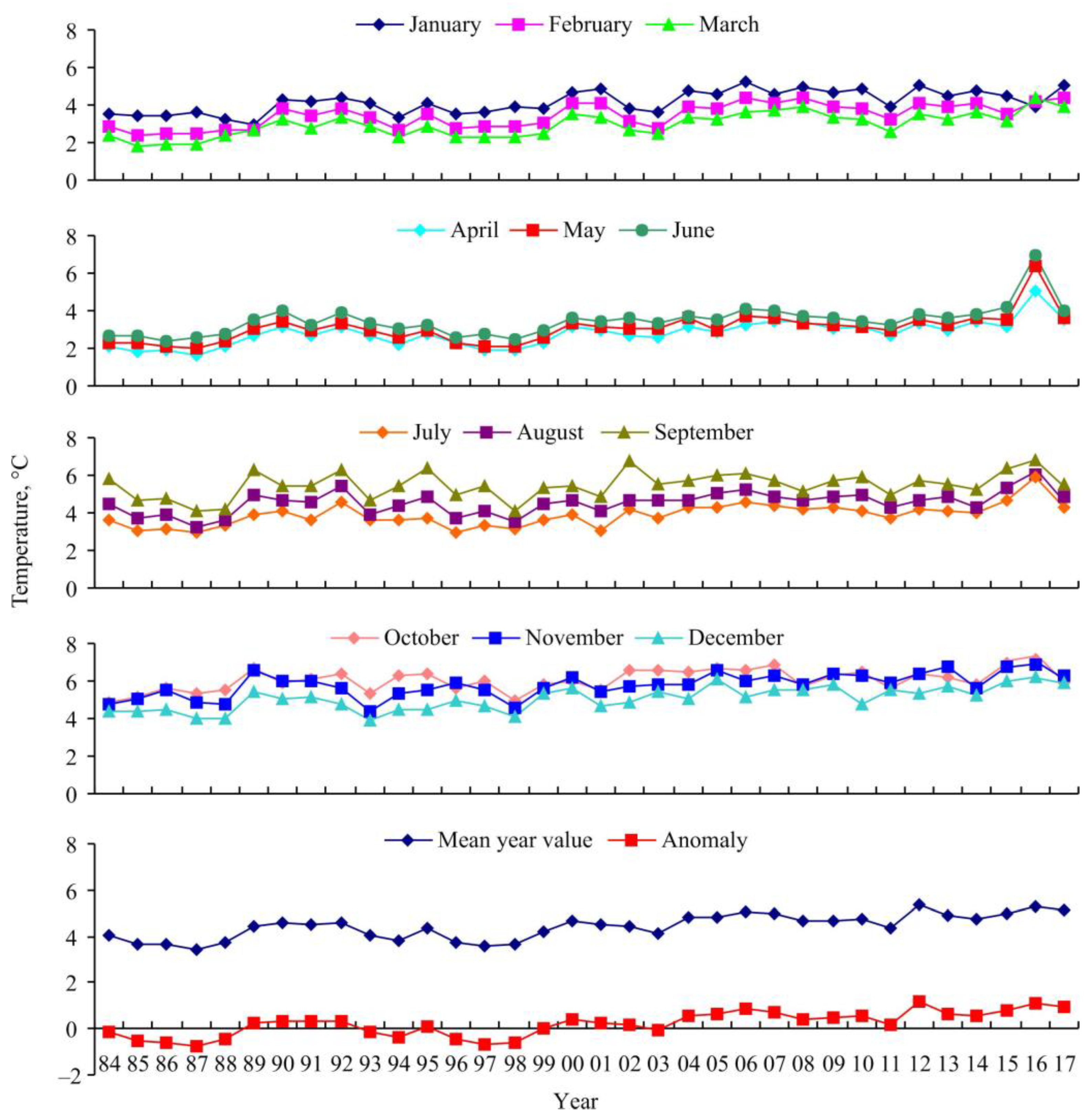
3.1. Environmental Conditions
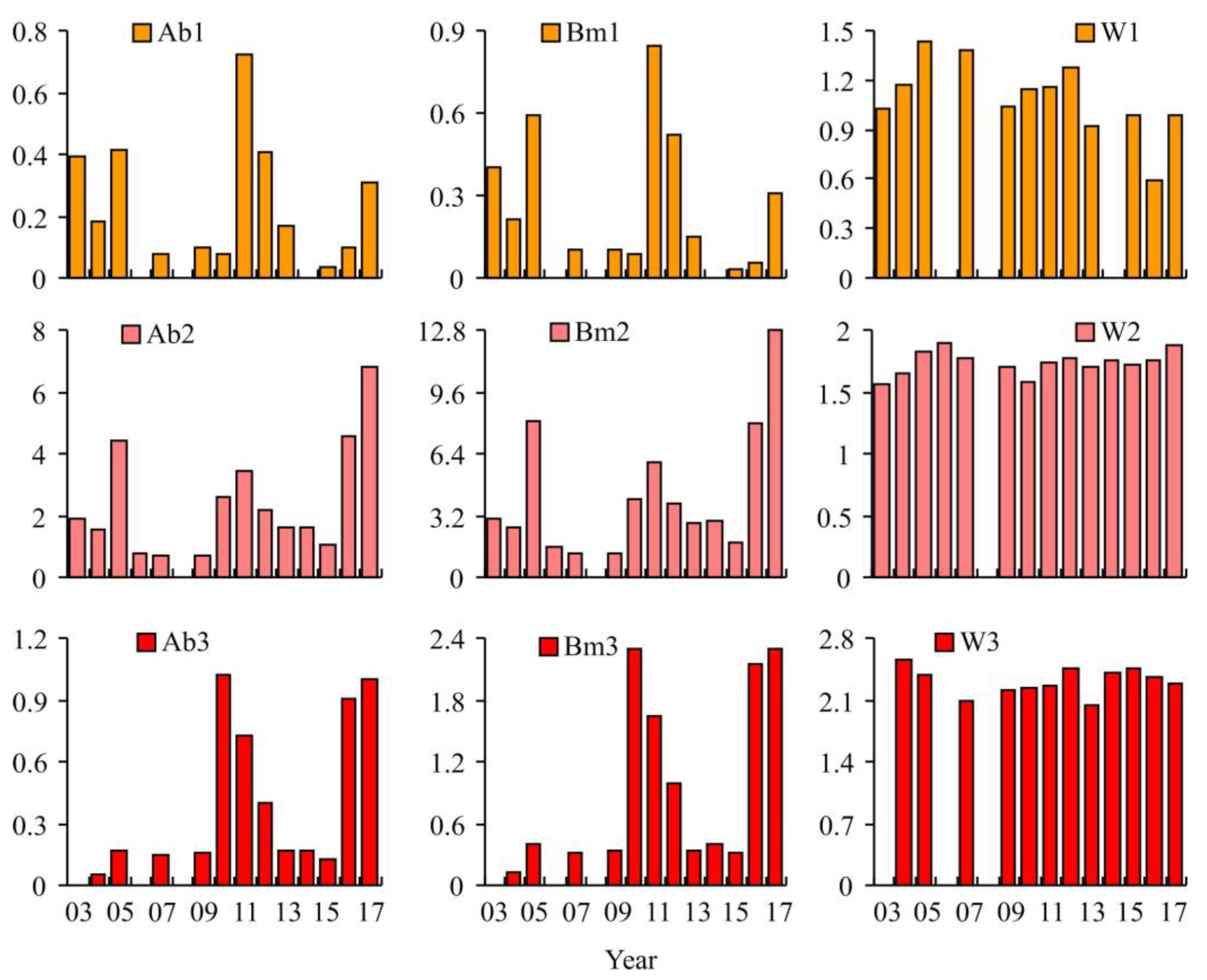
3.2. Red King Crab Stock Indices
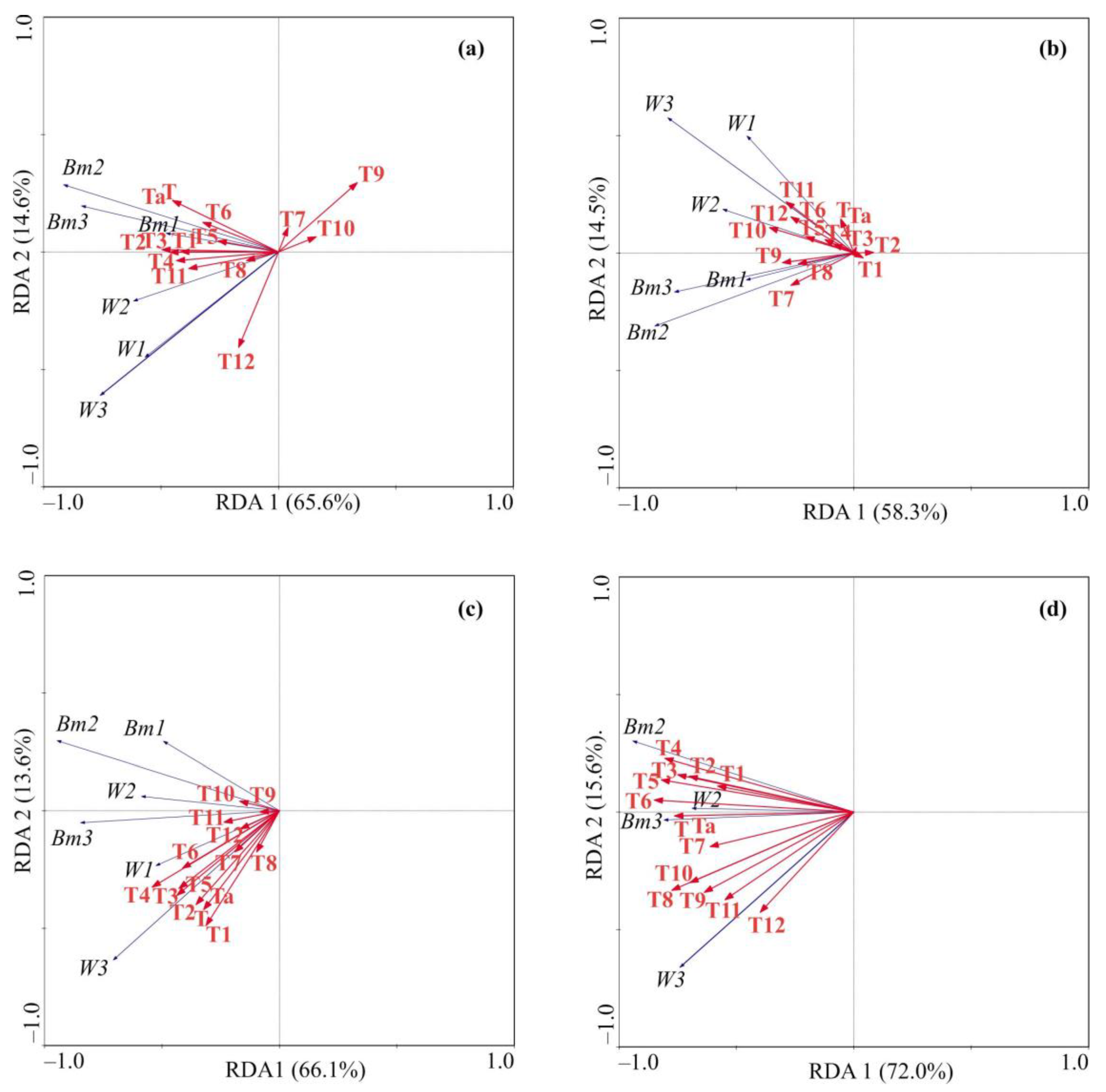
3.3. Relationships Between Crab Abundance and Environmental Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dvoretsky, A.G.; Dvoretsky, V.G. Red king crab (Paralithodes camtschaticus) fisheries in Russian waters: Historical review and present status. Rev. Fish Biol. Fish. 2018, 28, 331–353. [Google Scholar] [CrossRef]
- Loeng, H.; Ozhigin, V.; Ådlandsvik, B. Water fluxes through the Barents Sea. ICES J. Mar. Sci. 1997, 54, 310–317. [Google Scholar] [CrossRef]
- Dvoretsky, V.G.; Dvoretsky, A.G. Local variability of Arctic mesozooplankton biomass and production: A case summer study. Environ. Res. 2024, 241, 117416. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, P.; Reigstad, M.; Haug, T.; Rudels, B.; Carroll, M.L.; Hop, H.; Gabrielsen, G.W.; Falk-Petersen, S.; Denisenko, S.G.; Arashkevich, E.; et al. Food Webs and Carbon Flux in the Barents Sea. Prog. Oceanogr. 2006, 71, 232–287. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Filling knowledge gaps in Arctic marine biodiversity: Environment, plankton, and benthos of Franz Josef Land, Barents Sea. Ocean Coast. Manag. 2024, 249, 106987. [Google Scholar] [CrossRef]
- Kuzmin, S.A.; Olsen, S. Barents sea king crab Paralithodes camtschatica. The transplantation experiments were successful. ICES CM. K 1994, 12, 1–12. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibionts of an introduced king crab in the Barents Sea: A second five-year study. Diversity 2023, 15, 29. [Google Scholar] [CrossRef]
- Bakanev, S.V.; Stesko, A.V. Red king crab. In Materials of Total Allowable Catches of Water Biological Resources in Fishing Areas in Inland Seas of the Russian Federation, on the Continental Shelf of the Russian Federation, in the Exclusive Economical Zone of the Russian Federation, in the Azov and Caspian Seas in 2023; Goryanina, S.V., Ed.; FGBUN VNIRO: Murmansk, Russia, 2023; pp. 5–19. (In Russian) [Google Scholar]
- Sokolov, K.M. Status of the Living Marine Resources in the Barents, White and Kara Seas and the North Atlantic in 2024; PINRO: Murmansk, Russia, 2024. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Limb autotomy patterns in Paralithodes camtschaticus (Tilesius, 1815), an invasive crab, in the coastal Barents Sea. J. Exp. Mar. Biol. Ecol. 2009, 377, 20–27. [Google Scholar] [CrossRef]
- Kuzmin, S.A.; Gudimova, E.N. Introduction of the Kamchatka (Red King) Crab in the Barents Sea. Peculiarities of Biology, Perspectives of Fishery; KSC RAS Press: Apatity, Russia, 2002. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Population dynamics of the invasive lithodid crab, Paralithodes camtschaticus, in a typical bay of the Barents Sea. ICES J. Mar. Sci. 2013, 70, 1255–1262. [Google Scholar] [CrossRef]
- Sokolov, V.I.; Milyutin, D.M. Distribution, size-sex composition, and reserves of the red king crab (Paralithodes camtschaticus) in the upper sublittoral of the Kola Peninsula (the Barents Sea). Zool. Zhurnal 2006, 85, 158–171, (In Russian with English abstract). [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Ecology of Red King Crab in the Coastal Barents Sea; SSC RAS Press: Rostov-on-Don, Russia, 2018. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Epibiotic communities of common crab species in the coastal Barents Sea: Biodiversity and infestation patterns. Diversity 2022, 14, 6. [Google Scholar] [CrossRef]
- Stesko, A.V. Distribution and status of the king crab stock in the Russian territorial waters of the Barents Sea. Probl. Fish. 2015, 16, 175–192, (In Russian with English abstract). [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Ecology and distribution of red king crab larvae in the Barents Sea: A review. Water 2022, 14, 2328. [Google Scholar] [CrossRef]
- Zheng, J.; Kruse, G.H. King Crab Stock Assessments in Alaska. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 539–558. [Google Scholar]
- Piepenburg, D. Recent research on Arctic benthos: Common notions need to be revised. Polar Biol. 2005, 28, 733–755. [Google Scholar] [CrossRef]
- Drinkwater, K. The influence of climate variability and change on the ecosystems of the Barents Sea and adjacent waters: Review and synthesis of recent studies from the NESSAS project. Prog. Oceanogr. 2011, 90, 47–61. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Effects of environmental factors on the abundance, biomass, and individual weight of juvenile red king crabs in the Barents Sea. Front. Mar. Sci. 2020, 7, 726. [Google Scholar] [CrossRef]
- Oleszczuk, B.; Grzelak, K.; Kędra, M. Community structure and productivity of Arctic benthic fauna across depth gradients during springtime. Deep Sea Res. I 2021, 170, 103457. [Google Scholar] [CrossRef]
- Pavlova, L.V.; Dvoretsky, A.G.; Frolov, A.A.; Zimina, O.L.; Evseeva, O.Y.; Dikaeva, D.R.; Rumyantseva, Z.Y.; Panteleeva, N.N. The impact of sea ice loss on benthic communities of the Makarov Strait (northeastern Barents Sea). Animals 2023, 13, 2320. [Google Scholar] [CrossRef]
- Denisenko, S.G. The Barents Sea zoobenthos under the conditions of changing climate and anthropogenic impact. In Dynamics of Marine Ecosystems and Current Preservation Problems of Biological Potential of the Seas of Russia; Tarasov, V.G., Ed.; Dalnauka: Vladivostok, Russia, 2007; pp. 418–511. (In Russian) [Google Scholar]
- Frolova, E.A.; Lyubina, O.S.; Dikayeva, D.R.; Ahmetchina, O.Y.; Frolov, A.A. Effect of climatic changes on the zoobenthos of the Barents Sea (on the example of several abundant species). Dokl. Biol. Sci. 2007, 416, 349–351. [Google Scholar] [CrossRef]
- Renaud, P.E.; Ambrose, W.G.; Węsławski, J.M. Benthic communities in the polar night. In Polar Night Marine Ecology: Life and Light in the Dead of Night; Berge, J., Johnsen, G., Cohen, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 161–179. [Google Scholar]
- Turner, J.; Overland, J. Contrasting climate change in the two polar regions. Polar Res. 2009, 28, 146–164. [Google Scholar] [CrossRef]
- Johannesen, E.; Ingvaldsen, R.B.; Bogstad, B.; Dalpadado, P.; Eriksen, E.; Gjøsæter, H.; Knutsen, T.; Skern-Mauritzen, M.; Stiansen, J.E. Changes in Barents Sea ecosystem state, 1970–2009: Climate fluctuations, human impact, and trophic interactions. ICES J. Mar. Sci. 2012, 69, 880–889. [Google Scholar] [CrossRef]
- Smedsrud, L.H.; Muilwijk, M.; Brakstad, A.; Madonna, E.; Lauvset, S.K.; Spensberger, C.; Born, A.; Eldevik, T.; Drange, H.; Jeansson, E.; et al. Nordic Seas heat loss, Atlantic inflow, and Arctic sea ice cover over the last century. Rev. Geophys. 2022, 60, e2020RG000725. [Google Scholar] [CrossRef]
- Dikaeva, D.R.; Dvoretsky, A.G. Spatial patterns and environmental control of polychaete communities in the southwestern Barents Sea. Biology 2024, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Dvoretsky, V.G.; Dvoretsky, A.G. Effects of water temperature on zooplankton abundance and biomass in the southwestern Barents Sea: Implications for Arctic monitoring and management. Ocean Coast. Manag. 2025, 261, 107506. [Google Scholar] [CrossRef]
- Russell, B.D.; Connell, S.D.; Mellin, C.; Brook, B.W.; Burnell, O.W.; Fordham, D.A. Predicting the distribution of commercially important invertebrate stocks under future climate. PLoS ONE 2012, 7, e46554. [Google Scholar] [CrossRef][Green Version]
- Heath, M.R.; Neat, F.C.; Pinnegar, J.K.; Reid, D.G.; Sims, D.W.; Wright, P.J. Review of climate change impacts on marine fish and shellfish around the UK and Ireland. Aquat. Conserv. 2012, 22, 337–367. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Inter-annual dynamics of the Barents Sea red king crab (Paralithodes camtschaticus) stock indices in relation to environmental factors. Polar Sci. 2016, 10, 541–552. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Size at maturity of female red king crab, Paralithodes camtschaticus, from the costal zone of Kola Peninsula (southern Barents Sea). Cah. Biol. Mar. 2015, 56, 49–54. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Commercial fish and shellfish in the Barents Sea: Have introduced crab species affected the population trajectories of commercial fish? Rev. Fish Biol. Fish. 2015, 25, 297–322. [Google Scholar] [CrossRef]
- Pavlova, L.V. Red king crab trophic relations and its influence on bottom biocenoses. In Biology and Physiology of the Red King Crab from the Coastal Zone of the Barents Sea; Matishov, G.G., Ed.; Publishing Kola Scientific Centre Russian Academy of Sciences: Apatity, Russia, 2008; pp. 77–104. (In Russian) [Google Scholar]
- Britayev, T.A.; Rzhavsky, A.V.; Pavlova, L.V.; Dvoretskij, A.G. Studies on impact of the alien red king crab (Paralithodes camtschaticus) on the shallow water benthic communities of the Barents Sea. J. Appl. Ichthyol. 2010, 26 (Suppl. S2), 66–73. [Google Scholar] [CrossRef]
- Falk-Petersen, J.; Renaud, P.; Anisimova, N. Establishment and ecosystem effects of the alien invasive red king crab (Paralithodes camtschaticus) in the Barents Sea—A review. ICES J. Mar. Sci. 2011, 68, 479–488. [Google Scholar] [CrossRef]
- Oug, E.; Cochrane, S.K.J.; Sundet, J.H.; Norling, K.; Nilsson, H.C. Effects of the invasive red king crab (Paralithodes camtschaticus) on soft-bottom fauna in Varangerfjorden, northern Norway. Mar. Biodivers. 2011, 41, 467–479. [Google Scholar] [CrossRef]
- Oug, E.; Sundet, J.H.; Cochrane, S.K.J. Structural and functional changes of soft-bottom ecosystems in northern fjords invaded by the red king crab (Paralithodes camtschaticus). J. Mar. Syst. 2018, 180, 255–264. [Google Scholar] [CrossRef]
- Pavlova, L.V.; Dvoretsky, A.G. Prey selectivity in juvenile red king crabs from the coastal Barents Sea. Diversity 2022, 14, 568. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Aquaculture of green sea urchin in the Barents Sea: A brief review of Russian studies. Rev. Aquac. 2020, 12, 2080–2090. [Google Scholar] [CrossRef]
- Evseeva, O.Y.; Ishkulova, T.G.; Dvoretsky, A.G. Environmental drivers of an intertidal bryozoan community in the Barents Sea: A case study. Animals 2022, 12, 552. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. New echinoderm-crab epibiotic associations from the coastal Barents Sea. Animals 2021, 11, 917. [Google Scholar] [CrossRef]
- Pinchukov, M.A. Estimation of age in females of the red king crab Paralithodes camtschaticus (Decapoda, Lithodidae) from the Barents Sea Based on their size composition. Proc. Kazan Univ. Nat. Sci. Ser. 2017, 159, 480–491. (In Russian) [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Size-at-age of juvenile red king crab (Paralithodes camtschaticus) in the coastal Barents Sea. Cah. Biol. Mar. 2014, 55, 43–48. [Google Scholar]
- Bizikov, V.A.; Goncharov, S.M.; Polyakov, A.V. The geographical informational system CardMaster. Rybn. Khoz. 2007, 1, 96–99. (In Russian) [Google Scholar]
- McDonald, J.H. Handbook of Biological Statistics, 2nd ed.; Sparky House Publishing: Baltimore, MD, USA, 2009. [Google Scholar]
- Pyper, B.J.; Peterman, R.M. Comparison of methods to account for autocorrelation analyses of fish data. Can. J. Fish. Aquat. Sci. 1998, 55, 2127–2140. [Google Scholar] [CrossRef]
- Leps, J.; Smilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: New York, NY, USA, 2002. [Google Scholar]
- Karamushko, O.V.; Zhuravleva, N.G.; Karamushko, L.I.; Kudryavtseva, O.Y.; Raskhozheva, E.V.; Smirnova, E.V. Modern research of the Barents Sea and adjacent waters ichthyofauna. In Marine Ecosystems and Communities in the Conditions of Current Climate Change; Matishov, G.G., Ed.; Renome: Saint Petersburg, Russia, 2014; pp. 223–243. (In Russian) [Google Scholar]
- Stone, R.P.; O’Clair, C.E.; Shirley, T.C. Aggregating behavior of ovigerous female red king crab, Paralithodes camtschaticus, in Auke Bay, Alaska. Can. J. Fish. Aquat. Sci. 1993, 50, 750–758. [Google Scholar] [CrossRef]
- Dvoretsky, A.G.; Dvoretsky, V.G. Renewal of the amateur red king crab fishery in Russian waters of the Barents Sea: Potential benefits and costs. Mar. Policy 2022, 136, 104916. [Google Scholar] [CrossRef]
- Slizkin, A.G.; Safronov, S.G. Commercial Crabs of the Waters of Kamchatka; North Pacific: Petropavlovsk-Kamchatsky, Russia, 2000. [Google Scholar]
- Bahr, G.; Gulliksen, B. Variation of the epifauna on pier-pilings between 1980 and 1992 near the city of Tromsø, Northern Norway. Polar Biol. 2001, 24, 282–291. [Google Scholar]
- Beuchel, F.; Bahr, G.; Gulliksen, B. Long-term patterns of rocky bottom macrobenthic community structure in an Arctic fjord (Kongsfjorden, Svalbard) in relation to climate variability (1980–2003). J. Mar. Syst. 2003, 63, 35–48. [Google Scholar] [CrossRef]
- Sanchez-Rubio, G.; Perry, H.M.; Biesiot, P.M.; Johnson, D.R.; Lipcius, R.N. Climate-related hydrological regimes and their effects on abundance of juvenile blue crabs (Callinectes sapidus) in the northcentral Gulf of Mexico. Fish. Bull. 2011, 396, 139–146. [Google Scholar]
- Noskovich, A.E.; Dvoretsky, A.G. Spatial distribution and growth patterns of a common bivalve mollusk (Macoma calcarea) in Svalbard fjords in relation to environmental factors. Animals 2024, 14, 3352. [Google Scholar] [CrossRef]
- Hiddink, J.G.; Burrows, M.T.; García Molinos, J. Temperature tracking by North Sea benthic invertebrates in response to climate change. Glob. Change Biol. 2015, 21, 117–129. [Google Scholar] [CrossRef]
- Tremblay, J.É.; Robert, D.; Varela, D.E.; Lovejoy, C.; Darnis, G.; Nelson, R.J.; Sastri, A.R. Current state and trends in Canadian Arctic marine ecosystems: I. Primary production. Clim. Change 2012, 115, 161–178. [Google Scholar] [CrossRef]
- Renaud, P.E.; Sejr, M.K.; Bluhm, B.A.; Sirenko, B.; Ellingsen, I.H. The future of Arctic benthos: Expansion, invasion, and biodiversity. Prog. Oceanog. 2015, 139, 244–257. [Google Scholar] [CrossRef]
- Roy, V.; Iken, K.; Gosselin, M.; Tremblay, J.É.; Bélanger, S.; Archambault, P. Benthic faunal assimilation pathways and depth-related changes in food-web structure across the Canadian Arctic. Deep Sea Res I 2015, 102, 55–71. [Google Scholar] [CrossRef]
- Anisimova, N.A.; Manushin, I.E. Anisimova, N.A.; Manushin, I.E. A diet of red king crab in the Barents Sea. In The Red King Crab in the Barents Sea; Berenboim, B.I., Ed.; PINRO Press: Murmansk, Russia, 2003; pp. 170–189. (In Russian) [Google Scholar]
- Pedersen, T.; Fuhrmann, M.M.; Lindstrøm, U.; Nilssen, E.M.; Ivarjord, T.; Ramasco, V.; Jørgensen, L.L.; Sundet, J.H.; Sivertsen, K.; Källgren, E.; et al. Effects of the invasive red king crab on food web structure and ecosystem properties in an Atlantic fjord. Mar. Ecol. Prog. Ser. 2018, 596, 13–31. [Google Scholar] [CrossRef]
- Stevens, B.G. Temperature-dependent growth of juvenile red king crab (Paralithodes camtschatica) and its effects on size-at-age and subsequent recruitment in the eastern Bering Sea. Can. J. Fish. Aquat. Sci. 1990, 47, 1307–1317. [Google Scholar] [CrossRef]
- Webb, J. Reproduction Ecology of Commercially Important Lithodid Crabs. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 285–314. [Google Scholar]
- Tyler, A.V.; Kruse, G.C. Conceptual modeling of brood strength of red king crabs in the Bristol Bay region of the Bering Sea. In High Latitude Crabs: Biology, Management and Economics; University of Alaska Sea Grant Program Report 96-02; University of Alaska: Fairbanks, AK, USA, 1996; pp. 511–544. [Google Scholar]
- Zheng, J.; Kruse, G.H. Recruitment Patterns of Alaskan Crabs in Relation to Decadal Shifts in Climate and Physical Oceanography. ICES J. Mar. Sci. 2000, 57, 438–451. [Google Scholar] [CrossRef]
- Barber, D.G.; Lukovich, J.V.; Keogak, J.; Baryluk, S.; Fortier, L.; Henry, G.H.R. The changing climate of the Arctic. Arctic 2008, 61 (Suppl. S1), 7–26. [Google Scholar] [CrossRef]
- Koenig, Z.; Fer, I.; Chierici, M.; Fransson, A.; Jones, E.; Kolås, E. Diffusive and advective cross-frontal fluxes of inorganic nutrients and dissolved inorganic carbon in the Barents Sea in autumn. Prog. Oceanogr. 2023, 219, 103161. [Google Scholar] [CrossRef]
- Jakobsen, T.; Ozhigin, V.K. (Eds.) The Barents Sea: Ecosystem, Resources, Management: Half a Century of Russian-Norwegian Cooperation; Tapir Academic Press: Trondheim, Norway, 2011. [Google Scholar]
- Rawlins, M.A.; Karmalkar, A.V. Regime shifts in Arctic terrestrial hydrology manifested from impacts of climate warming. Cryosphere 2024, 18, 1033–1052. [Google Scholar] [CrossRef]
- Nixon, S.W.; Fulweiler, R.W.; Buckley, B.A.; Granger, S.L.; Nowicki, B.L.; Henry, K.M. The impact of changing climate on phenology, productivity, and benthic–pelagic coupling in Narragansett Bay. Estuar. Coast. Shelf Sci. 2009, 82, 1–18. [Google Scholar] [CrossRef]
- Sakshaug, E.; Johnsen, G.; Kovacs, K. (Eds.) Ecosystem Barents Sea; Tapir Academic Press: Trondheim, Norway, 2009. [Google Scholar]
- McGovern, M.; Poste, A.E.; Oug, E.; Renaud, P.E.; Trannum, H.C. Riverine impacts on benthic biodiversity and functional traits: A comparison of two sub-Arctic fjords. Estuar. Coast. Shelf Sci. 2020, 240, 106774. [Google Scholar] [CrossRef]
- Fuhrmann, M.M.; Pedersen, T.; Ramasco, V.; Nilssen, E.M. Macrobenthic biomass and production in a heterogenic subarctic fjord after invasion by the red king crab. J. Sea Res. 2015, 106, 1–13. [Google Scholar] [CrossRef]
- Mueter, F.J.; Norcross, B.L.; Royer, T.C. Do cyclic temperatures cause cyclic fisheries? Can. Spec. Publ. Fish. Aquat. Sci. 1995, 121, 119–129. [Google Scholar]
- Loher, T.; Armstrong, D.A. Historical changes in the abundance and distribution of ovigerous red king crabs (Paralithodes camtschaticus) in Bristol Bay (Alaska), and potential relationship with bottom temperature. Fish. Oceanogr. 2005, 14, 292–306. [Google Scholar] [CrossRef]
- Stevens, B.G. Biology and Ecology of Juvenile King Crabs. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 261–401. [Google Scholar]
- Stevens, B.G. Development and Biology of King Crab Larvae. In King Crabs of the World: Biology and Fisheries Management; Stevens, B.G., Ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 233–260. [Google Scholar]
- Long, W.C.; Daly, B.J.; Cummiskey, P.A. Optimizing release strategies for red king crab stock enhancement: Effects of release timing. Fish. Res. 2024, 274, 106975. [Google Scholar] [CrossRef]
- Armstrong, D.A.; Kruse, G.H.; Hines, A.H.; Orensanz, J.M.; McDonald, P.S. A crab for all seasons: The confluence of fisheries and climate as drivers of crab abundance and distribution. In Biology and Management of Exploited Crab Populations under Climate Change; Kruse, G.H., Eckert, G.L., Foy, R.J., Lipcius, R.N., Sainte-Marie, B., Stram, D.L., Woodby, D., Eds.; Alaska Sea Grant Publications: Fairbanks, AL, USA, 2010; pp. 1–48. [Google Scholar]
- Dvoretsky, A.G.; Dvoretsky, V.G. Distribution of amphipods Ischyrocerus on the red king crab, Paralithodes camtschaticus: Possible interactions with the host in the Barents Sea. Estuar. Coast. Shelf Sci. 2009, 82, 390–396. [Google Scholar] [CrossRef]
| Parameter | Ab1 | Ab2 | Ab3 | Bm1 | Bm2 | Bm3 | W1 | W2 | W3 |
|---|---|---|---|---|---|---|---|---|---|
| Lag 4 | |||||||||
| T1 | 0.104 | 0.333 | 0.501 | 0.127 | 0.312 | 0.508 | −0.211 | −0.154 | 0.261 |
| T2 | 0.281 | 0.408 | 0.524 | 0.298 | 0.388 | 0.532 | −0.055 | −0.108 | 0.359 |
| T3 | 0.360 | 0.366 | 0.514 | 0.375 | 0.349 | 0.522 | 0.010 | −0.067 | 0.283 |
| T4 | 0.242 | 0.306 | 0.546 | 0.255 | 0.293 | 0.556 | −0.142 | 0.043 | 0.256 |
| T5 | 0.089 | 0.203 | 0.556 | 0.102 | 0.187 | 0.554 | 0.000 | 0.002 | 0.018 |
| T6 | 0.168 | 0.260 | 0.643 | 0.167 | 0.245 | 0.637 | 0.107 | 0.063 | −0.130 |
| T7 | −0.151 | −0.116 | 0.435 | −0.223 | −0.130 | 0.425 | −0.292 | −0.152 | −0.257 |
| T8 | −0.149 | −0.014 | 0.466 | −0.217 | −0.031 | 0.447 | −0.330 | −0.220 | −0.445 |
| T9 | −0.427 | −0.280 | 0.041 | −0.464 | −0.278 | 0.027 | −0.463 | 0.161 | −0.490 |
| T10 | −0.173 | −0.184 | 0.248 | −0.220 | −0.186 | 0.228 | −0.261 | 0.060 | −0.602 |
| T11 | −0.042 | 0.348 | 0.476 | −0.147 | 0.355 | 0.473 | −0.471 | 0.065 | −0.246 |
| T12 | 0.129 | 0.012 | 0.141 | 0.039 | 0.013 | 0.137 | 0.187 | −0.224 | −0.261 |
| T | 0.024 | 0.444 | 0.739 | −0.029 | 0.423 | 0.743 | −0.373 | −0.059 | 0.024 |
| Ta | 0.024 | 0.444 | 0.739 | −0.029 | 0.423 | 0.743 | −0.373 | −0.059 | 0.024 |
| Lag 8 | |||||||||
| T1 | −0.380 | 0.003 | 0.076 | −0.423 | 0.022 | 0.094 | −0.717 | 0.176 | 0.057 |
| T2 | −0.408 | −0.022 | 0.063 | −0.458 | −0.010 | 0.081 | −0.701 | 0.022 | 0.061 |
| T3 | −0.336 | 0.013 | 0.161 | −0.393 | 0.022 | 0.178 | −0.666 | −0.007 | 0.078 |
| T4 | −0.194 | 0.050 | 0.267 | −0.273 | 0.045 | 0.282 | −0.649 | −0.192 | 0.109 |
| T5 | −0.070 | 0.043 | 0.310 | −0.128 | 0.038 | 0.322 | −0.593 | −0.173 | 0.105 |
| T6 | −0.120 | 0.103 | 0.367 | −0.172 | 0.096 | 0.375 | −0.638 | −0.183 | −0.006 |
| T7 | −0.104 | 0.218 | 0.387 | −0.144 | 0.212 | 0.394 | −0.530 | −0.042 | −0.035 |
| T8 | 0.072 | 0.189 | 0.312 | 0.008 | 0.177 | 0.310 | −0.569 | −0.249 | −0.315 |
| T9 | 0.190 | 0.192 | 0.341 | 0.160 | 0.156 | 0.330 | −0.158 | −0.592 | −0.230 |
| T10 | 0.298 | 0.184 | 0.278 | 0.272 | 0.162 | 0.274 | −0.205 | −0.375 | −0.040 |
| T11 | 0.058 | 0.245 | 0.244 | 0.012 | 0.247 | 0.244 | −0.266 | −0.182 | −0.099 |
| T12 | 0.009 | 0.249 | 0.338 | −0.049 | 0.257 | 0.336 | −0.207 | 0.026 | −0.381 |
| T | −0.253 | −0.036 | 0.208 | −0.318 | −0.035 | 0.215 | −0.647 | −0.154 | −0.124 |
| Ta | −0.253 | −0.036 | 0.208 | −0.318 | −0.035 | 0.215 | −0.647 | −0.154 | −0.124 |
| Lag 9 | |||||||||
| T1 | −0.416 | 0.179 | 0.421 | −0.471 | 0.181 | 0.415 | −0.414 | −0.060 | −0.174 |
| T2 | −0.364 | 0.259 | 0.469 | −0.432 | 0.257 | 0.466 | −0.461 | −0.090 | −0.070 |
| T3 | −0.265 | 0.351 | 0.529 | −0.347 | 0.347 | 0.530 | −0.500 | −0.084 | −0.058 |
| T4 | −0.072 | 0.439 | 0.558 | −0.151 | 0.430 | 0.563 | −0.485 | −0.140 | 0.097 |
| T5 | −0.016 | 0.274 | 0.471 | −0.099 | 0.258 | 0.478 | −0.389 | −0.248 | 0.062 |
| T6 | −0.004 | 0.284 | 0.485 | −0.100 | 0.269 | 0.492 | −0.508 | −0.202 | 0.112 |
| T7 | −0.012 | 0.138 | 0.154 | −0.112 | 0.141 | 0.165 | −0.609 | 0.014 | 0.096 |
| T8 | −0.052 | 0.029 | 0.105 | −0.128 | 0.024 | 0.121 | −0.615 | −0.163 | 0.451 |
| T9 | 0.312 | −0.006 | −0.028 | 0.262 | −0.021 | −0.020 | −0.354 | −0.153 | 0.432 |
| T10 | 0.161 | 0.061 | 0.038 | 0.100 | 0.043 | 0.059 | −0.560 | −0.127 | 0.419 |
| T11 | −0.073 | 0.229 | 0.140 | −0.085 | 0.234 | 0.159 | −0.688 | 0.185 | 0.356 |
| T12 | −0.120 | 0.224 | 0.190 | −0.136 | 0.244 | 0.208 | −0.690 | 0.290 | 0.219 |
| T | −0.182 | 0.188 | 0.387 | −0.252 | 0.178 | 0.393 | −0.645 | −0.205 | 0.102 |
| Ta | −0.182 | 0.188 | 0.387 | −0.252 | 0.178 | 0.393 | −0.645 | −0.205 | 0.102 |
| Lag 10 | |||||||||
| T1 | - | 0.554 | 0.718 | - | 0.531 | 0.725 | - | −0.026 | 0.084 |
| T2 | - | 0.670 | 0.786 | - | 0.646 | 0.792 | - | −0.029 | 0.140 |
| T3 | - | 0.712 | 0.795 | - | 0.689 | 0.800 | - | −0.057 | 0.147 |
| T4 | - | 0.763 | 0.726 | - | 0.743 | 0.734 | - | 0.020 | 0.202 |
| T5 | - | 0.731 | 0.706 | - | 0.708 | 0.715 | - | −0.058 | 0.152 |
| T6 | - | 0.729 | 0.711 | - | 0.707 | 0.724 | - | −0.079 | 0.255 |
| T7 | - | 0.514 | 0.471 | - | 0.508 | 0.493 | - | −0.013 | 0.415 |
| T8 | - | 0.562 | 0.512 | - | 0.553 | 0.527 | - | 0.052 | 0.327 |
| T9 | - | 0.390 | 0.248 | - | 0.394 | 0.270 | - | 0.302 | 0.487 |
| T10 | - | 0.514 | 0.348 | - | 0.524 | 0.364 | - | 0.317 | 0.327 |
| T11 | - | 0.315 | 0.476 | - | 0.326 | 0.479 | - | 0.402 | 0.015 |
| T12 | - | 0.157 | 0.414 | - | 0.162 | 0.411 | - | 0.121 | −0.140 |
| T | - | 0.657 | 0.693 | - | 0.643 | 0.707 | - | 0.051 | 0.291 |
| Ta | - | 0.657 | 0.693 | - | 0.643 | 0.707 | - | 0.051 | 0.291 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvoretsky, A.G.; Dvoretsky, V.G. Stock Dynamics of Female Red King Crab in a Small Bay of the Barents Sea in Relation to Environmental Factors. Animals 2025, 15, 99. https://doi.org/10.3390/ani15010099
Dvoretsky AG, Dvoretsky VG. Stock Dynamics of Female Red King Crab in a Small Bay of the Barents Sea in Relation to Environmental Factors. Animals. 2025; 15(1):99. https://doi.org/10.3390/ani15010099
Chicago/Turabian StyleDvoretsky, Alexander G., and Vladimir G. Dvoretsky. 2025. "Stock Dynamics of Female Red King Crab in a Small Bay of the Barents Sea in Relation to Environmental Factors" Animals 15, no. 1: 99. https://doi.org/10.3390/ani15010099
APA StyleDvoretsky, A. G., & Dvoretsky, V. G. (2025). Stock Dynamics of Female Red King Crab in a Small Bay of the Barents Sea in Relation to Environmental Factors. Animals, 15(1), 99. https://doi.org/10.3390/ani15010099







