Scent Detection Threshold of Trained Dogs to Eucalyptus Hydrolat
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of In-House Eucalyptus Hydrolat
2.2. Preparation of In-House Eucalyptus Hydrolat Dilutions
2.3. Preparation of Target and Non-Target Samples
2.4. Scent Line-Up Tracks for a Three-Alternative Forced-Choice Method
2.5. Test Protocol
2.6. Study 1
2.7. Study 2
2.8. Study 3
2.9. Ethics Statement
2.10. NMR Analysis of the Eucalyptus Oil and Hydrolats
3. Results
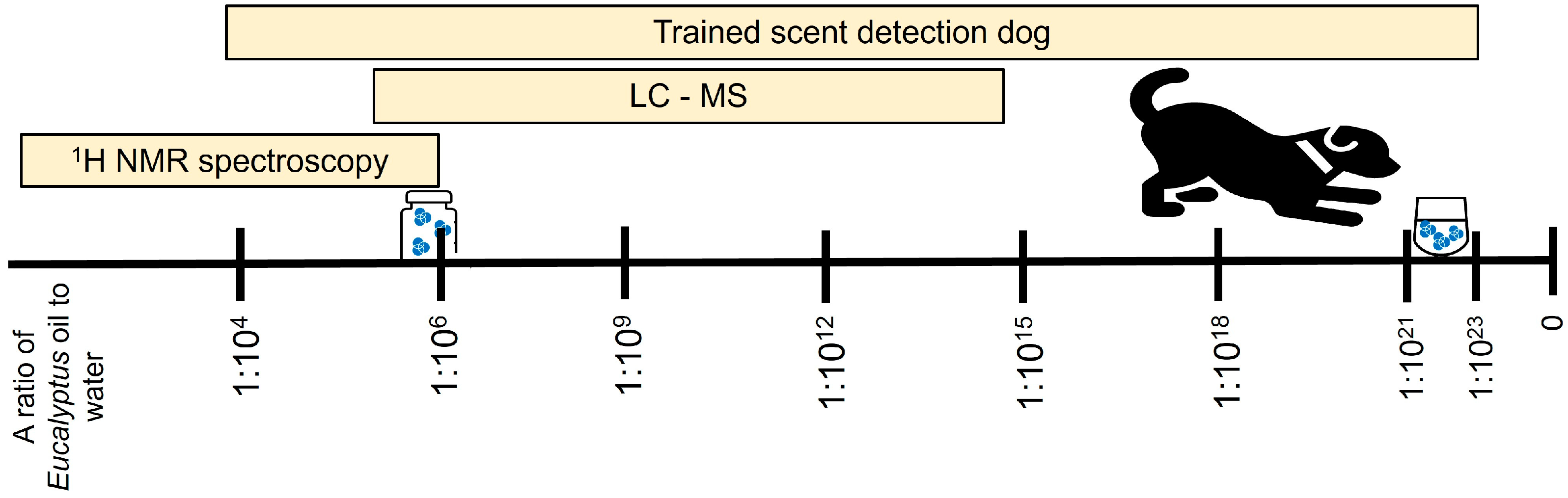
3.1. Scent Detection Threshold
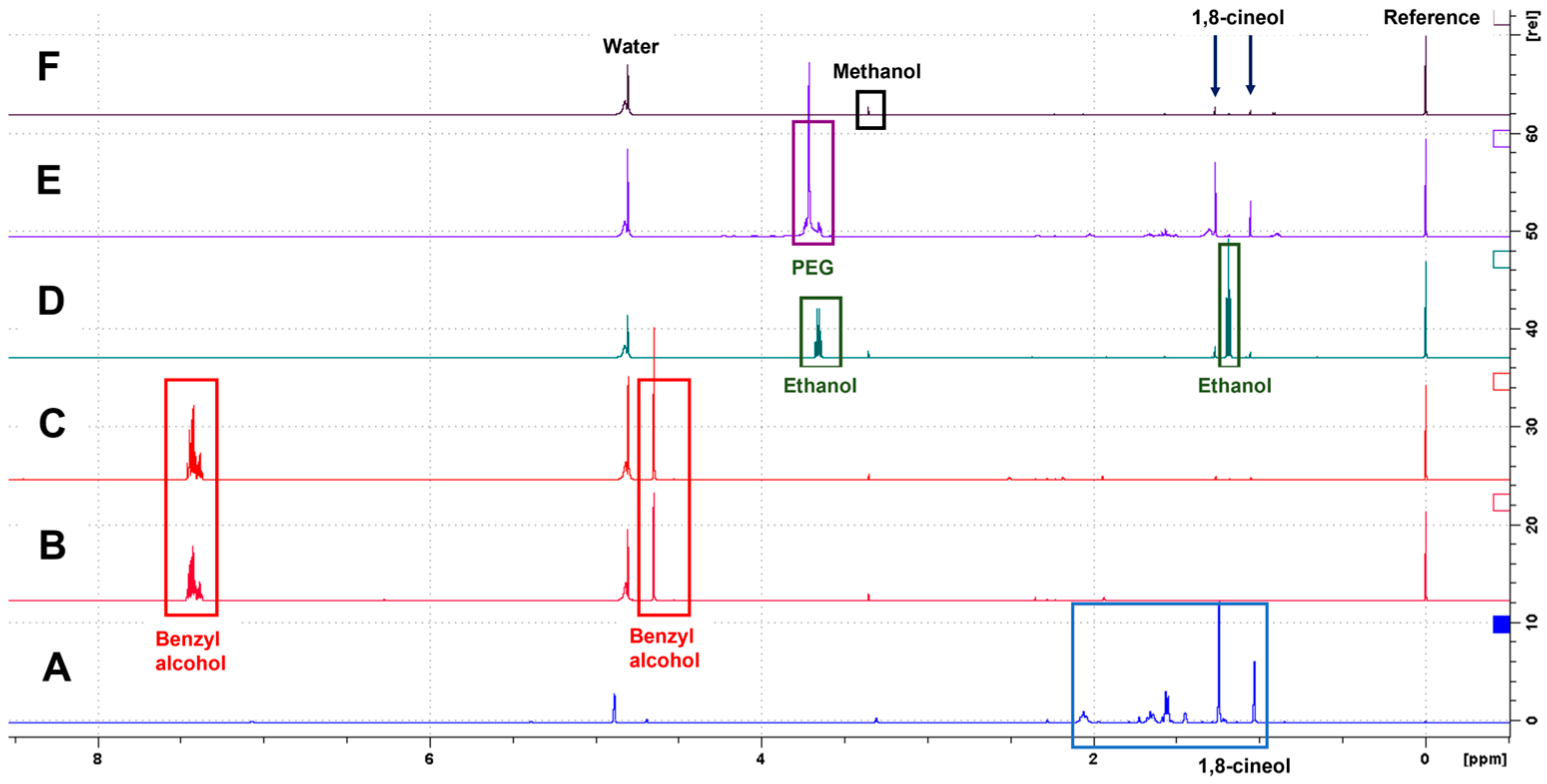
3.2. NMR Detection and Identification of Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, D.B.; Walker, J.C.; Cavnar, P.J.; Taylor, J.L.; Pickel, D.H.; Hall, S.B.; Suarez, J.C. Naturalistic quantification of canine olfactory sensitivity. Appl. Anim. Behav. Sci. 2006, 97, 241–254. [Google Scholar] [CrossRef]
- Craven, B.A.; Paterson, E.G.; Settles, G.S. The fluid dynamics of canine olfaction: Unique nasal airflow patterns as an explanation of macrosmia. J. R. Soc. Interface 2010, 7, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Tacher, S.; Quignon, P.; Rimbault, M.; Dreano, S.; Andre, C.; Galibert, F. Olfactory receptor sequence polymorphism within and between breeds of dogs. J. Hered. 2005, 96, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Quignon, P.; Kirkness, E.; Cadieu, E.; Touleimat, N.; Guyon, R.; Renier, C.; Hitte, C.; André, C.; Fraser, C.; Galibert, F. Comparison of the canine and human olfactory receptor gene repertoires. Genome Biol. 2003, 4, R80. [Google Scholar] [CrossRef] [PubMed]
- Kavoi, B.M.; Jameela, H. Comparative Morphometry of the Olfactory Bulb, Tract and Stria in the Human, Dog and Goat. Int. J. Morphol. 2011, 29, 939–946. [Google Scholar] [CrossRef]
- Jezierski, T.; Adamkiewicz, E.; Walczak, M.; Sobczy’nska, M.; G’orecka-Bruzda, A.; Ensminger, J.; Papet, E. Efficacy of drug detection by fully-trained police dogs varies by breed, training level, type of drug and search environment. Forensic Sci. Int. 2014, 237, 112–118. [Google Scholar] [CrossRef]
- van Dam, A.; Schoon, A.; Wierda, S.F.; Heeringa, E.; Aalders, M.C.G. The use of crime scene detection dogs to locate semen stains on different types of fabric. Forensic Sci. Int. 2019, 302, 109907. [Google Scholar] [CrossRef] [PubMed]
- Abel, R.J.; Lunder, J.L.; Harynuk, J.J. A novel protocol for producing low-abundance targets to characterize the sensitivity limits of ignitable liquid detection canines. Forensic Chem. 2020, 18, 100230. [Google Scholar] [CrossRef]
- Gazit, I.; Goldblatt, A.; Grinstein, D.; Terkel, J. Dogs can detect the individual odors in a mixture of explosives. Appl. Anim. Behav. Sci. 2021, 235, 105212. [Google Scholar] [CrossRef]
- Jenkins, E.K.; DeChant, M.T.; Perry, E.B. When the Nose Doesn’t Know: Canine Olfactory Function Associated with Health, Management, and Potential Links to Microbiota. Front. Vet. Sci. 2018, 5, 56. [Google Scholar] [CrossRef]
- Jinn, J.; Connor, E.G.; Jacobs, L.F. How Ambient Environment Influences Olfactory Orientation in Search and Rescue Dogs. Chem. Senses 2020, 45, 625–634. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, B.; Pinelli, C.; Varcamonti, M.; Rendine, M.; Lombardi, P.; Scandurra, A. COVID Sniffer Dogs: Technical and Ethical Concerns. Front. Vet. Sci. 2021, 8, 669712. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, D.; Sarkis, R.; Lecoq-Julien, C.; Benard, A.; Roger, V.; Levesque, E.; Bernes-Luciani, E.; Maestracci, B.; Morvan, P.; Gully, E.; et al. Can the detection dog alert on COVID-19 positive persons by sniffing axillary sweat samples? A proof-of-concept study. PLoS ONE 2020, 15, e0243122. [Google Scholar] [CrossRef] [PubMed]
- Jendrny, P.; Schulz, C.; Twele, F.; Meller, S.; von Köckritz-Blickwede, M.; Osterhaus, A.D.M.E.; Ebbers, J.; Pilchová, V.; Pink, I.; Welte, T.; et al. Scent dog identification of samples from COVID-19 patients—A pilot study. BMC Infect. Dis. 2020, 20, 536. [Google Scholar] [CrossRef] [PubMed]
- Kantele, A.; Paajanen, J.; Turunen, S.; Pakkanen, S.H.; Patjas, A.; Itkonen, L.; Heiskanen, E.; Lappalainen, M.; Desquilbet, L.; Vapalahti, O.; et al. Scent dogs in detection of COVID-19: Triple-blinded randomised trial and operational real-life screening in airport setting. BMJ Glob. Health 2022, 7, e008024. [Google Scholar] [CrossRef] [PubMed]
- Meller, S.; Caraguel, C.; Twele, F.; Charalambous, M.; Schoneberg, C.; Chaber, A.L.; Desquilbet, L.; Grandjean, D.; Mardones, F.O.; Kreienbrock, L.; et al. Canine Olfactory Detection of SARS-CoV-2-Infected Humans—A Systematic Review. Ann. Epidemiol. 2023, 85, 68–85. [Google Scholar] [CrossRef]
- Willis, C.M.; Church, S.M.; Guest, C.M.; Cook, W.A.; McCarthy, N.; Bransbury, A.J.; Church, M.R.T.; Church, J.C.T. Olfactory detection of human bladder cancer by dogs: Proof of principle study. BMJ 2004, 329, 712. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, M.; Jezierski, T.; Broffman, M.; Hubbard, A.; Turner, K.; Janecki, T. Diagnostic Accuracy of Canine Scent Detection in Early- and Late-Stage Lung and Breast Cancers. Integr. Cancer Ther. 2006, 5, 30–39. [Google Scholar] [CrossRef]
- Junqueira, H.; Quinn, T.A.; Biringer, R.; Hussein, M.; Smeriglio, C.; Barrueto, L.; Finizio, J.; Huang, X.Y. Accuracy of Canine Scent Detection of Non–Small Cell Lung Cancer in Blood Serum. J. Osteopath. Med. 2019, 119, 413–418. [Google Scholar] [CrossRef]
- Taverna, G.; Tidu, L.; Grizzi, F.; Torri, V.; Mandressi, A.; Sardella, P.; La Torre, G.; Cocciolone, G.; Seveso, M.; Giusti, G.; et al. Olfactory system of highly trained dogs detects prostate cancer in urine samples. J. Urol. 2015, 193, 1382–1387. [Google Scholar] [CrossRef]
- Muppidi, S.S.; Katragadda, R.; Lega, J.; Alford, T.; Aidman, C.B.; Moore, C. A review of the efficacy of a low-cost cancer screening test using cancer sniffing canines. J. Breath Res. 2021, 15, 024001. [Google Scholar] [CrossRef]
- Gao, C.; Wang, S.; Wang, M.; Li, J.; Qiao, J.; Huang, J.; Zhang, X.; Xiang, Y.; Xu, Q.; Wang, J.; et al. Sensitivity of sniffer dogs for a diagnosis of Parkinson’s disease: A diagnostic accuracy study. Mov. Disord. 2022, 37, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Guest, C.; Pinder, M.; Doggett, M.; Squires, C.; Affara, M.; Kandeh, B.; Dewhirst, S.; Morant, S.V.; D’Alessandro, U.; Logan, J.G.; et al. Trained dogs identify people with malaria parasites by their odour. Lancet Infect. Dis. 2019, 19, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Maurer, M.; McCulloch, M.; Willey, A.M.; Hirsch, W.; Dewey, D. Detection of Bacteriuria by Canine Olfaction. Open Forum Infect. Dis. 2016, 3, ofw051. [Google Scholar] [CrossRef]
- Taylor, M.T.; McCready, J.; Broukhanski, G.; Kirpalaney, S.; Lutz, H.; Powis, J. Using Dog Scent Detection as a Point-of-Care Tool to Identify Toxigenic Clostridium difficile in Stool. Open Forum Infect. Dis. 2018, 5, ofy179. [Google Scholar] [CrossRef]
- Büntgen, U.; Bagi, I.; Fekete, O.; Molinier, V.; Peter, M.; Splivallo, R.; Vahdatzadeh, M.; Richard, F.; Murat, C.; Tegel, W.; et al. New Insights into the Complex Relationship between Weight and Maturity of Burgundy Truffles (Tuber aestivum). PLoS ONE 2017, 12, e0170375. [Google Scholar] [CrossRef]
- Kauhanen, E.; Harri, M.; Nevalainen, A.; Nevalainen, T. Validity of detection of microbial growth in buildings by trained dogs. Environ. Int. 2002, 28, 153–157. [Google Scholar] [CrossRef]
- Pfiester, M.; Koehler, P.G.; Pereira, R.M. Ability of bed bug-detecting canines to locate live bed bugs and viable bed bug eggs. J. Econ. Entomol. 2008, 101, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- National Association of Canine Scent Work®. About Us, Trial Rule Book. 2022. Available online: https://www.nacsw.net/ (accessed on 24 February 2024).
- Finnish Kennel Club. The Rule Book of Finnish Kennel Club for Official Nose Work Test. 2020. Available online: https://www.kennelliitto.fi/koiraharrastukset/kokeet-ja-kilpailut/nose work/nose work-kokeen-jarjestajalle (accessed on 24 February 2024).
- Paolini, J.; Leandri, C.; Desjobert, J.M.; Barboni, T.; Costa, J. Comparison of liquid-liquid extraction with headspace methods for the characterization of volatile fractions of commercial hydrolats from typically Mediterranean species. J. Chromatogr. A 2008, 1193, 37–49. [Google Scholar] [CrossRef]
- Chemical Datasheet, N-Amyl Acetate. Available online: https://cameochemicals.noaa.gov/chemical/2465 (accessed on 13 March 2024).
- Aviles-Rosa, E.O.; Kane, S.A.; Mizuho, N.; Feuerbacher, E.; Hall, N.J. Olfactory threshold of dogs (Canis lupus familiaris) to cold-killed spotted lantern fly eggs. Appl. Anim. Behav. Sci. 2023, 261, 105880. [Google Scholar] [CrossRef]
- DeChant, M.T.; Bunker, P.C.; Hall, N.J. Stimulus Control of Odorant Concentration: Pilot Study of Generalization and Discrimination of Odor Concentration in Canines. Animals 2021, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Concha, A.R.; Guest, C.M.; Harris, R.; Pike, T.W.; Feugier, A.; Zulch, H.; Mills, D.S. Canine Olfactory Thresholds to Amyl Acetate in a Biomedical Detection Scenario. Front. Vet. Sci. 2018, 5, 345. [Google Scholar] [CrossRef] [PubMed]
- DeChant, M.T.; Hall, N.J. Training with varying odor concentrations: Implications for odor detection thresholds in canines. Anim. Cogn. 2021, 24, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Browne, C.M.; Stafford, K.J.; Fordham, R.A. The detection and identification of tuatara and gecko scents by dogs. J. Vet. Behav. 2015, 10, 496–503. [Google Scholar] [CrossRef]
- Collins, M.A.; Browne, C.M.; Edwards, T.L.; Ling, N.; Tempero, G.W.; Gleeson, D.M.; Crockett, K.; Quaife, J. How low can they go: A comparison between dog (Canis familiaris) and environmental DNA detection of invasive koi carp (Cyprinus rubrofuscus). Appl. Anim. Behav. Sci. 2022, 255, 105729. [Google Scholar] [CrossRef]
- Polgár, Z.; Kinnunen, M.; Újváry, D.; Miklósi, Á.; Gácsi, M. A Test of Canine Olfactory Capacity: Comparing Various Dog Breeds and Wolves in a Natural Detection Task. PLoS ONE 2016, 11, e0154087. [Google Scholar] [CrossRef] [PubMed]
- Wise, P.M.; Bien, N.; Wysocki, C.J. Two rapid odor threshold methods compared to a modified method of constant stimuli. Chem. Percept. 2008, 1, 16–23. [Google Scholar] [CrossRef]
- Wang, H.-W.; Wysocki, C.J.; Gold, G.H. Induction of Olfactory Receptor Sensitivity in Mice. Science 1993, 260, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Youngentob, S.L.; Kent, P.F. Enhancement of odorant-induced mucosal activity patterns in rats trained on an odorant identification task. Brain Res. 1995, 670, 82–88. [Google Scholar] [CrossRef]
- Health, H.B. Source Book of Flavours, 2nd ed.; Aspen Publishers, Inc.: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Williams, M.; James, M.J. Training and maintaining the performance of dogs (Canis familiaris) on an increasing number of odor discriminations in a controlled setting. Appl. Anim. Behav. Sci. 2002, 78, 55–65. [Google Scholar] [CrossRef]
- Reeve, C.; Wilson, C.; Hanna, D.; Gadbois, S. Dog Owners’ Survey reveals Medical Alert Dogs can alert to multiple conditions and multiple people. PLoS ONE 2021, 16, e0249191. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.Y.; Brown, W.Y.; Bizo, L.A.; Andrew, N.R.; Taylor, M.K. Biosecurity Dogs Detect Live Insects after Training with Odor-Proxy Training Aids: Scent Extract and Dead Specimens. Chemical Senses. 2020, 45, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Quignon, P.; Rimbault, M.; Robin, S.; Galibert, F. Genetics of canine olfaction and receptor diversity. Mamm. Genome 2012, 23, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Robin, S.; Tacher, S.; Rimbault, M.; Vaysse, A.; Dréano, S.; André, C.; Hitte, C.; Galibert, F. Genetic diversity of canine olfactory receptors. BMC Genom. 2009, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Ieri, F.; Cecchi, L.; Giannini, E.; Clemente, C.; Romani, A. GC-MS and HS-SPME-GCxGC-TOFMS Determination of the Volatile Composition of Essential Oils and Hydrosols (By-Products) from Four Eucalyptus Species Cultivated in Tuscany. Molecules 2019, 24, 226. [Google Scholar] [CrossRef]
- Campos, J.F.; Berteina-Raboin, S. Eucalyptol, an All-Purpose Product. Catalysts 2022, 12, 48. [Google Scholar] [CrossRef]
- Drugbank Online. Eucalyptol. 2021. Available online: https://go.drugbank.com/drugs/DB03852 (accessed on 24 February 2024).
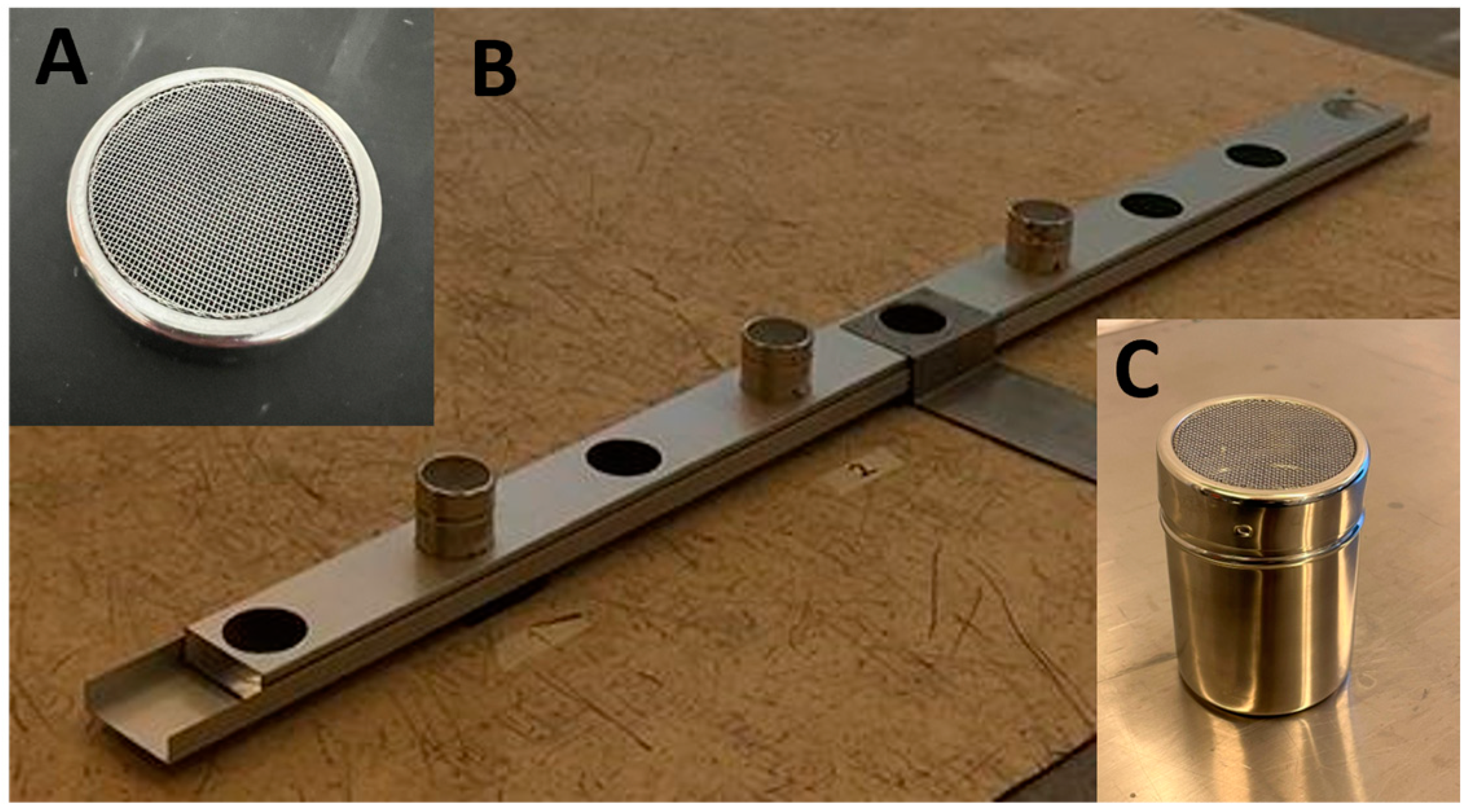

| Solution | Dilution Ration |
|---|---|
| stock solution | 1:104 |
| dilution 1 | 1:106 |
| dilution 2 | 1:108 |
| dilution 3 | 1:1010 |
| dilution 4 | 1:1012 |
| dilution 5 | 1:1014 |
| dilution 6 | 1:1016 |
| dilution 7 | 1:1018 |
| dilution 8 | 1:1020 |
| dilution 9 | 1:1022 |
| dilution 10 | 1:1024 |
| Dog | Breed | Age | Sex | Neutered Status | Trained Target Scents | Level in Nose Work Sports | Last Ratio of E. hydrolat Successfully Indicated by Dog | ||
|---|---|---|---|---|---|---|---|---|---|
| Study 1 | Study 2 | Study 3 | |||||||
| 1 | Spanish Galgo mix | 5 y | M | Y | bedbugs, tracking of rats, molds, floor carpet glue residues, cancer | no experience | 1:1022 | 1:1023 | 1:1021 |
| 2 | Parson Russell terrier | 1 y 9 m | F | N | eucalyptus | hobbyist | 1:104 | ||
| 3 | Parson Russell terrier | 2 y 7 m | M | Y | eucalyptus, bay leaf, lavender, pieces of Kong toy | contestant | 1:106 | ||
| 4 | Beagle | 2 y 2 m | M | Y | eucalyptus, bay leaf, lavender, chanterelle, tracking of blood, human, and dog scents | contestant | 1:106 | ||
| 5 | Cavalier King Charles spaniel | 5 y 2 m | M | N | eucalyptus, bay leaf, lavender | hobbyist | 1:108 | ||
| 6 | Giant schnauzer | 8 y 5 m | F | Y | eucalyptus, bay leaf, lavender | hobbyist | 1:106 | ||
| 7 | French bulldog | 2 y 6 m | M | N | eucalyptus, bay leaf | hobbyist | 1:108 | ||
| 8 | Mittelspitz | 6 y 6 m | F | N | eucalyptus | contestant | 1:1010 | ||
| 9 | Shepherd mix | 4 y 0 m | F | N | eucalyptus, human scent | hobbyist | 1:1010 | ||
| 10 | Parson Russell terrier | 5 y 10 m | F | N | eucalyptus, chanterelle | hobbyist | 1:104 | ||
| 11 | Dogo Argentino | 5 y 0 m | F | N | ID tracking of human, district heating water, eucalyptus | hobbyist | 1:104 | ||
| 12 | Long-haired Dutch shepherd | 4 y 2 m | M | NA | eucalyptus | hobbyist | 1:108 | ||
| 13 | rough collie | 5 y 7 m | M | N | eucalyptus | hobbyist | 1:1019 | ||
| 14 | Danish-Swedish farm dog | 5 y 4 m | F | Y | cancer | no experience | 1:1017 | ||
| 15 | Australian kelpie | 5 y 7 m | F | Y | eucalyptus, bay leaf, lavender | hobbyist | 1:1021 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turunen, S.; Paavilainen, S.; Vepsäläinen, J.; Hielm-Björkman, A. Scent Detection Threshold of Trained Dogs to Eucalyptus Hydrolat. Animals 2024, 14, 1083. https://doi.org/10.3390/ani14071083
Turunen S, Paavilainen S, Vepsäläinen J, Hielm-Björkman A. Scent Detection Threshold of Trained Dogs to Eucalyptus Hydrolat. Animals. 2024; 14(7):1083. https://doi.org/10.3390/ani14071083
Chicago/Turabian StyleTurunen, Soile, Susanna Paavilainen, Jouko Vepsäläinen, and Anna Hielm-Björkman. 2024. "Scent Detection Threshold of Trained Dogs to Eucalyptus Hydrolat" Animals 14, no. 7: 1083. https://doi.org/10.3390/ani14071083
APA StyleTurunen, S., Paavilainen, S., Vepsäläinen, J., & Hielm-Björkman, A. (2024). Scent Detection Threshold of Trained Dogs to Eucalyptus Hydrolat. Animals, 14(7), 1083. https://doi.org/10.3390/ani14071083





