First Results on the Presence of Mycotoxins in the Liver of Pregnant Fallow Deer (Dama dama) Hinds and Fetuses
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
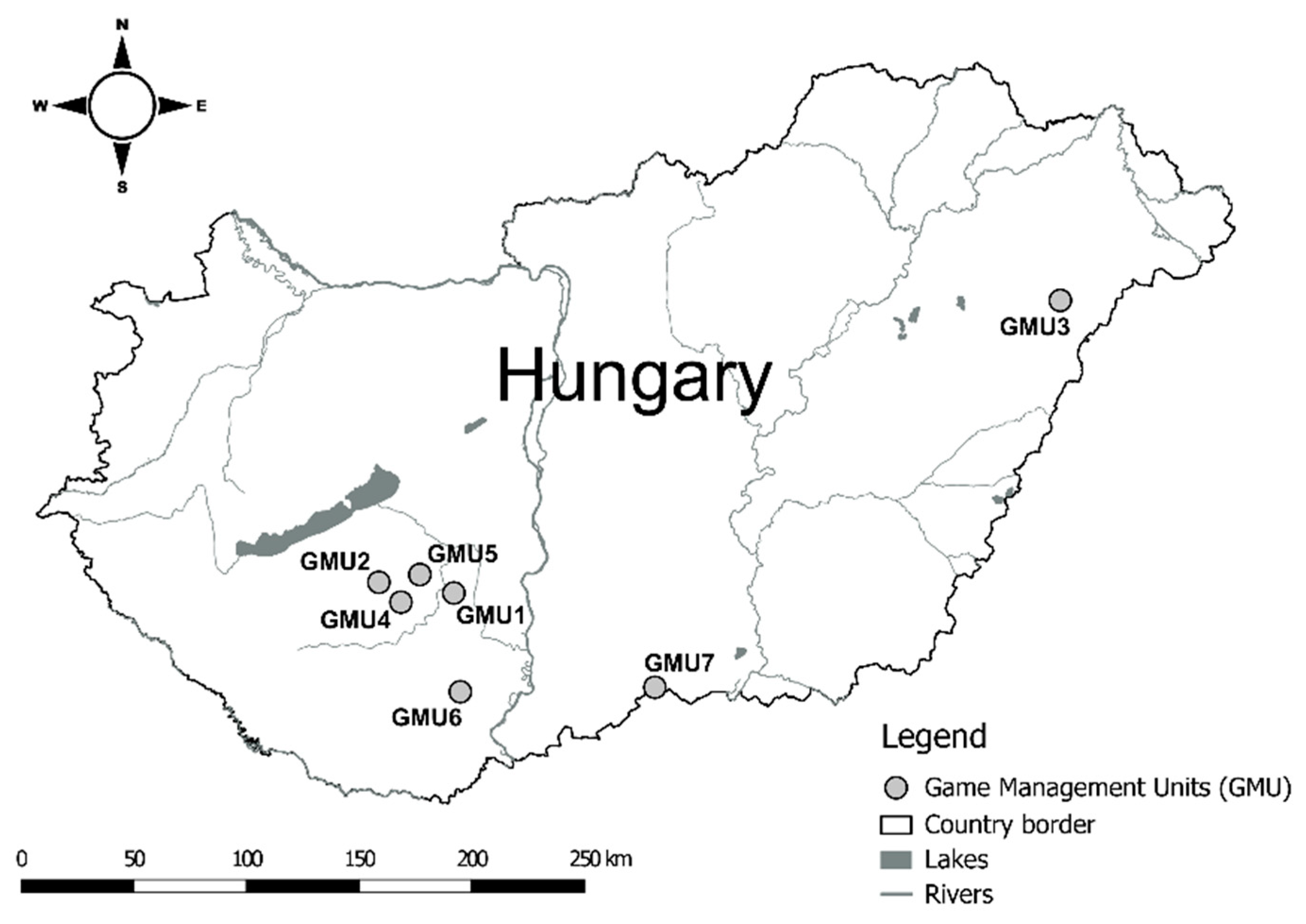
2.1. Study Areas
2.2. Sampling
2.3. Toxin Analyses
2.4. Statistical Analysis
3. Results
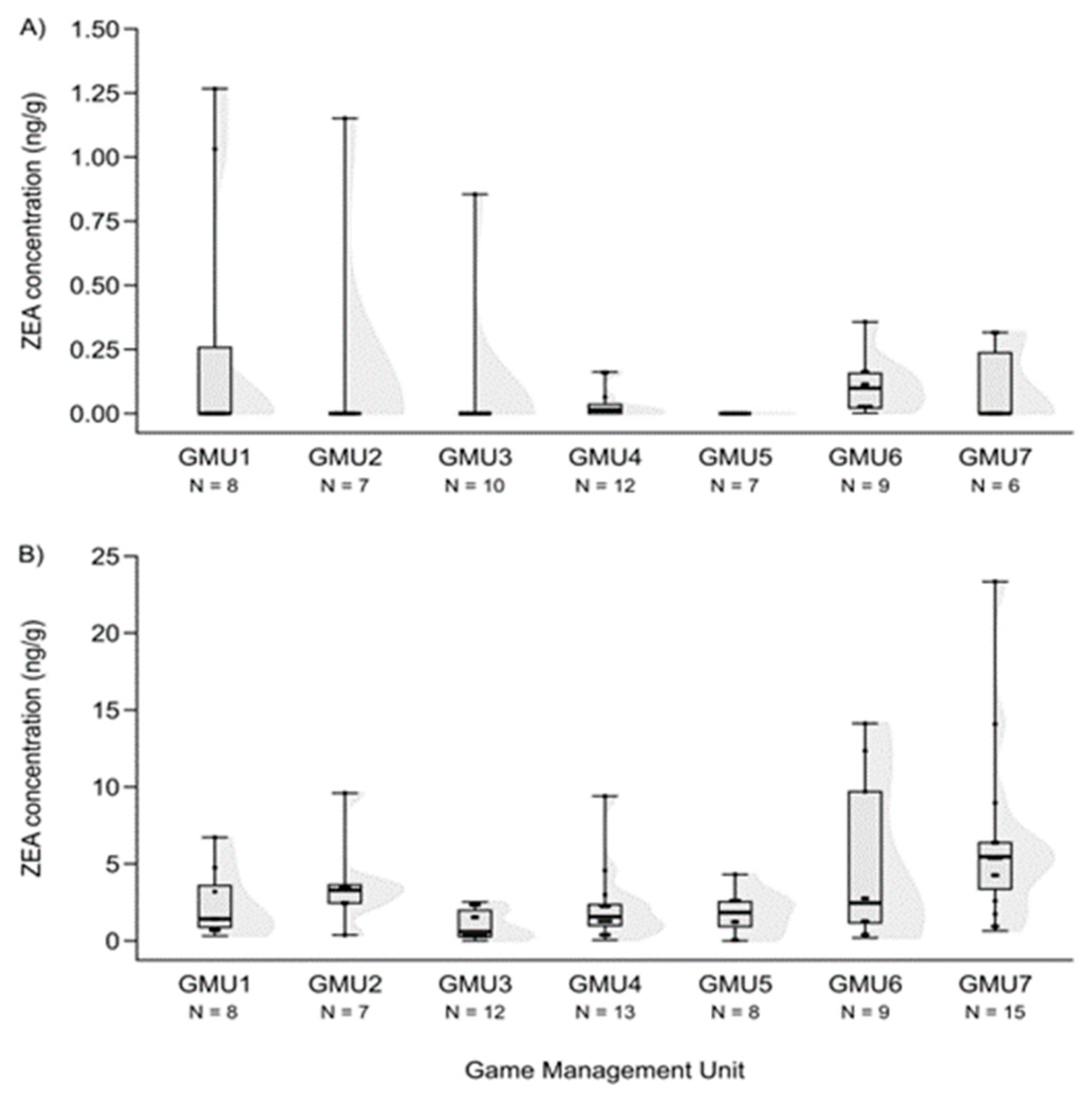
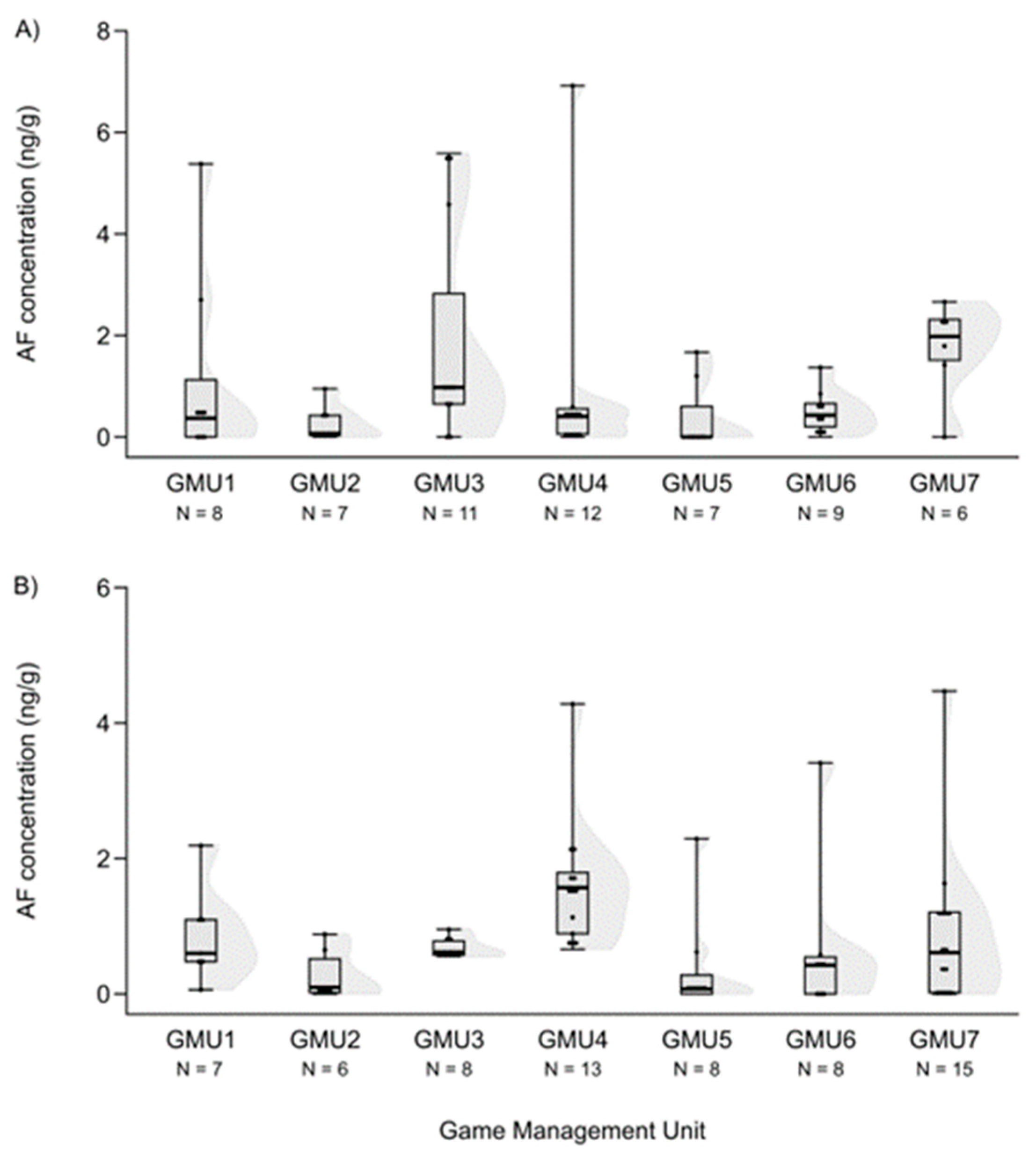
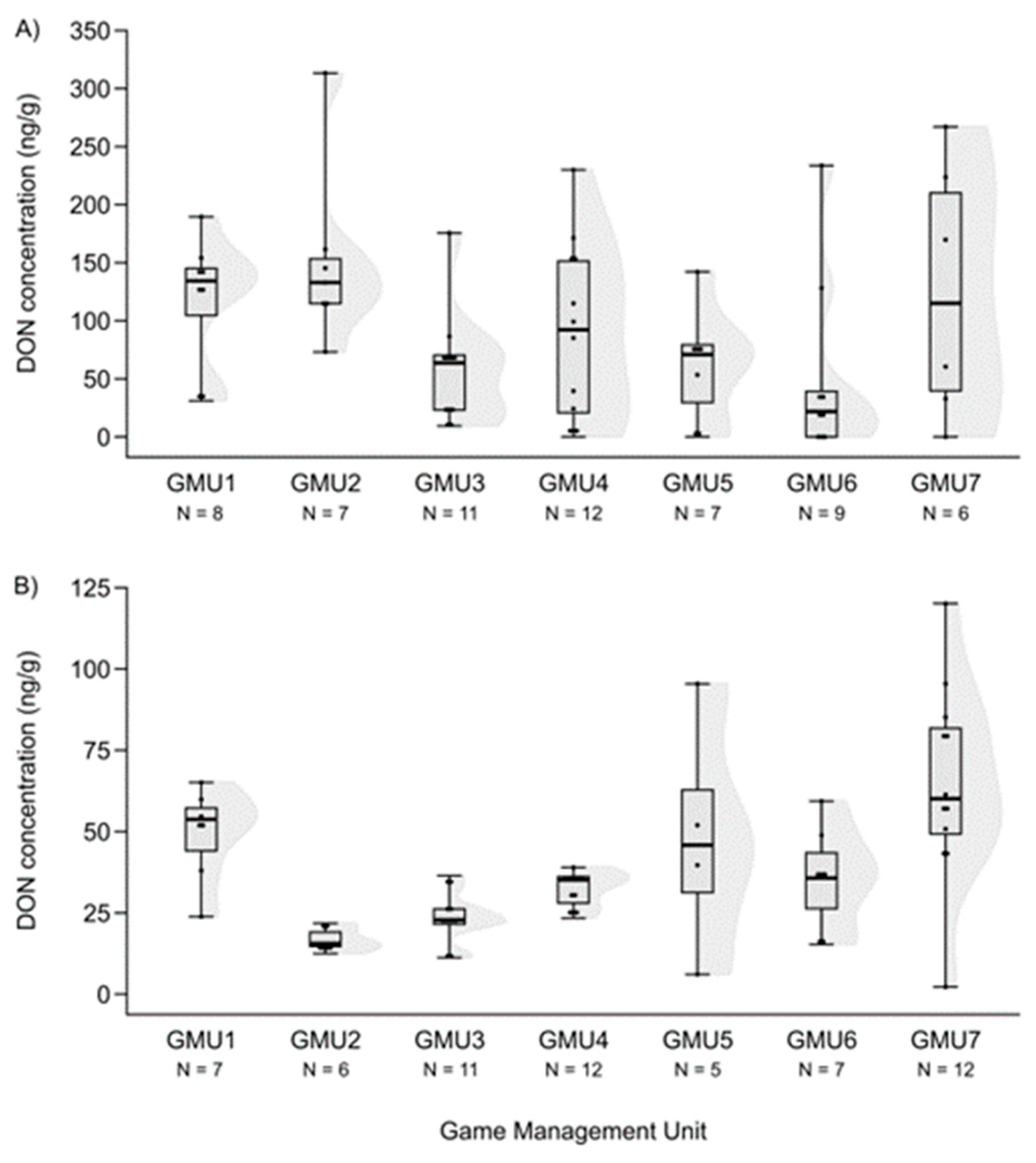
3.1. Mycotoxin Levels in the Liver of Fallow Deer Hinds and Fetuses
3.2. Spatial Patterns of Mycotoxin Concentrations in the Hinds and Fetuses
3.3. Comparison of the Mycotoxin Levels between Fallow Deer Hinds and Fetuses
3.4. Difference in Mycotoxin Levels among the Three Age Groups of Fetuses
3.5. Relationships between the Mycotoxin Level of the Hinds and Their Fetuses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crudo, F.; Varga, E.; Aichinger, G.; Galaverna, G.; Marko, D.; Dall’Asta, C.; Dellafiora, L. Co-Occurrence and Combinatory Effects of Alternaria Mycotoxins and other Xenobiotics of Food Origin: Current Scenario and Future Perspectives. Toxins 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed]
- Czéh, Á.; Mézes, M.; Mandy, F.; Szőke, Z.; Nagyéri, G.; Laufer, N.; Kőszegi, B.; Koczka, T.; Kunsági-Máté, S.; Lustyik, G. Flow cytometry based rapid duplexed immunoassay for fusarium mycotoxins. Cytom. A 2017, 1, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Czéh, Á.; Mandy, F.; Feher-Toth, S.; Torok, L.; Mike, Z.; Koszegi, B.; Lustyik, G. A flow cytometry based competitive fluorescent microsphere immunoassay (CFIA) system for detecting up to six mycotoxins. J. Immunol. Methods 2012, 384, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.T.; Gao, Y.J.; Zhang, W.J.; Bi, T.C.; Wang, X.; Ma, C.X.; Rong, R. Development a multi-immunoaffinity column LC-MS-MS method for comprehensive investigation of mycotoxins contamination and co-occurrence in traditional Chinese medicinal materials. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1178, 122730. [Google Scholar] [CrossRef] [PubMed]
- Szőke, Z.; Babarczi, B.; Mézes, M.; Lakatos, I.; Poór, M.; Fliszár-Nyúl, E.; Oldal, M.; Czéh, Á.; Bodó, K.; Nagyéri, G.; et al. Analysis and Comparison of Rapid Methods for the Determination of Ochratoxin a Levels in Organs and Body Fluids Obtained from Exposed Mice. Toxins 2022, 14, 634. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Rossi, V.; Giorni, P.; Pietri, A.; Gualla, A.; Van der Fels-Klerx, H.J.; Booij, C.J.H.; Moretti, A.; Logrieco, A.; Miglietta, F.; et al. Modelling, predicting and mapping the emergence of aflatoxins in cereals in the EU due to climate change. EFSA Support. Publ. 2012, 9, 223E. [Google Scholar] [CrossRef]
- Valencia-Quintana, R.; Milić, M.; Jakšić, D.; Šegvić Klarić, M.; Tenorio-Arvide, M.G.; Pérez-Flores, G.A.; Bonassi, S.; Sánchez-Alarcón, J. Environment Changes, Aflatoxins, and Health Issues, a Review. Int. J. Environ. Res. Public Health 2020, 17, 7850. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.; Paterson, M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Farkas, J.; Bencze, J.; Szeitzné Szabó, M.; Kovács, M.; Varga, J.; Varga, L. The impact of climate change in the Carpathian Basin on our food security. Hung. Magy. Tudomány 2013, 174, 147–158. [Google Scholar]
- Benkerroum, N. Chronic and Acute Toxicities of Aflatoxins: Mechanisms of Action. Int. J. Environ. Res. Public Health 2020, 17, 423. [Google Scholar] [CrossRef]
- Smith, L.E.; Prendergast, A.J.; Turner, P.C.; Humphrey, J.H.; Stoltzfus, R.J. Aflatoxin Exposure During Pregnancy, Maternal Anemia, and Adverse Birth Outcomes. Am. J. Trop. Med. Hyg. 2017, 96, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Lamplugh, S.M.; Hendrickse, R.G.; Apeagyei, F.; Mwanmut, D.D. Aflatoxins in breast milk, neonatal cord blood, and serum of pregnant women. Br. Med. J. (Clin. Res. Ed.) 1988, 296, 968. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazzaq, Y.M.; Osman, N.; Ibrahim, A. Fetal exposure to aflatoxins in the United Arab Emirates. Ann. Trop. Paediatr. 2002, 22, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.C.; Collinson, A.C.; Cheung, Y.B.; Gong, Y.; Hall, A.J.; Prentice, A.M.; Wild, C.P. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int. J. Epidemiol. 2007, 36, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Groopman, J.D.; Egner, P.A.; Schulze, K.J.; Wu, L.S.; Merrill, R.; Mehra, S.; Shamim, A.A.; Ali, H.; Shaikh, S.; Gernand, A.; et al. Aflatoxin exposure during the first 1000 days of life in rural South Asia assessed by aflatoxin B₁-lysine albumin biomarkers. Food Chem. Toxicol. 2014, 74, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Bondy, G.S.; Pestka, J.J. Immunomodulation by fungal toxins. J. Toxicol. Environ. Health B Crit. Rev. 2000, 3, 109–143. [Google Scholar] [CrossRef] [PubMed]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C.; Taranu, I. Zearalenone and the Immune Response. Toxins 2021, 13, 248. [Google Scholar] [CrossRef]
- Kovalsky Paris, M.P.; Schweiger, W.; Hametner, C.; Stückler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16-O-glucoside: A new masked mycotoxin. J. Agric. Food Chem. 2014, 62, 1181–1189. [Google Scholar] [CrossRef]
- Kriszt, R.; Winkler, Z.; Polyák, Á.; Kuti, D.; Molnár, C.; Hrabovszky, E.; Kalló, I.; Szőke, Z.; Ferenczi, S.; Kovács, K.J. Xenoestrogens Ethinyl Estradiol and Zearalenone Cause Precocious Puberty in Female Rats via Central Kisspeptin Signaling. Endocrinology 2015, 156, 3996–4007. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, X.; Ni, C.; Dai, Y.; Guo, Y. Zearalenone regulates endometrial stromal cell apoptosis and migration via the promotion of mitochondrial fission by activation of the JNK/Drp1 pathway. Mol. Med. Rep. 2018, 17, 7797–7806. [Google Scholar] [CrossRef]
- Afriyie-Gyawu, E.; Wiles, M.C.; Huebner, H.J.; Richardson, M.B.; Fickey, C.; Phillips, T.D. Prevention of Zearalenone-Induced Hyperestrogenism in Prepubertal Mice. J. Toxicol. Environ. Health Part A 2005, 68, 353–368. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Goyarts, T.; Dänicke, S.; Brüssow, K.P.; Valenta, H.; Ueberschär, K.H.; Tiemann, U. On the transfer of the Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) from sows to their fetuses during days 35–70 of gestation. Toxicol. Lett. 2007, 171, 38–49. [Google Scholar] [CrossRef]
- Tiemann, U.; Brüssow, K.P.; Danicke, S.; Vanselow, J. Feeding of pregnant sows with mycotoxin-contaminated diets and their non-effect on foetal and maternal hepatic transcription of genes of the insulin-like growth factor system. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.F.; Collins, R.L.; Sprando, T.N.; Black, N.; Olejnik, R.M.; Eppley, F.A.; Hines, D.I.; Rorie, J.; Ruggles, D.I. Ruggles Effects of deoxynivalenol (DON, vomitoxin) on in utero development in rats. Food Chem. Toxicol. 2006, 44, 747–757. [Google Scholar] [CrossRef]
- Yu, M.; Chen, L.; Peng, Z.; Nüssler, A.K.; Wu, Q.; Liu, L.; Yang, W. Mechanism of deoxynivalenol effects on the reproductive system and fetus malformation: Current status and future challenges. Toxicol. Vitr. 2017, 41, 150–158. [Google Scholar] [CrossRef]
- Yang, X.; Liu, P.; Cui, Y.; Xiao, B.; Liu, M.; Song, M.; Huang, W.; Li, Y. Review of the Reproductive Toxicity of T-2 Toxin. J. Agric. Food Chem. 2020, 68, 727–734. [Google Scholar] [CrossRef]
- Gelineau-van Waes, J.; Starr, L.; Maddox, J.; Aleman, F.; Voss, K.A.; Wilberding, J.; Riley, R.T. Maternal fumonisin exposure and risk for neural tube defects: Mechanisms in an in vivo mouse model. Birth Defects Res. A Clin. Mol. Teratol. 2005, 73, 487–497. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on Mycotoxin Issues in Ruminants: Occurrence in Forages, Effects of Mycotoxin Ingestion on Health Status and Animal Performance and Practical Strategies to Counteract Their Negative Effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef] [PubMed]
- Guitart, R.; Croubels, S.; Caloni, F.; Sachana, M.; Davanzo, F.; Vandenbroucke, V.; Berny, P. Animal poisoning in Europe. Part 1: Farm livestock and poultry. Vet. J. 2009, 183, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Giannoccaro, A.; Fornelli, F.; Dell’Aquila, M.E.; Minoia, P.; Visconti, A. Influence of mycotoxin zearalenone and its derivatives (alpha and beta zearalenol) on apoptosis and proliferation of cultured granulosa cells from equine ovaries. Reprod. Biol. Endocrinol. 2006, 4, 62. [Google Scholar] [CrossRef]
- Mostrom, M.S.; Jacobsen, B.J. Ruminant Mycotoxicosis: An Update. Vet. Clin. North Am. Food Anim. Pract. 2020, 36, 745–774. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Tabares, F.; Khiaosa-ard, R.; Schmidt, M.; Bartl, E.-M.; Kehrer, J.; Nagl, V.; Faas, J.; Sulyok, M.; Krska, R.; Zebeli, Q. Cocktails of Mycotoxins, Phytoestrogens, and Other Secondary Metabolites in Diets of Dairy Cows in Austria: Inferences from Diet Composition and Geo-Climatic Factors. Toxins 2022, 14, 493. [Google Scholar] [CrossRef] [PubMed]
- Nichea, M.J.; Cendoya, E.; Zachetti, V.G.L.; Chiacchiera, S.M.; Sulyok, M.; Krska, R.; Torres, A.M.; Chulze, S.N.; Ramirez, M.L. Mycotoxin profile of Fusarium armeniacum isolated from natural grasses intended for cattle feed. World Mycotoxin J. 2015, 8, 451–457. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Sulyok, M.; Nagl, V.; Faas, J.; Krska, R.; Khiaosa-Ard, R.; Zebeli, Q. Mixtures of mycotoxins, phytoestrogens and pesticides co-occurring in wet spent brewery grains (BSG) intended for dairy cattle feeding in Austria. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2022, 39, 1855–1877. [Google Scholar] [CrossRef] [PubMed]
- Csányi, S.; Márton, M.; Bőti, S.; Schally, G. Vadgazdálkodási Adattár—2021/2022; Országos Vadgazdálkodási Adattár: Gödöllő, Hungary, 2022; p. 70. ISSN 1417-4308. [Google Scholar]
- Sándor, G.; László, R.; Náhlik, A. Determination of time of conception of fallow deer in a Hungarian free range habitat. Folia Zool. 2014, 63, 122–126. [Google Scholar] [CrossRef][Green Version]
- Ács, K.; Lanszky, J. Pre-, postnatal growth and maternal condition in a free ranging fallow deer population. Folia Zool. 2017, 66, 72–78. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis; Prentice-Hall: Hoboken, NJ, USA, 2010; p. 944. [Google Scholar]
- Wood, S.N. Fast stable direct fitting and smoothness selection for generalized additive models. J. Roy. Stat. Soc. Ser. B-Stat. Met. 2008, 70, 495–518. [Google Scholar] [CrossRef]
- Wood, S.N.; Augustin, N.H. GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecol. Model. 2002, 157, 157–177. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biomety: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W. H. Freeman and Company: New York, NY, USA, 1995. [Google Scholar]
- Toutounchi, N.S.; Braber, S.; Land, B.V.; Thijssen, S.; Garssen, J.; Folkerts, G.; Hogenkamp, A. Deoxynivalenol exposure during pregnancy has adverse effects on placental structure and immunity in mice model. Reprod. Toxicol. 2022, 112, 109–118. [Google Scholar] [CrossRef]
- Huston, J.E.; Rector, B.S.; Ellis, W.C.; Allen, M.L. Dynamics of digestion in cattle, sheep, goats and deer. J. Anim. Sci. 1986, 62, 208–215. [Google Scholar] [CrossRef]
- Collins, T.F.; Sprando, R.L.; Black, T.N.; Shackelford, M.E.; Laborde, J.B.; Hansen, D.K.; Eppley, R.M.; Trucksess, M.W.; Howard, P.C.; Bryant, M.A.; et al. Effects of fumonisin B1 in pregnant rats. Part 2. Food Chem. Toxicol. 1998, 36, 673–685. [Google Scholar] [CrossRef]
- Obremski, K.; Zalewski, K.; Gajęcka, M.; Giżejewski, Z.; Zielonka, Ł.; Gajęcki, M.; Nitkiewicz, B. Zearalenone intoxication of game animals. Pol. J. Nat. Sci. 2006, 21, 1099–1106. [Google Scholar]
- Thompson, C.; Henke, S.E. Effect of climate and type of storage container on aflatoxin production in corn and its associated risks to wildlife species. J. Wild. Dis. 2000, 36, 172–179. [Google Scholar] [CrossRef]
- Mesterházy Szieberth, D.; Szabó, B.; Berényi, A.; Tóth, B. Mycotoxin contamination of maize (Zea mays L.) samples in Hungary, 2012–2017. Cereal Res. Commun. 2022, 50, 1065–1073. [Google Scholar] [CrossRef]
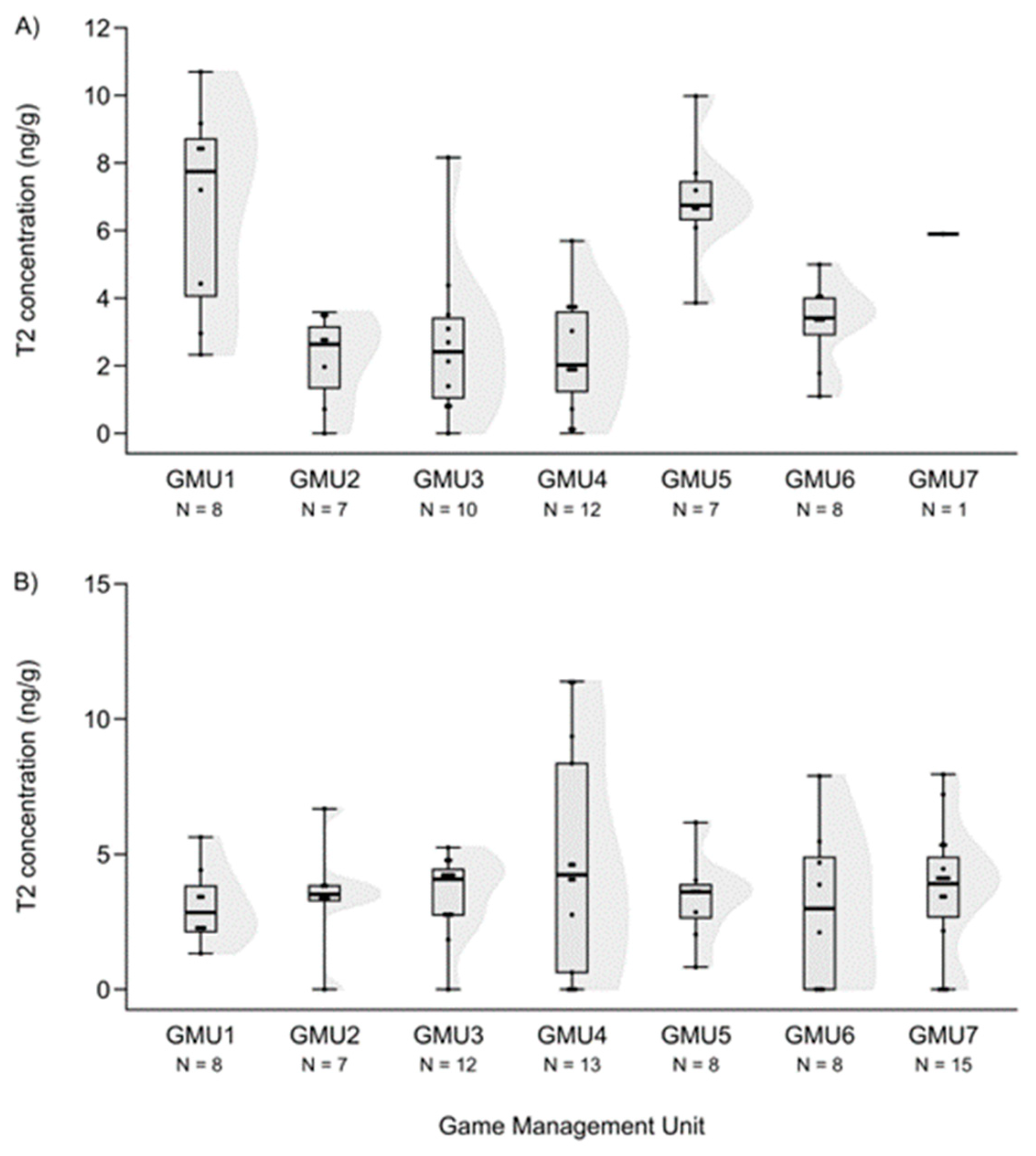
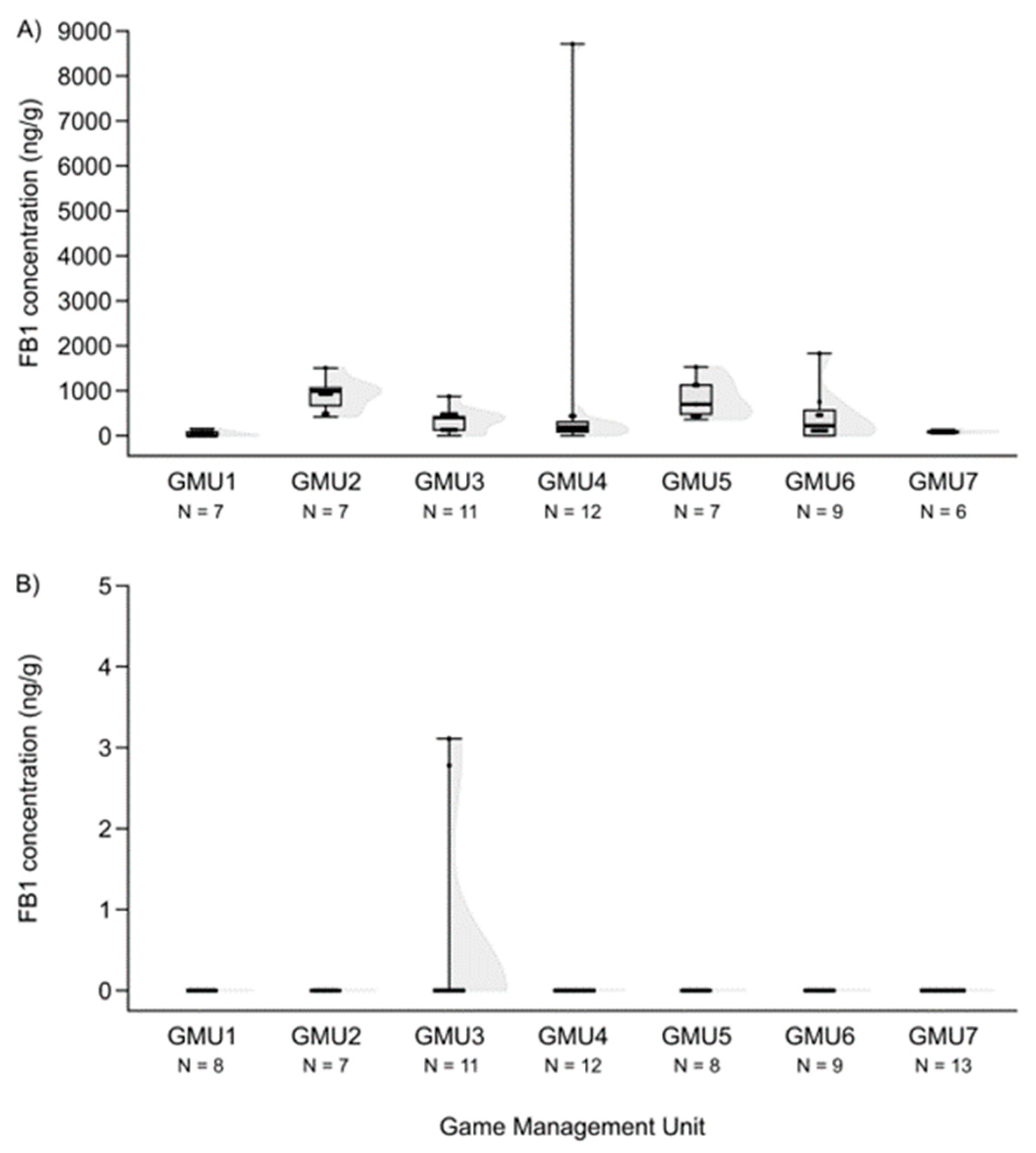

| Mycotoxin | Valid N | Median | Minimum | Maximum | Number of “0” Concentration |
|---|---|---|---|---|---|
| AF | 60 | 0.475 | 0 | 6.918 | 18 |
| ZEA | 59 | 0.000 | 0 | 1.267 | 35 |
| DON | 60 | 72.740 | 0 | 313.240 | 6 |
| T2-toxin | 53 | 3.426 | 0 | 16.850 | 2 |
| FB1 | 59 | 256.987 | 0 | 8711.522 | 10 |
| Mycotoxin | Valid N | Median | Minimum | Maximum | Number of “0” Concentration |
|---|---|---|---|---|---|
| AF | 65 | 0.610 | 0 | 4.470 | 12 |
| ZEA | 72 | 2.217 | 0 | 23.343 | 3 |
| DON | 60 | 35.685 | 0 | 120.120 | 1 |
| T2-toxin | 71 | 3.810 | 0 | 11.392 | 11 |
| FB1 | 68 | 0.000 | 0 | 3.110 | 66 |
| Mycotoxin | ZEA | AF | DON | T2-Toxin | ||||
|---|---|---|---|---|---|---|---|---|
| Sampling Area | U | Z | U | Z | U | Z | U | Z |
| GMU1 | 6 | 2.678 ** | 20 | n.s. | 11 | n.s. | 10 | −2.258 * |
| GMU2 | - | - | 20 | n.s. | 0 | −2.929 ** | 11.5 | n.s. |
| GMU3 | 16 | 2.868 ** | 24 | n.s. | 37 | n.s. | 38.5 | n.s. |
| GMU4 | 3 | 4.052 *** | 13 | 3.508 *** | 47 | n.s. | 63.5 | n.s. |
| GMU5 | 4 | 2.720 ** | 24 | n.s. | 12.5 | n.s. | 3 | −2.835 ** |
| GMU6 | 1 | 3.444 *** | 30.5 | n.s. | 24 | n.s. | 30 | n.s. |
| GMU7 | 0 | 3.464 *** | 19.5 | n.s. | 29 | n.s. | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakatos, I.; Babarczi, B.; Molnár, Z.; Tóth, A.; Skoda, G.; Horváth, G.F.; Horváth, A.; Tóth, D.; Sükösd, F.; Szemethy, L.; et al. First Results on the Presence of Mycotoxins in the Liver of Pregnant Fallow Deer (Dama dama) Hinds and Fetuses. Animals 2024, 14, 1039. https://doi.org/10.3390/ani14071039
Lakatos I, Babarczi B, Molnár Z, Tóth A, Skoda G, Horváth GF, Horváth A, Tóth D, Sükösd F, Szemethy L, et al. First Results on the Presence of Mycotoxins in the Liver of Pregnant Fallow Deer (Dama dama) Hinds and Fetuses. Animals. 2024; 14(7):1039. https://doi.org/10.3390/ani14071039
Chicago/Turabian StyleLakatos, István, Bianka Babarczi, Zsófia Molnár, Arnold Tóth, Gabriella Skoda, Győző F. Horváth, Adrienn Horváth, Dániel Tóth, Farkas Sükösd, László Szemethy, and et al. 2024. "First Results on the Presence of Mycotoxins in the Liver of Pregnant Fallow Deer (Dama dama) Hinds and Fetuses" Animals 14, no. 7: 1039. https://doi.org/10.3390/ani14071039
APA StyleLakatos, I., Babarczi, B., Molnár, Z., Tóth, A., Skoda, G., Horváth, G. F., Horváth, A., Tóth, D., Sükösd, F., Szemethy, L., & Szőke, Z. (2024). First Results on the Presence of Mycotoxins in the Liver of Pregnant Fallow Deer (Dama dama) Hinds and Fetuses. Animals, 14(7), 1039. https://doi.org/10.3390/ani14071039






