Early Transport Patterns and Influencing Factors of Different Stocks of Uroteuthis edulis in the East China Sea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Measurements
2.2. Environmental Data
2.3. Measurements of Statolith Trace Elements
2.4. Tracer Experiments
2.5. Statistical Analysis
3. Results
3.1. Tracer Trajectories
3.2. Environmental Variable Importance
3.3. Relationship between the Element Ratio and Environmental Variables
4. Discussion
4.1. Early Transport Trajectory
4.2. Relationship between Trace Element and Environmental Variables
4.3. Early Potential Transport Process
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, B.L.; Cao, J.; Truesdell, S.B.; Chen, Y.; Chen, X.J.; Tian, S.Q. Reconstructing cephalopod migration with statolith elemental signatures: A case study using Dosidicus gigas. Fish. Sci. 2016, 82, 425–433. [Google Scholar] [CrossRef]
- Umezawa, Y.; Yamaguchi, A.; Ishizaka, J.; Hasegawa, T.; Yoshimizu, C.; Tayasu, I.; Yoshimura, H.; Morii, Y.; Aoshima, T.; Yamawaki, N. Seasonal shifts in the contributions of the Changjiang River and the Kuroshio Current to nitrate dynamics in the continental shelf of the northern East China Sea based on a nitrate dual isotopic composition approach. Biogeosciences 2014, 11, 1297–1317. [Google Scholar] [CrossRef]
- Vidal, E.A.G.; Shea, E.K. Cephalopod ontogeny and life cycle patterns. Front. Mar. Sci. 2023, 10, 1162735. [Google Scholar] [CrossRef]
- Li, N.; Han, P.W.; Wang, C.; Chen, X.J.; Fang, Z. Migration routes of the swordtip squid Uroteuthis edulis in the East China Sea determined based on the statolith trace element information. Hydrobiologia 2023, 850, 861–880. [Google Scholar] [CrossRef]
- Martins, R.S.; de Camargo, R.; Gasalla, M.A. Effect of retention processes on the recruitment of tropical arrow squid (Doryteuthis pleii): An individual-based modeling case study in southeastern Brazil. Fish. Res. 2020, 224, 105455. [Google Scholar] [CrossRef]
- Higuchi, T.; Ito, S.; Ishimura, T.; Kamimura, Y.; Shirai, K.; Shindo, H.; Nishida, K.; Komatsu, K. Otolith oxygen isotope analysis and temperature history in early life stages of the chub mackerel Scomber japonicus in the Kuroshio-Oyashio transition region. Deep-Sea. Res. Pt. II 2019, 169, 104660. [Google Scholar] [CrossRef]
- Xing, Q.W.; Yu, H.Q.; Ito, S.; Ma, S.Y.; Yu, H.M.; Wang, H.; Tian, Y.J.; Sun, P.; Liu, Y.; Li, J.C.; et al. Using a larval growth index to detect the environment-recruitment relationships and its linkage with basin-scale climate variability: A case study for Japanese anchovy (Engraulis japonicus) in the Yellow Sea. Ecol. Indic. 2021, 122, 107301. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Jan, S.; Chen, C.C. Kuroshio intrusion and its impact on swordtip squid (Uroteuthis edulis) abundance in the southern East China Sea. Front. Mar. Sci. 2022, 9, 900299. [Google Scholar] [CrossRef]
- Garcia-Cordova, E.A.; De Silva-Davila, R.; Velazquez-Abunader, I.; Garcia-Maldonado, J.Q.; Ardisson, P.L. Distribution of squid paralarvae and related oceanographic features in the eastern Campeche Bank, Mexico. J. Plankton. Res. 2023, 45, 278–290. [Google Scholar] [CrossRef]
- Pecl, G.T.; Jackson, G.D. The potential impacts of climate change on inshore squid: Biology, ecology and fisheries. Rev. Fish. Biol. Fisher 2008, 18, 373–385. [Google Scholar] [CrossRef]
- Suca, J.J.; Santora, J.A.; Field, J.C.; Curtis, K.A.; Muhling, B.A.; Cimino, M.A.; Hazen, E.L.; Bograd, S.J. Temperature and upwelling dynamics drive market squid (Doryteuthis opalescens) distribution and abundance in the California Current. ICES. J. Mar. Sci. 2022, 79, 2489–2509. [Google Scholar] [CrossRef]
- Pang, Y.M.; Tian, Y.J.; Fu, C.H.; Wang, B.; Li, J.C.; Ren, Y.P.; Wan, R. Variability of coastal cephalopods in overexploited China Seas under climate change with implications on fisheries management. Fish. Res. 2018, 208, 22–33. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kawakami, Y.; Matsuyama, M. Analysis of the hatching site and migratory behaviour of the swordtip squid (Uroteuthis edulis) caught in the Japan Sea and Tsushima Strait in autumn estimated by statolith analysis. Mar. Biol. Res. 2018, 14, 105–112. [Google Scholar] [CrossRef]
- Vayghan, A.H.; Ray, A.; Mondal, S.; Lee, M.A. Modeling of swordtip squid (Uroteuthis edulis) monthly habitat preference using remote sensing environmental data and climate indices. Front. Mar. Sci. 2024, 11, 1329254. [Google Scholar] [CrossRef]
- Gao, X.D.; Jiang, Y.Z.; Yuan, X.W.; Yang, L.L.; Ling, J.Z.; Li, S.F. Modeling spatio-temporal variations in the habitat utilization of swordtip squid (Uroteuthis edulis) in the East China Sea and Southern Yellow Sea. Animals 2023, 13, 3492. [Google Scholar] [CrossRef]
- Qi, J.F.; Yin, B.S.; Zhang, Q.L.; Yang, D.Z.; Xu, Z.H. Seasonal variation of the Taiwan Warm Current Water and its underlying mechanism. Chin. J. Oceanol. Limnol. 2017, 35, 1045–1060. [Google Scholar] [CrossRef]
- Liao, C.H.; Lan, K.W.; Ho, H.Y.; Wang, K.Y.; Wu, Y.L. Variation in the catch rate and distribution of swordtip squid Uroteuthis edulis associated with factors of the oceanic environment in the Southern East China Sea. Mar. Coast. Fish. 2018, 10, 452–464. [Google Scholar] [CrossRef]
- Wang, K.Y.; Lee, K.T.; Liao, C.H. Age, growth and maturation of swordtip squid (Photololigo edulis) in the southern East China Sea. J. Mar. Sci. Technol. 2010, 18, 99–105. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takayama, K.; Hirose, N. Influence of migratory route on early maturation of swordtip squid, Uroteuthis edulis, caught off western Kyushu Island, Japan. Fish. Res. 2022, 249, 106233. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chang, K.Y.; Liao, C.H.; Lee, M.A.; Lee, K.T. Growth strategies of the swordtip squid, Uroteuthis edulis, in response to environmental changes in the southern East China Sea-A cohort analysis. Bull. Mar. Sci. 2013, 89, 677–698. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chen, R.G.; Liao, C.H.; Lee, K.T.; Wu, C.L.; Lee, M.A.; Chang, K.Y. Seasonal Growth Differences of Uroteuthis edulis in the Southern East China Sea, Based on Statolith Analysis. J. Taiwan. Fish. Res. 2011, 19, 1–13. [Google Scholar]
- Li, N.; Han, P.W.; Fang, Z.; Chen, X.J. Variation of statolith microchemistry among stocks of Uroteuthis edulis in the East China Sea. Fish. Sci. 2023, 89, 747–759. [Google Scholar] [CrossRef]
- Mitsuguchi, T.; Matsumoto, E.; Abe, O.; Uchida, T.; Isdale, P.J. Mg/Ca thermometry in coral skeletons. Science 1996, 274, 961–963. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Bizikov, V.A.; Doubleday, Z.A.; Laptikhovsky, V.V.; Lishchenko, F.V.; Perales-Raya, C.; Hollyman, P.R. Techniques for estimating the age and growth of Molluscs: Cephalopoda. J. Shellfish Res. 2018, 37, 783–792. [Google Scholar] [CrossRef]
- Jones, J.B.; Arkhipkin, A.I.; Marriott, A.L.; Pierce, G.J. Reprint of using statolith elemental signatures to confirm ontogenetic migrations of the squid Doryteuthis gahi around the Falkland Islands (Southwest Atlantic). Chem. Geol. 2019, 526, 165–174. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Campana, S.E.; FitzGerald, J.; Thorrold, S.R. Spatial and temporal variation in elemental signatures of statoliths from the Patagonian longfin squid (Loligo gahi). Can. J. Fish. Aquat. Sci. 2004, 61, 1212–1224. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takayama, K.; Hirose, N.; Matsuyama, M. Relationship between empirical water temperature and spring characteristics of swordtip squid (Uroteuthis edulis) caught in the eastern Tsushima Strait. Mar. Biol. Res. 2020, 16, 93–102. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Aketagawa, T.; Takayama, K.; Hirose, N.; Matsuyama, M. Migratory routes of different sized swordtip squid (Uroteuthis edulis) caught in the Tsushima Strait. Fish. Res. 2019, 209, 24–31. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kawakami, Y.; Matsuyama, M. Migratory routes of the swordtip squid Uroteuthis edulis inferred from statolith analysis. Aquat. Biol. 2015, 24, 53–60. [Google Scholar] [CrossRef]
- Wang, K.Y.; Liao, C.H.; Lee, K.T. Population and maturation dynamics of the swordtip squid (Photololigo edulis) in the southern East China Sea. Fish. Res. 2008, 90, 178–186. [Google Scholar] [CrossRef]
- Kako, S.I.; Nakagawa, T.; Takayama, K.; Hirose, N.; Isobe, A. Impact of Changjiang River discharge on sea surface temperature in the East China Sea. J. Phys. Oceanogr. 2016, 46, 1735–1750. [Google Scholar] [CrossRef]
- Gaylard, S.; Waycott, M.; Lavery, P. Review of coast and marine ecosystems in temperate Australia demonstrates a wealth of ecosystem services. Front. Mar. Sci. 2020, 7, 453. [Google Scholar] [CrossRef]
- Natsukari, Y.; Nakanose, T.; Oda, K. Age and growth of loliginid squid Photololig edulis (Hoyle, 1885). J. Exp. Mar. Biol. Ecol. 1988, 116, 177–190. [Google Scholar] [CrossRef]
- Hu, Z.C.; Gao, S.; Liu, Y.S.; Hu, S.H.; Chen, H.H.; Yuan, H.L. Signal enhancement in laser ablation ICP-MS by addition of nitrogen in the central channel gas. J. Anal. Atom. Spectrom. 2008, 23, 1093–1101. [Google Scholar] [CrossRef]
- Xing, Q.W.; Yu, H.M.; Yu, H.Q.; Sun, P.; Liu, Y.; Ye, Z.J.; Li, J.C.; Tian, Y.J. A comprehensive model -based index for identification of larval retention areas: A case study for Japanese anchovy Engraulis japonicus in the Yellow Sea. Ecol. Indic. 2020, 116, 106479. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Aketagawa, T.; Miyamoto, M.; Hirose, N.; Matsuyama, M. The use of statolith analyses and particle-tracking experiments to reveal the migratory route of the swordtip squid (Uroteuthis edulis) caught on the Pacific side of Japan. Fish. Oceanogr. 2018, 27, 517–524. [Google Scholar] [CrossRef]
- Ellis, N.; Smith, S.J.; Pitcher, C.R. Gradient forests: Calculating importance gradients on physical predictors. Ecology 2012, 93, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.H.; Zhang, D.W. Inference in generalized additive mixed models by using smoothing splines. J. R. Stat. Soc. B 1999, 61, 381–400. [Google Scholar] [CrossRef]
- Wagner, T.; Hayes, D.B.; Bremigan, M.T. Accounting for multilevel data structures in fisheries data using mixed models. Fisheries 2006, 31, 180–187. [Google Scholar] [CrossRef]
- Chang, K.Y.; Hsu, Y.J.; Ching, T.Y.; Liao, C.H.; Chen, C.S. Catch and effort standardization for Taiwanese swordtip squid Uroteuthis edulis fisheries in the southern East China Sea. J. Mar. Sci. Tech.-Taiw. 2022, 30, 37–47. [Google Scholar] [CrossRef]
- Ma, S.Y.; Liu, Y.; Li, J.C.; Fu, C.H.; Ye, Z.J.; Sun, P.; Yu, H.Q.; Cheng, J.H.; Tian, Y.J. Climate-induced long-term variations in ecosystem structure and atmosphere-ocean-ecosystem processes in the Yellow Sea and East China Sea. Prog. Oceanogr. 2019, 175, 183–197. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takayama, K.; Hirose, N.; Matsuyama, M. The Sea of Amakusa playing the role of a distributor of swordtip squid (Uroteuthis edulis) migrating from the East China Sea to the east and west sides of Japan. Fish. Res. 2020, 225, 105475. [Google Scholar] [CrossRef]
- Ito, T.; Takayama, K.; Hirose, N. Prediction of potential fishing grounds of swordtip squid (Uroteuthis edulis) based on a physical-biochemical coupled model. Fish. Oceanogr. 2023, 32, 559–570. [Google Scholar] [CrossRef]
- Chen, J.Y.; Ching, Z.Y.; Chen, C.S. Inter-annual variability in growth and maturation of the swordtip squid Uroteuthis edulis in Yilan Bay off Northeastern Taiwan. J. Mar. Sci. Tech.-Taiw. 2022, 30, 158–171. [Google Scholar] [CrossRef]
- Jacobs, Z.; Kelly, S.; Jebri, F.; Roberts, M.; Srokosz, M.; Sauer, W.; Hancke, L.; Popova, E. Retention properties of the Agulhas bank and their relevance to the chokka squid life cycle. Deep-Sea. Res. Pt. II 2022, 202, 105151. [Google Scholar] [CrossRef]
- Jebri, F.; Raitsos, D.E.; Gittings, J.A.; Jacobs, Z.L.; Srokosz, M.; Gornall, J.; Sauer, W.H.H.; Roberts, M.J.; Popova, E. Unravelling links between squid catch variations and biophysical mechanisms in South African waters. Deep-Sea. Res. Pt. II 2022, 196, 105028. [Google Scholar] [CrossRef]
- Ji, F.; Guo, X.Y.; Wang, Y.C.; Takayama, K. Response of the Japanese flying squid (Todarodes pacificus) in the Japan Sea to future climate warming scenarios. Climatic. Chang. 2020, 159, 601–618. [Google Scholar] [CrossRef]
- Arkhipkin, A.I. Statoliths as ‘black boxes’ (life recorders) in squid. Mar. Freshwater. Res. 2005, 56, 573–583. [Google Scholar] [CrossRef]
- Daryanani, D.S.; Martino, J.C.; Doubleday, Z.A. Statolith chemistry: A new tool to understand the ecology and provenance of octopus. Rev. Fish. Biol. Fisher 2021, 31, 923–934. [Google Scholar] [CrossRef]
- Warner, R.R.; Hamilton, S.L.; Sheehy, M.S.; Zeidberg, L.D.; Brady, B.C.; Caselle, J.E. Geographic variation in natal and early larval trace-elemental signatures in the statoliths of the market squid Doryteuthis (formerly Loligo) opalescens. Mar. Ecol. Prog. Ser. 2009, 379, 109–121. [Google Scholar] [CrossRef]
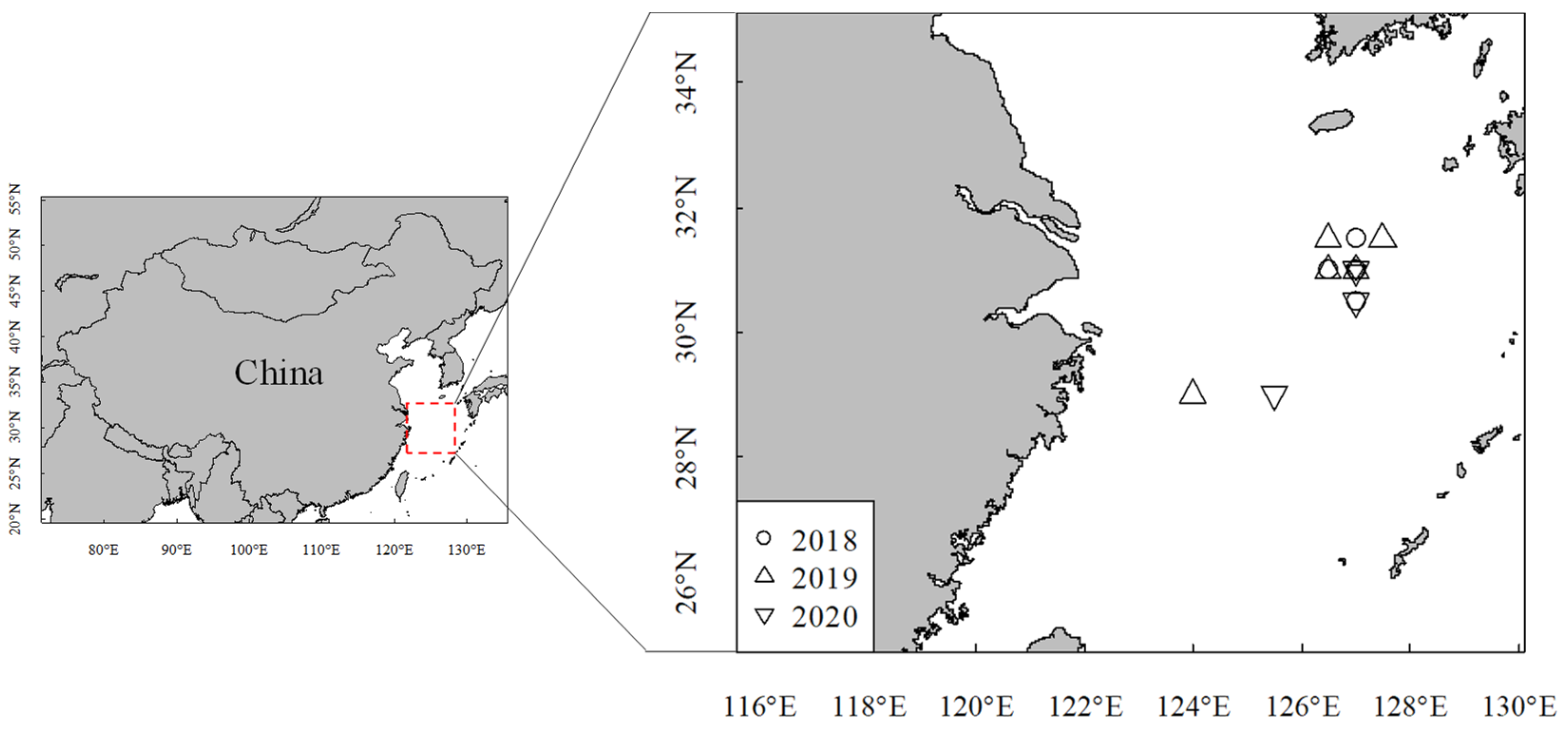

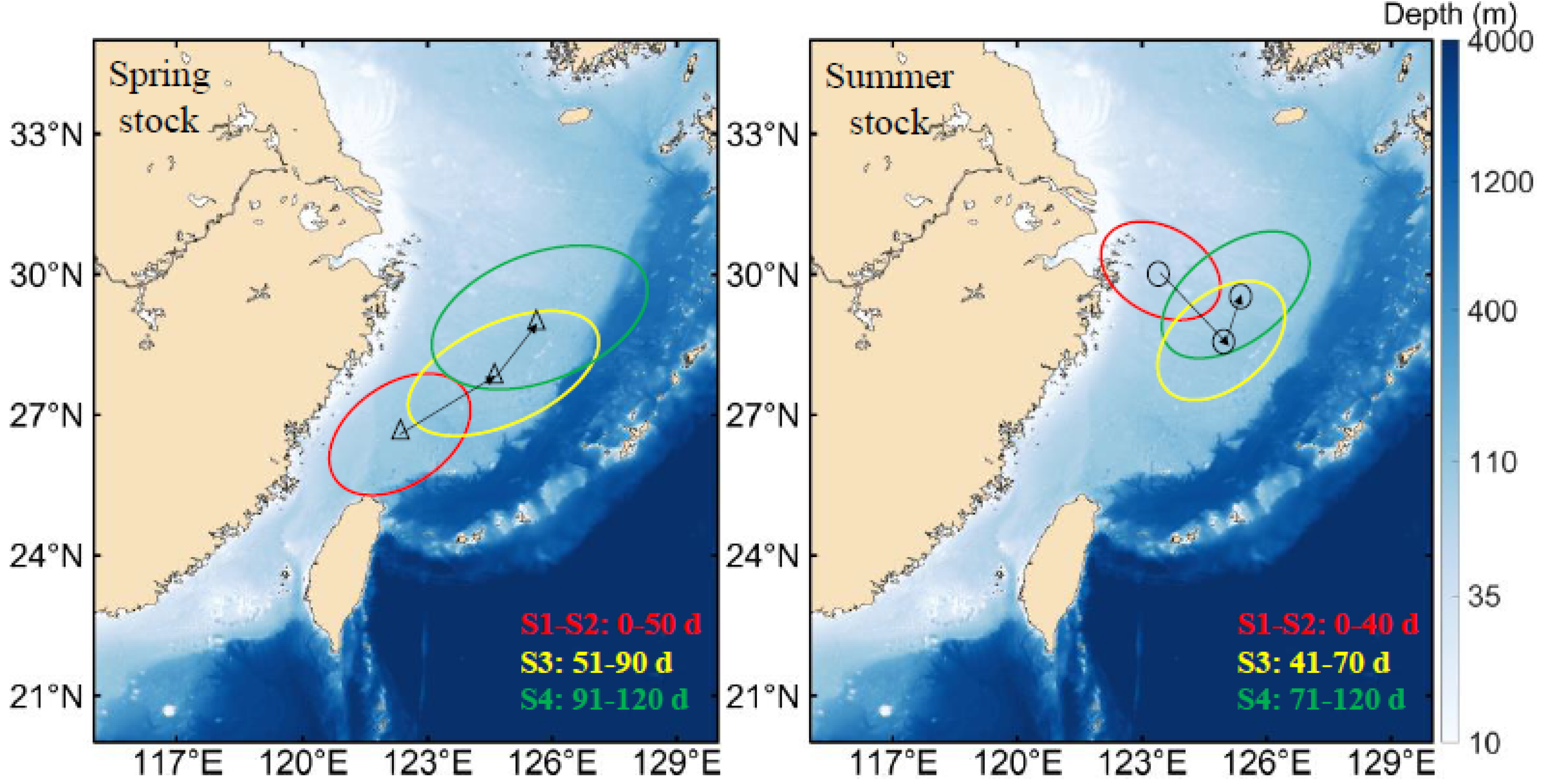
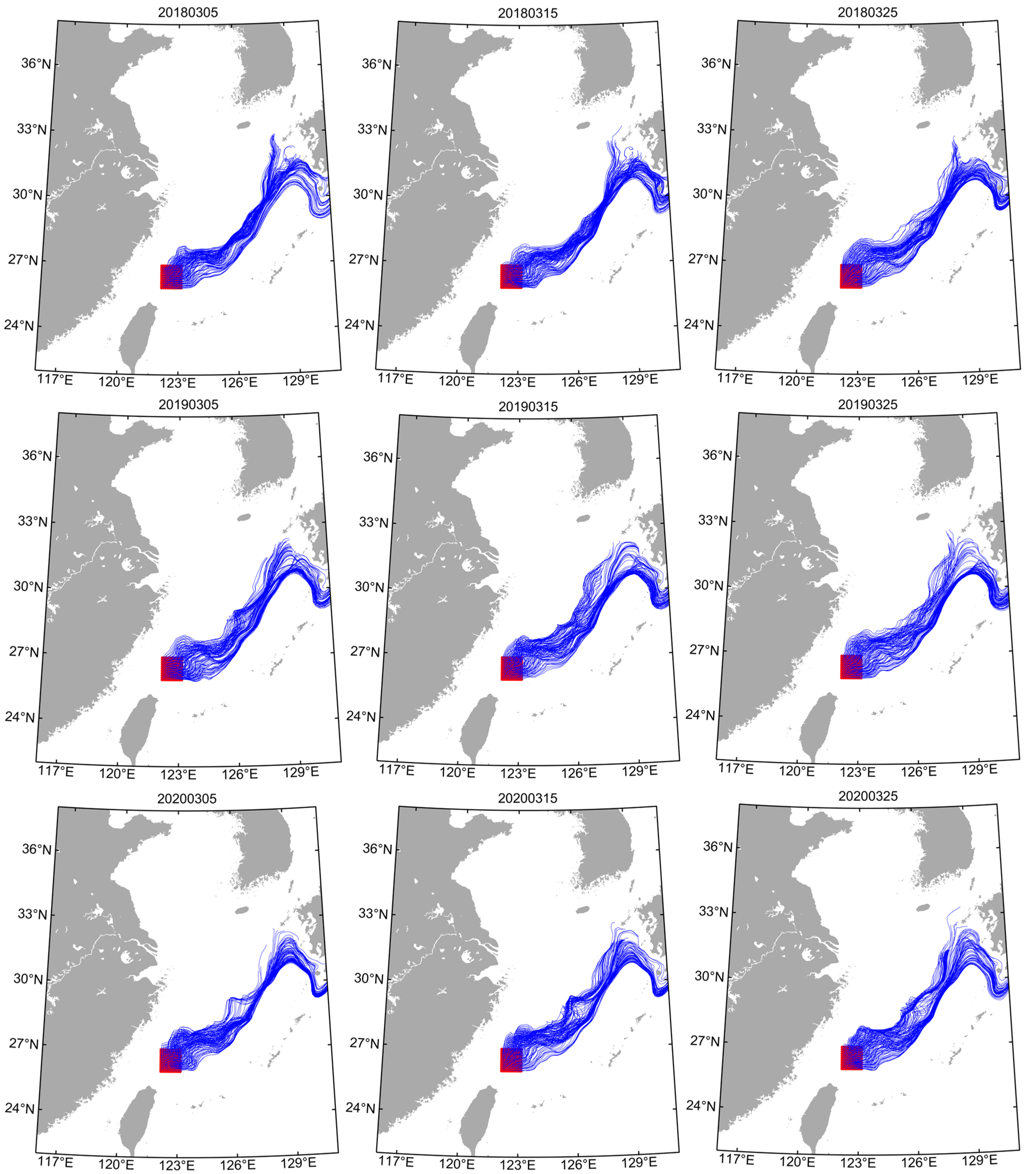


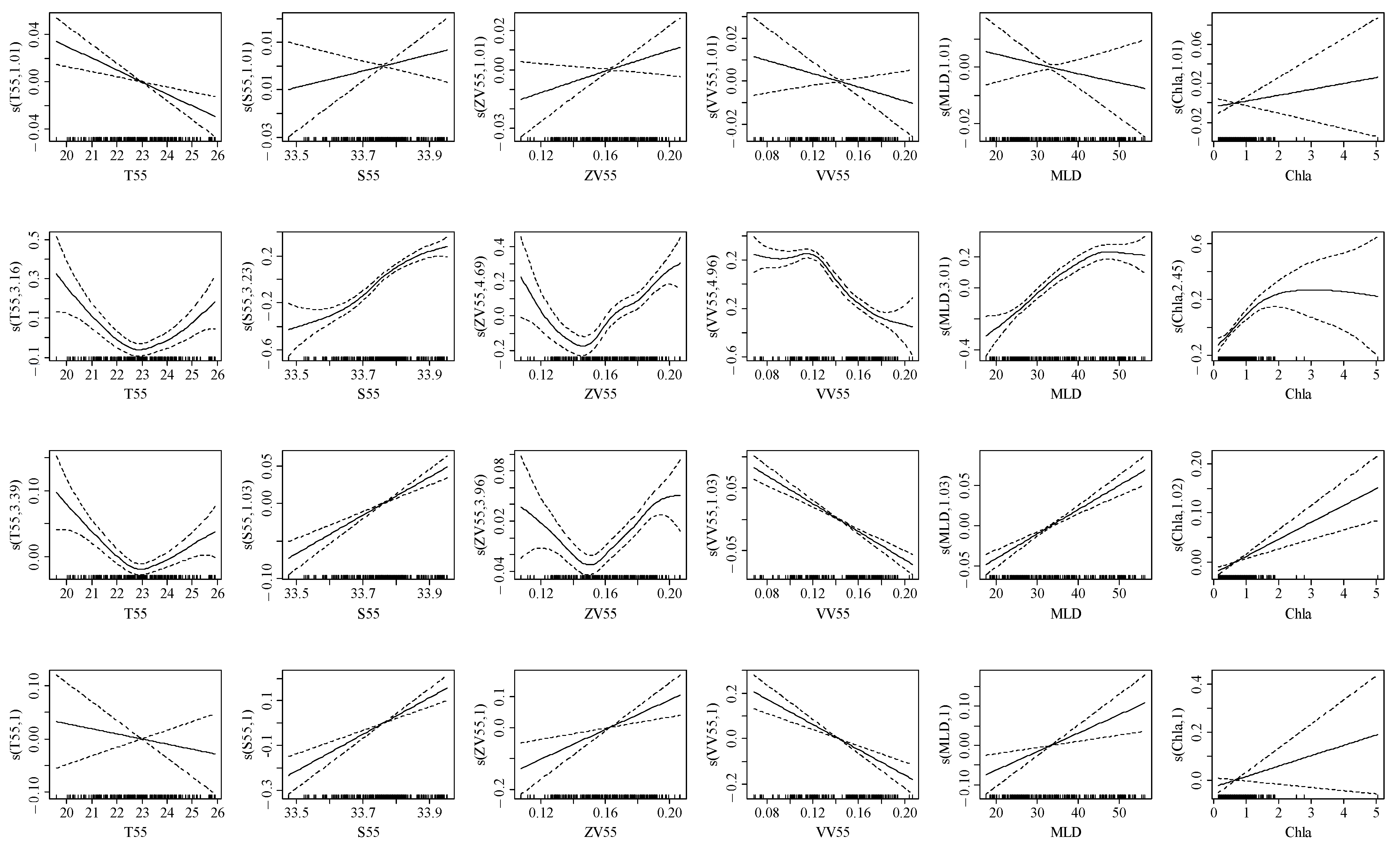
| Stock | Sampling Years | Quantity | Mantle Length/mm | Body Weight/g | Age/d | Maturity |
|---|---|---|---|---|---|---|
| Spring | 2018 | 17 | 116–204 | 68–304 | 164–236 | II–IV |
| 2019 | 13 | 128–284 | 70–356 | 182–301 | II–IV | |
| 2020 | 14 | 123–210 | 81–274 | 174–247 | II–IV | |
| Summer | 2018 | 9 | 60–151 | 52–143 | 150–212 | II–IV |
| 2019 | 8 | 68–149 | 43–137 | 153–213 | II–IV | |
| 2020 | 8 | 73–166 | 51–152 | 143–204 | II–IV |
| Element Ratios | Environment Variables | Random Effects | Fixed Effects | Smooth Terms | Model Statistic | |||
|---|---|---|---|---|---|---|---|---|
| fYear SD | Std.Error | edf | F | p | BIC | Deviance Explained/% | ||
| Na:Ca | T55 | 1.07 × 10−11 | 3.60 × 10−3 | 1.01 | 12.18 | *** | −808.87 | 23.49 |
| S55 | 9.05 × 10−11 | 3.68 × 10−3 | 1.01 | 0.99 | ns | −798.09 | 7.23 | |
| ZV55 | 4.04 × 10−11 | 3.69 × 10−3 | 1.01 | 2.48 | * | −799.54 | 16.49 | |
| VV55 | 4.51 × 10−11 | 3.70 × 10−3 | 1.01 | 1.66 | * | −798.81 | 12.25 | |
| MLD | 1.96 × 10−11 | 3.72 × 10−3 | 1.01 | 0.79 | ns | −797.93 | 6.03 | |
| Chla | 2.11 × 10−11 | 3.61 × 10−3 | 1.01 | 0.74 | ns | −797.83 | 4.01 | |
| Mg:Ca | T55 | 5.27 × 10−5 | 7.09 × 10−2 | 3.16 | 8.83 | *** | 123.76 | 7.53 |
| S55 | 9.46 × 10−2 | 7.70 × 10−2 | 3.23 | 48.54 | *** | 16.61 | 26.90 | |
| ZV55 | 5.12 × 10−5 | 1.14 × 10−1 | 4.69 | 18.55 | *** | 80.02 | 22.10 | |
| VV55 | 3.90 × 10−2 | 1.27 × 10−1 | 4.96 | 66.43 | *** | −75.44 | 53.50 | |
| MLD | 4.68 × 10−2 | 7.32 × 10−2 | 3.01 | 47.59 | *** | 33.41 | 31.10 | |
| Chla | 6.81 × 10−2 | 3.82 × 10−2 | 2.45 | 21.03 | *** | 103.25 | 9.43 | |
| Sr:Ca | T55 | 1.04 × 10−2 | 2.09 × 10−2 | 3.39 | 9.76 | *** | −736.96 | 9.97 |
| S55 | 2.39 × 10−11 | 4.09 × 10−3 | 1.03 | 44.27 | *** | −750.91 | 12.60 | |
| ZV55 | 1.99 × 10−2 | 2.71 × 10−2 | 3.96 | 11.10 | *** | −733.88 | 8.50 | |
| VV55 | 2.66 × 10−11 | 3.93 × 10−3 | 1.03 | 79.48 | *** | −779.68 | 20.50 | |
| MLD | 1.03 × 10−10 | 4.01 × 10−3 | 1.03 | 58.71 | *** | −762.68 | 15.90 | |
| Chla | 1.14 × 10−11 | 3.89 × 10−3 | 1.02 | 21.28 | *** | −729.44 | 6.06 | |
| Ba:Ca | T55 | 3.27 × 10−7 | 1.58 × 10−2 | 1.00 | 0.56 | ns | 109.60 | 7.54 |
| S55 | 6.74 × 10−9 | 1.53 × 10−2 | 1.00 | 31.00 | *** | 80.71 | 19.12 | |
| ZV55 | 3.97 × 10−10 | 1.57 × 10−2 | 1.00 | 10.27 | ** | 100.80 | 12.92 | |
| VV55 | 3.95 × 10−2 | 1.50 × 10−2 | 1.00 | 30.76 | *** | 82.76 | 17.86 | |
| MLD | 3.53 × 10−6 | 1.51 × 10−2 | 1.00 | 9.02 | ** | 102.00 | 9.50 | |
| Chla | 1.09 × 10−7 | 1.47 × 10−2 | 1.00 | 2.33 | ns | 107.99 | 5.42 | |
| Element Ratios | Environment Variables | Random Effects | Fixed Effects | Smooth Terms | Model Statistic | |||
|---|---|---|---|---|---|---|---|---|
| fYear SD | Std.Error | edf | F | p | BIC | Deviance Explained/% | ||
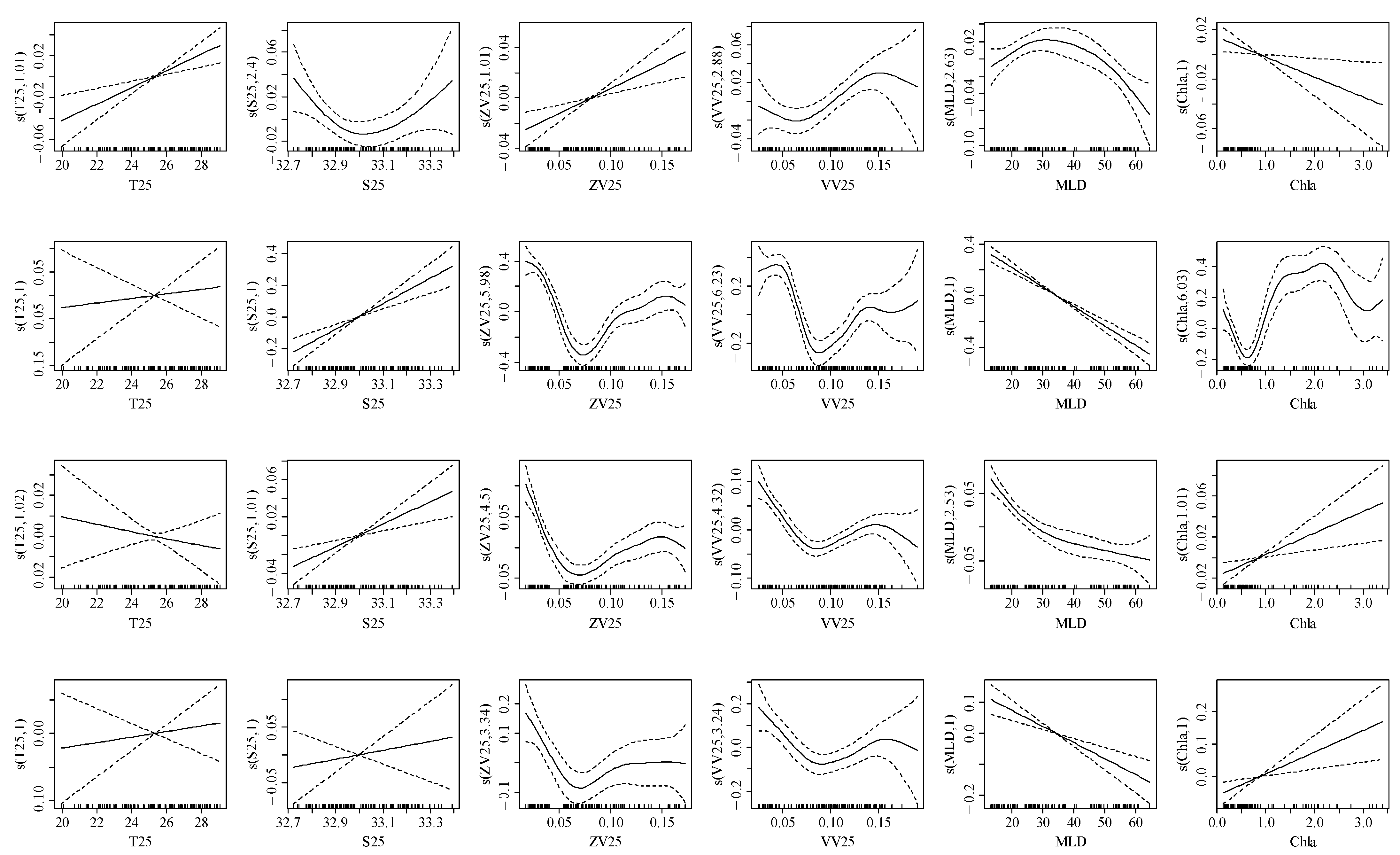
| Na:Ca | T25 | 9.15 × 10−7 | 5.74 × 10−3 | 1.01 | 12.25 | *** | −334.69 | 18.62 |
| S25 | 2.99 × 10−2 | 1.83 × 10−2 | 2.40 | 4.05 | ** | −332.98 | 29.20 | |
| ZV25 | 3.47 × 10−2 | 4.77 × 10−3 | 1.01 | 12.65 | *** | −303.66 | 10.91 | |
| VV25 | 3.63 × 10−2 | 2.37 × 10−2 | 2.89 | 5.38 | ** | −335.88 | 12.06 | |
| MLD | 3.41 × 10−2 | 2.41 × 10−2 | 2.63 | 7.76 | ** | −312.66 | 12.11 | |
| Chla | 3.14 × 10−2 | 5.01 × 10−3 | 1.00 | 5.86 | *** | −342.66 | 28.62 | |
| Mg:Ca | T25 | 2.24 × 10−10 | 2.89 × 10−2 | 1.00 | 0.18 | ns | 84.54 | 10.71 |
| S25 | 2.46 ×10−10 | 2.50 × 10−2 | 1.00 | 26.30 | *** | 63.10 | 17.20 | |
| ZV25 | 1.56 ×10−1 | 2.26 × 10−1 | 5.98 | 34.85 | *** | 1.55 | 56.10 | |
| VV25 | 1.11 ×10−1 | 2.49 × 10−1 | 6.24 | 22.04 | *** | 27.82 | 49.10 | |
| MLD | 3.14 × 10−12 | 2.30 × 10−2 | 1.00 | 109.70 | *** | 21.07 | 50.90 | |
| Chla | 1.48 × 10−5 | 2.74 × 10−1 | 6.03 | 20.99 | *** | 5.33 | 49.20 | |
| Sr:Ca | T25 | 2.23 × 10−11 | 5.94 × 10−3 | 1.02 | 0.58 | ns | −299.11 | 3.38 |
| S25 | 1.99 × 10−11 | 5.59 × 10−3 | 1.01 | 12.18 | *** | −310.00 | 8.52 | |
| ZV25 | 1.03 × 10−6 | 4.22 × 10−2 | 4.50 | 17.80 | *** | −345.06 | 39.70 | |
| VV25 | 7.32 × 10−7 | 3.85 × 10−2 | 4.32 | 16.20 | *** | −340.04 | 36.20 | |
| MLD | 1.54 × 10−16 | 3.38 × 10−2 | 2.53 | 27.79 | *** | −345.63 | 36.10 | |
| Chla | 1.10 × 10−11 | 5.48 × 10−3 | 1.01 | 8.44 | ** | −306.46 | 5.70 | |
| Ba:Ca | T25 | 4.73 × 10−7 | 1.92 × 10−2 | 1.00 | 0.29 | ns | −12.93 | 10.67 |
| S25 | 6.98 × 10−7 | 1.91 × 10−2 | 1.00 | 0.45 | ns | −13.08 | 10.47 | |
| ZV25 | 3.83 × 10−6 | 1.10 × 10−1 | 3.34 | 5.40 | *** | −21.52 | 23.90 | |
| VV25 | 4.70 × 10−6 | 1.01 × 10−1 | 3.24 | 6.62 | *** | −23.31 | 24.90 | |
| MLD | 3.17 × 10−6 | 1.83 × 10−2 | 1.00 | 20.58 | *** | −31.47 | 24.60 | |
| Chla | 2.32 × 10−6 | 1.71 × 10−2 | 1.00 | 8.61 | ** | −20.12 | 15.56 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Xing, Q.; Feng, Z.; Chen, X.; Fang, Z. Early Transport Patterns and Influencing Factors of Different Stocks of Uroteuthis edulis in the East China Sea. Animals 2024, 14, 941. https://doi.org/10.3390/ani14060941
Li N, Xing Q, Feng Z, Chen X, Fang Z. Early Transport Patterns and Influencing Factors of Different Stocks of Uroteuthis edulis in the East China Sea. Animals. 2024; 14(6):941. https://doi.org/10.3390/ani14060941
Chicago/Turabian StyleLi, Nan, Qinwang Xing, Zhiping Feng, Xinjun Chen, and Zhou Fang. 2024. "Early Transport Patterns and Influencing Factors of Different Stocks of Uroteuthis edulis in the East China Sea" Animals 14, no. 6: 941. https://doi.org/10.3390/ani14060941
APA StyleLi, N., Xing, Q., Feng, Z., Chen, X., & Fang, Z. (2024). Early Transport Patterns and Influencing Factors of Different Stocks of Uroteuthis edulis in the East China Sea. Animals, 14(6), 941. https://doi.org/10.3390/ani14060941







