Training the Concept of Innovate in Dolphins (Tursiops truncatus) Is Both Creative and Cognitively Stimulating
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Ways to Enrich Managed Care Animals
1.2. Training
1.3. Creativity
1.3.1. Spontaneous Creativity
1.3.2. Trained Creativity
1.4. Measures
1.5. Purpose
- How much variation in creativity do bottlenose dolphins show while under stimulus control?
- Do dolphins show individual creativity profiles?
- Is there evidence that learning this task is cognitively stimulating?
2. Materials and Methods
2.1. Subjects
2.2. Measures
2.3. Procedure
2.3.1. Training Procedure
2.3.2. Test Trials
2.3.3. Coding
2.4. Statistical Analyses
2.4.1. Validation Measures
2.4.2. Individual Construct Overall Rankings
2.4.3. Overall Creativity Score Rankings
3. Results
3.1. Variation across Constructs and Individual Differences
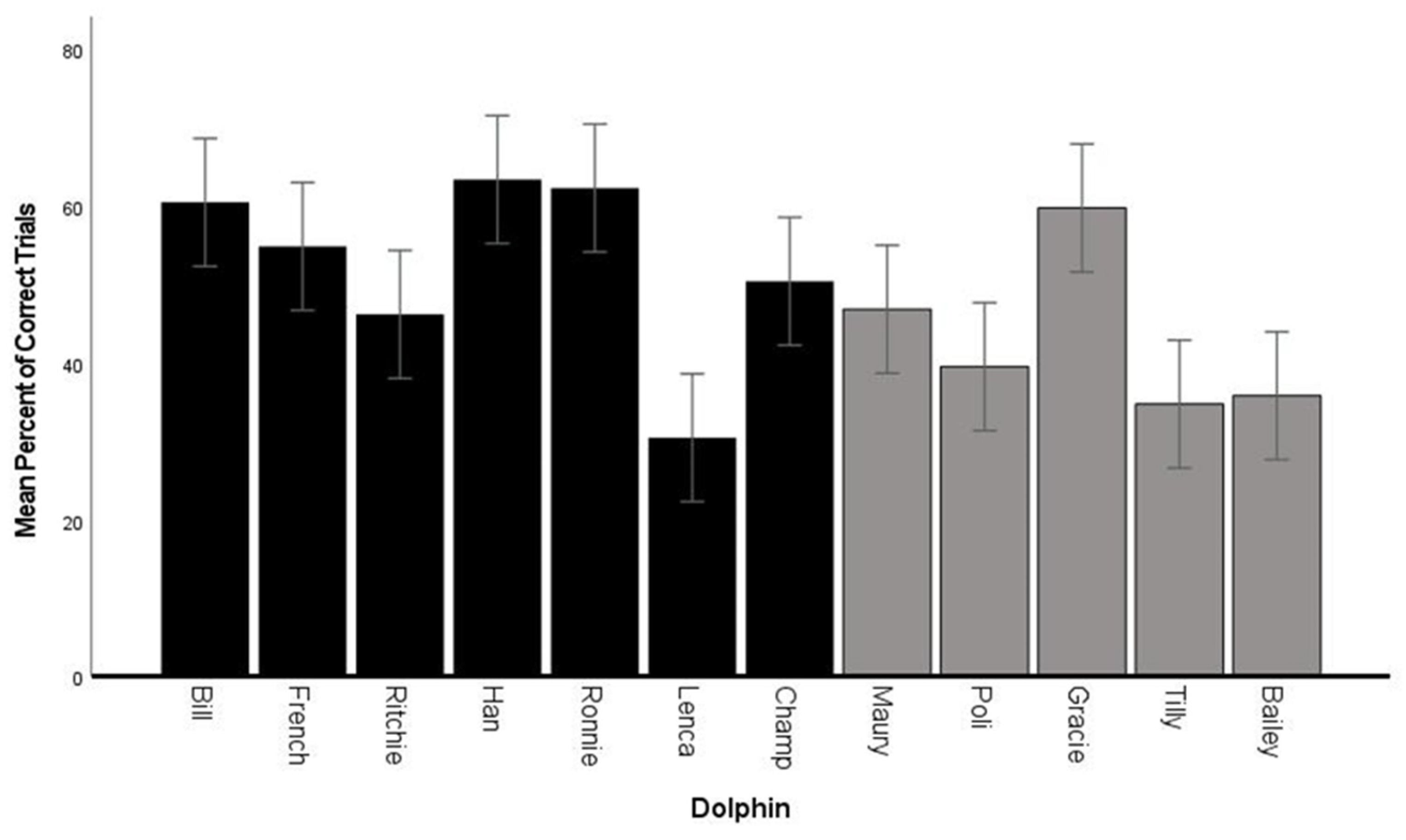
3.2. Fluency—Percent Correct
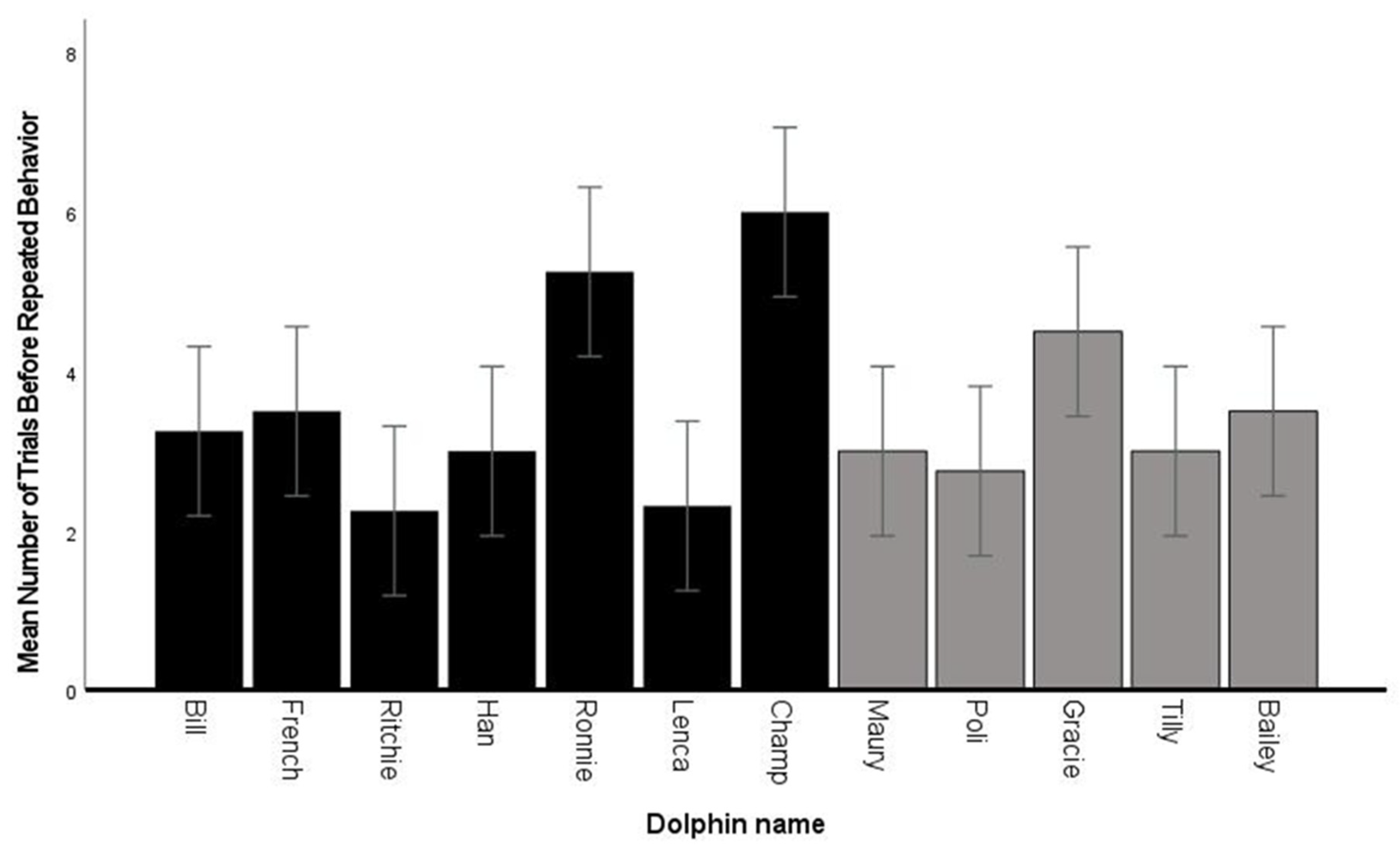
3.3. Fluency—Number of Trials before Repeated Behaviors
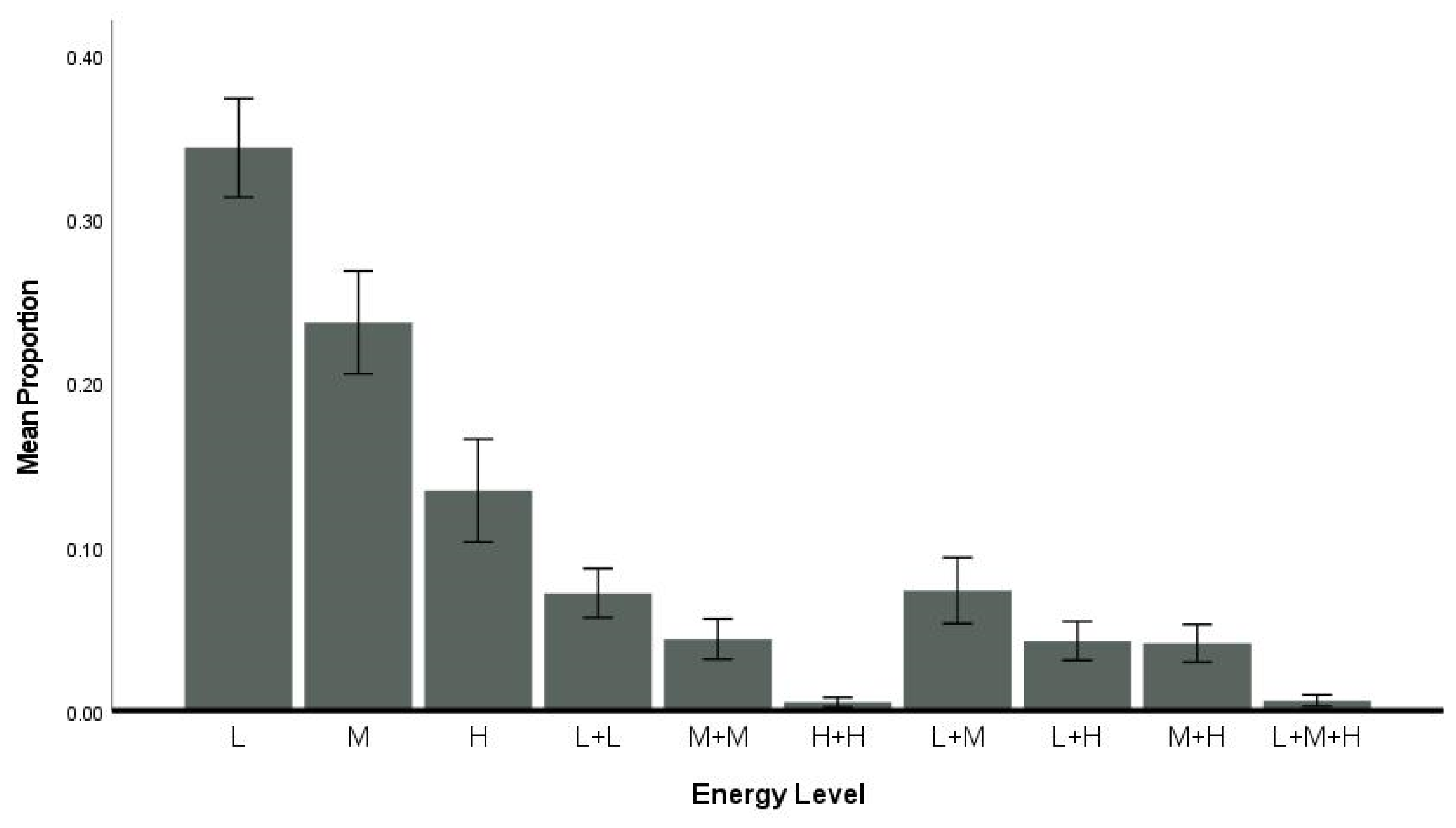
3.4. Flexibility—Energy
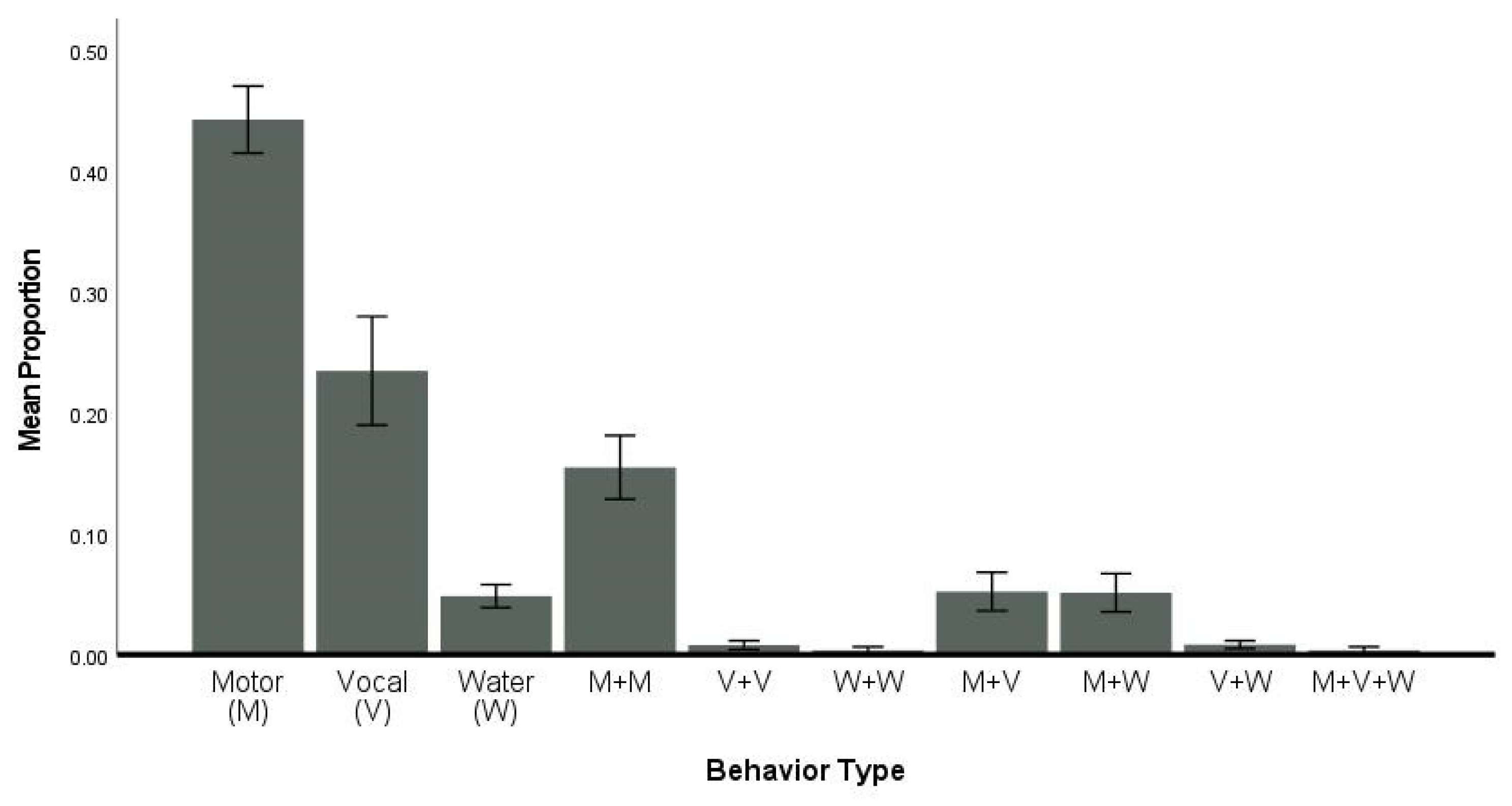
3.5. Flexibility—Type
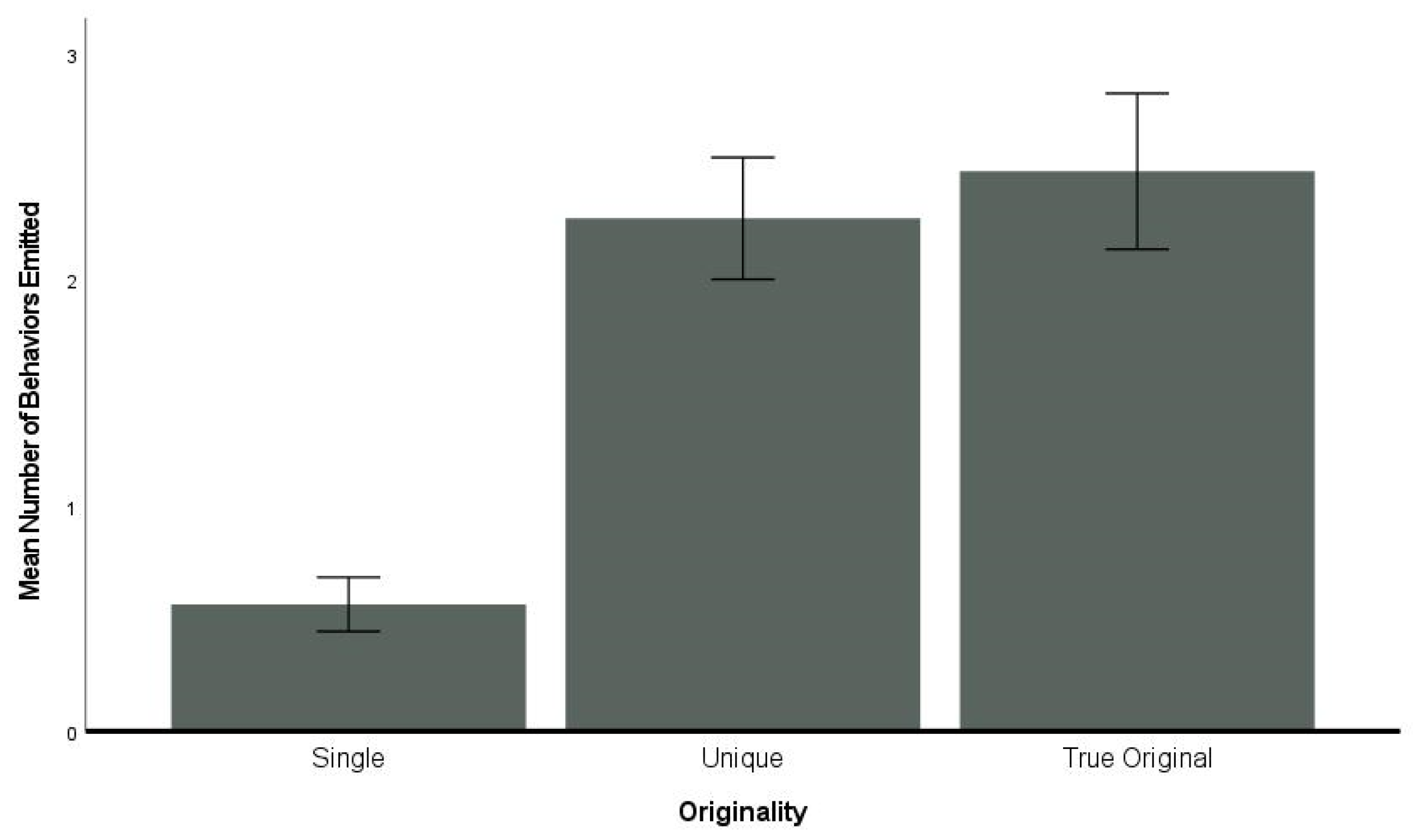
3.6. Originality
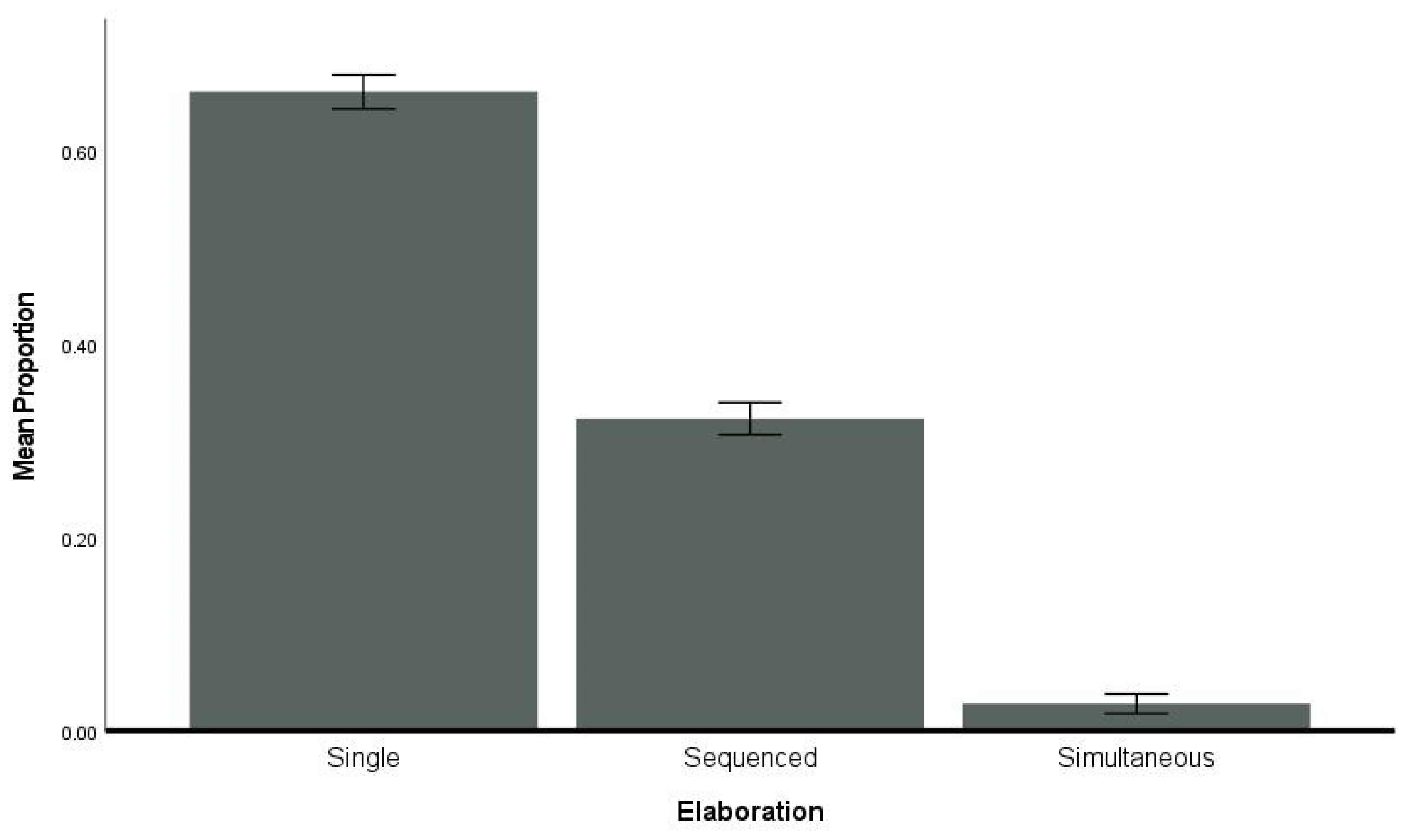
3.7. Elaboration
3.8. Profile of Creativity Based on Z-Scores
3.9. Cognitive Stimulation
4. Discussion
4.1. Fluency
4.2. Flexibility
4.3. Originality
4.4. Elaboration
4.5. Overall Creativity
4.6. Cognitive Stimulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, S.J.; Bejder, L.; Krützen, M. Why do Indo-Pacific bottlenose dolphins (Tursiops sp.) carry conch shells (Turbinella sp.) in Shark Bay, Western Australia? Mar. Mammal Sci. 2011, 27, 449–454. [Google Scholar] [CrossRef]
- Smolker, R.; Richards, A.; Connor, R.; Mann, J.; Berggren, P. Sponge carrying by dolphins (Delphinidae, Tursiops sp.): A foraging specialization involving tool use? Ethology 1997, 103, 454–465. [Google Scholar] [CrossRef]
- McCowan, B.; Reiss, D. Maternal aggressive contact vocalizations in captive bottlenose dolphins (Tursiops truncatus): Wide-band, low-frequency signals during mother/aunt-infant interactions. Zoo Biol. 1995, 14, 293–309. [Google Scholar] [CrossRef]
- Vollmer, N.L.; Hayek, L.A.C.; Heithaus, M.R.; Connor, R.C. Further evidence of a context-specific agonistic signal in bottlenose dolphins: The influence of consortships and group size on the pop vocalization. Behaviour 2015, 152, 1979–2000. [Google Scholar] [CrossRef]
- Kuczaj, S.A., II; Eskelinen, H.C. The “creative dolphin” revisited: What do dolphins do when asked to vary their behavior? Anim. Behav. Cogn. 2014, 1, 66–77. [Google Scholar] [CrossRef]
- Kuczaj, S.A.; Highfill, L.E. Dolphin play: Evidence for cooperation and culture? Beh. Brain Sci. 2005, 28, 705–706. [Google Scholar] [CrossRef]
- Kuczaj, S.A.; Makecha, R.; Trone, M.; Paulos, R.D.; Ramos, J.A. Role of peers in cultural innovation and cultural transmission: Evidence from the play of dolphin calves. Int. J. Comp. Psychol. 2006, 19, 223–240. [Google Scholar] [CrossRef]
- Weaver, A.; Kuczaj, S. Neither toy nor tool: Grass-wearing behavior among free-ranging bottlenose dolphins in Western Florida. Int. J. Comp. Psychol. 2016, 29, 31885. [Google Scholar] [CrossRef]
- Connor, R.C.; Wells, R.S.; Mann, J.; Read, A.J. The bottlenose dolphin In Cetacean Societies: Field Studies of Dolphins and Whales; Mann, J., Connor, R., Tyack, P., Whitehead, H., Eds.; University of Chicago Press: Chicago, IL, USA, 2000; pp. 91–125. [Google Scholar]
- McHugh, K.A.; Allen, J.B.; Barleycorn, A.A.; Wells, R.S. Natal philopatry, ranging behavior, and habitat selection of juvenile bottlenose dolphins in Sarasota Bay, Florida. J. Mammal. 2011, 92, 1298–1313. [Google Scholar] [CrossRef]
- Tsai, Y.J.J.; Mann, J. Dispersal, philopatry, and the role of fission-fusion dynamics in bottlenose dolphins. Mar. Mammal Sci. 2013, 29, 261–279. [Google Scholar] [CrossRef]
- Brando, S.; Broom, D.M.; Acasuso-Rivero, C.; Clark, F. Optimal marine mammal welfare under human care: Current efforts and future directions. Behav. Process. 2018, 156, 16–36. [Google Scholar] [CrossRef]
- Hill, H.; Lackups, M. Journal publication trends regarding cetaceans found in both wild and captive environments: What do we study and where do we publish? Int. J. Comp. Psychol. 2010, 23, 414–534. [Google Scholar] [CrossRef]
- Hill, H.M.; Guarino, S.; Dietrich, S.; St Leger, J. An inventory of peer-reviewed articles on killer whales (Orcinus orca) with a comparison to bottlenose dolphins (Tursiops truncatus). Anim. Behav. Cogn. 2016, 3, 135–149. [Google Scholar] [CrossRef]
- Jaakkola, K.; Willis, K. How long do dolphins live? Survival rates and life expectancies for bottlenose dolphins in zoological facilities vs. wild populations. Mar. Mammal Sci. 2019, 35, 1418–1437. [Google Scholar] [CrossRef]
- Brando, S.; Kooistra, N.; Hosey, G. Pre and post session behaviour of captive bottlenose dolphins Tursiops truncatus involved in “Swim-with-Dolphin” events. J. Zoo Aquar. Res. 2019, 7, 195–202. [Google Scholar]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 five domains model: Including human–animal interactions in assessments of animal welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Kuczaj, S.A. Animal creativity and innovation. In APA Handbook of Comparative Psychology: Perception, Learning, and Cognition; Call, J., Burghardt, G.M., Pepperberg, I.M., Snowdon, C.T., Zentall, T., Eds.; American Psychological Association: Washington, DC, USA, 2017; pp. 627–641. [Google Scholar] [CrossRef]
- Clark, F.E. Marine mammal cognition and captive care: A proposal for cognitive enrichment in zoos and aquariums. J. Zoo Aquar. Res. 2013, 1, 1–6. [Google Scholar]
- Clegg, I.L.; Domingues, M.; Ström, E.; Berggren, L. Cognitive foraging enrichment (but not non-cognitive enrichment) improved several longer-term welfare indicators in bottlenose dolphins. Animals 2023, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Delfour, F.; Vaicekauskaite, R.; García-Párraga, D.; Pilenga, C.; Serres, A.; Brasseur, I.; Pascaud, A.; Perlado-Campos, E.; Sanchez-Contreras, G.J.; Baumgartner, K.; et al. Behavioural diversity study in bottlenose dolphin (Tursiops truncatus) groups and its implications for welfare assessments. Animals 2021, 11, 1715. [Google Scholar] [CrossRef] [PubMed]
- Huettner, T.; Dollhaeupl, S.; Simon, R.; Baumgartner, K.; von Fersen, L. Activity budget comparisons using long-term observations of a group of bottlenose dolphins (Tursiops truncatus) under human care: Implications for animal welfare. Animals 2021, 11, 2107. [Google Scholar] [CrossRef] [PubMed]
- Lauderdale, L.K.; Miller, L.J. Efficacy of an interactive apparatus as environmental enrichment for common bottlenose dolphins (Tursiops truncatus). Anim. Welf. 2021, 29, 379–386. [Google Scholar] [CrossRef]
- Miller, L.J.; Vicino, G.A.; Sheftel, J.; Lauderdale, L.K. Behavioral diversity as a potential indicator of positive animal welfare. Animals 2020, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Clegg, I.L.; Rödel, H.G.; Boivin, X.; Delfour, F. Looking forward to interacting with their caretakers: Dolphins’ anticipatory behaviour indicates motivation to participate in specific events. Appl. Anim. Behav. Sci. 2018, 202, 85–93. [Google Scholar] [CrossRef]
- Lauderdale, L.K.; Walsh, M.T.; Mellen, J.D.; Granger, D.A.; Miller, L.J. Environmental enrichment, training, and habitat characteristics of common bottlenose dolphins (Tursiops truncatus) and Indo-Pacific bottlenose dolphins (Tursiops aduncus). PLoS ONE 2021, 16, e0253688. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.; Guarino, S.; Crandall, S.; Lenhart, E.; Dietrich, S. Young belugas diversify adult beluga (Delphinapterus leucas) behavior. Anim. Beh. Cogn. 2015, 2, 267–284. [Google Scholar] [CrossRef]
- Melfi, V.A.; Dorey, N.R.; Ward, S.J. Zoo Animal Learning and Training; Wiley-Blackwell: Hoboken, NJ, USA, 2020. [Google Scholar]
- Ramirez, K. Marine mammal training: The history of training animals for medical behaviors and keys to their success. Vet. Clin. N. Am. Exot. Anim. Pract. 2012, 15, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Clegg, I.L.; Rödel, H.G.; Mercera, B.; van der Heul, S.; Schrijvers, T.; de Laender, P.; Gojceta, R.; Zimmitti, M.; Verhoeven, E.; Burger, J.; et al. Dolphins’ willingness to participate (WtP) in positive reinforcement training as a potential welfare indicator, where WtP predicts early changes in health status. Front. Psychol. 2019, 10, 2112. [Google Scholar] [CrossRef]
- Bigiani, S.; Pilenga, C. A fast technique to induce and measure anticipatory behavior in bottlenose dolphins (Tursiops truncatus). J. Appl. Anim. Welf. Sci. 2022, 1–13. [Google Scholar] [CrossRef]
- Clegg, I.L.; Delfour, F. Cognitive judgement bias is associated with frequency of anticipatory behavior in bottlenose dolphins. Zoo Biol. 2018, 37, 67–73. [Google Scholar] [CrossRef]
- Jensen, A.L.M.; Delfour, F.; Carter, T. Anticipatory behavior in captive bottlenose dolphins (Tursiops truncatus): A preliminary study. Zoo Biol. 2013, 32, 436–444. [Google Scholar] [CrossRef]
- Pryor, K.; Chase, S. Training for variable and innovative behavior. Int. J. Comp. Psychol. 2014, 27, 361–368. Available online: https://escholarship.org/uc/item/9cs2q3nr (accessed on 20 February 2022). [CrossRef]
- Clegg, I.L.; Rödel, H.G.; Delfour, F. Bottlenose dolphins engaging in more social affiliative behaviour judge ambiguous cues more optimistically. Behav. Brain Res. 2017, 322, 115–122. [Google Scholar] [CrossRef]
- Dibble, D.S.; Van Alstyne, K.R.; Ridgway, S. Dolphins signal success by producing a victory squeal. Int. J. Comp. Psychol. 2016, 29, 32031. [Google Scholar] [CrossRef]
- Hill, H.M.; Weiss, M.; Brasseur, I.; Manibusan, A.; Sandoval, I.R.; Robeck, T.; Sigman, J.; Werner, K.; Dudzinski, K.M. Killer whale innovation: Teaching animals to use their creativity upon request. Anim. Cogn 2022, 25, 1091–1108. [Google Scholar] [CrossRef]
- Pryor, K.W.; Haag, R.; O’Reilly, J. The creative porpoise: Training for novel behavior. J. Exp. Anal. Behav. 1969, 12, 653–661. [Google Scholar] [CrossRef]
- Dudzinski, K.M.; Yeater, D.; Bolton, T.; Eskelinen, H.; Hill, H. Defining creativity and confirming understanding of the concept in dolphins: Research and training perspectives. Aquat. Mamm. 2018, 44, 426–436. [Google Scholar] [CrossRef]
- Mann, J.; Sargeant, B.L.; Watson-Capps, J.J.; Gibson, Q.A.; Heithaus, M.R.; Connor, R.C.; Patterson, E. Why do dolphins carry sponges? PLoS ONE 2008, 3, e3868. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Hoppitt, W.J.; Allen, S.J.; Krützen, M. Integrating genetic, environmental, and social networks to reveal transmission pathways of a dolphin foraging innovation. Curr. Biol. 2020, 30, 3024–3030. [Google Scholar] [CrossRef]
- Bacher, K.; Allen, S.; Lindholm, A.K.; Bejder, L.; Krützen, M. Genes or culture: Are mitochondrial genes associated with tool use in bottlenose dolphins (Tursiops sp.)? Behav. Genet. 2010, 40, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Kopps, A.M.; Sherwin, W.B. Modelling the emergence and stability of a vertically transmitted cultural trait in bottlenose dolphins. Anim. Behav. 2012, 84, 1347–1362. [Google Scholar] [CrossRef]
- Krützen, M.; Mann, J.; Heithaus, M.R.; Connor, R.C.; Bejder, L.; Sherwin, W.B. Cultural transmission of tool use in bottlenose dolphins. Proc. Natl. Soc. Sci. USA 2005, 102, 8939–8943. [Google Scholar] [CrossRef]
- Sargeant, B.L.; Mann, J. Developmental evidence for foraging traditions in wild bottlenose dolphins. Anim. Behav. 2009, 78, 715–721. [Google Scholar] [CrossRef]
- Bizzozzero, M.R.; Allen, S.J.; Gerber, L.; Wild, S.; King, S.L.; Connor, R.C.; Friedman, W.R.; Wittwer, S.; Krützen, M. Tool use and social homophily among male bottlenose dolphins. Proc. R. Soc. B 2019, 286, 20190898. [Google Scholar] [CrossRef] [PubMed]
- Guinet, C. International stranding apprenticeship and social play in killer whales (Orcinus orca). Can. J. Zool. 1991, 9, 2712–2716. [Google Scholar] [CrossRef]
- Guinet, C.; Bouvier, J. Development of intentional stranding hunting techniques in killer whale (Orcinus orca) calves at Crozet Archipelago. Can. J. Zool. 1995, 73, 27–33. [Google Scholar] [CrossRef]
- Lopez, J.C.; Lopez, D. Killer whales (Orcinus orca) of Patagonia, and their behavior of intentional stranding while hunting nearshore. J. Mammal. 1985, 66, 181–183. [Google Scholar] [CrossRef]
- Duffy-Echevarria, E.E.; Connor, R.C.; St. Aubin, D.J. Observations of strand-feeding behavior by bottlenose dolphins (Tursiops truncatus) in Bull Creek, South Carolina. Mar. Mammal Sci. 2008, 24, 202–206. [Google Scholar] [CrossRef]
- Gisburne, T.J.; Connor, R.C. Group size and feeding success in strand-feeding bottlenose dolphins (Tursiops truncatus) in Bull Creek, South Carolina. Mar. Mammal Sci. 2015, 31, 1252–1257. [Google Scholar] [CrossRef]
- Jiménez, P.J.; Alava, J.J. Strand-feeding by coastal bottlenose dolphins (Tursiops truncatus) in the Gulf of Guayaquil, Ecuador. Lat. Am. J. Aquat. Mamm. 2015, 10, 33–37. [Google Scholar] [CrossRef]
- Lewis, J.S.; Schroeder, W.W. Mud plume feeding, a unique foraging behavior of the bottlenose dolphin in the Florida Keys. Gulf Mex. Sci. 2003, 21, 9. [Google Scholar] [CrossRef]
- Ramos, E.A.; Santoya, L.; Verde, J.; Walker, Z.; Castelblanco-Martínez, N.; Kiszka, J.J.; Rieucau, G. Lords of the Rings: Mud ring feeding by bottlenose dolphins in a Caribbean estuary revealed from sea, air, and space. Mar. Mammal Sci. 2022, 38, 364–373. [Google Scholar] [CrossRef]
- Melzer, D.; Yeater, D.; Bradley, M.; Manitzas Hill, H.; Guerra, G.; Salazar, E.; Bolton, T.; Dudzinski, K. A Comparative test of creative thinking in preschool children and dolphins. Anim. Behav. Cogn. 2022, 9, 349–362. [Google Scholar] [CrossRef]
- Mercado, E.; Murray, S.O.; Uyeyama, R.K.; Pack, A.A.; Herman, L.M. Memory for recent actions in the bottlenosed dolphin (Tursiops truncatus): Repetition of arbitrary behaviors using an abstract rule. Anim. Learn. Behav. 1998, 26, 210–218. [Google Scholar] [CrossRef]
- Lawrence, M.K.; Borger-Turner, J.L.; Turner, T.N.; Eskelinen, H.C. Investigating the effects of applied learning principles of the “create” response in Atlantic bottlenose dolphins (Tursiops truncates). Int. J. Comp. Psychol. 2016, 29, 32069. [Google Scholar] [CrossRef]
- Willgohs, K.R.; Williams, J.; Franklin, E.; Highfill, L.E. The creative canine: Investigating the concept of creativity in dogs (Canis lupus familiaris) using citizen science. Int. J. Comp. Psychol. 2022, 35, 58745. [Google Scholar] [CrossRef]
- Kaufman, A.B. Innovation in marine mammals. In The Cambridge Handbook of Animal Cognition; Kaufman, A.B., Call, J., Kaufman, J.C., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Kaufman, J.C.; Kaufman, A.B. Applying a creativity framework to animal cognition. New Ideas Psychol. 2004, 22, 143–155. [Google Scholar] [CrossRef]
- Mercado, E., III; Uyeyama, R.K.; Pack, A.A.; Herman, L.M. Memory for action events in the bottlenosed dolphin. Anim. Cogn. 1999, 2, 17–25. [Google Scholar]
- Xitco, M.J., Jr. Mimicry of Modeled Behaviors by Bottlenose Dolphins; University of Hawai’i at Manoa: Honolulu, HI, USA, 1988. [Google Scholar]
- Zhu, W.; Shang, S.; Jiang, W.; Pei, M.; Su, Y. Convergent thinking moderates the relationship between divergent thinking and scientific creativity. Creat. Res. J. 2019, 31, 320–328. [Google Scholar] [CrossRef]
- Wells, R.S. Common bottlenose dolphin foraging: Behavioral solutions that incorporate habitat features and social associates. In Ethology and Behavioral Ecology of Odontocetes; Wursig, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 331–344. [Google Scholar]
- Guilford, J.P. Measurement and creativity. Theory Pract. 1966, 5, 185–189. [Google Scholar] [CrossRef]
- Torrance, E.P. The Torrance Tests of Creative Thinking-Norms-Technical Manual Research Edition-Verbal Tests, Forms A and B- Figural Tests, Forms A and B; Personnel Press: Surrey, UK, 1974. [Google Scholar]
- Eisenberger, R.; Armeli, S.; Pretz, J. Can the promise of reward increase creativity? J. Pers. Soc. Psychol. 1998, 74, 704–714. [Google Scholar] [CrossRef]
- Eisenberger, R.; Cameron, J. Detrimental effects of reward: Reality or myth? Am. Psychol. 1996, 51, 1153–1166. [Google Scholar] [CrossRef]
- Bujacz, A.; Dunne, S.; Fink, D.; Gatej, A.R.; Karlsson, E.; Ruberti, V.; Wronska, M.K. Why do we enjoy creative tasks? Results from a multigroup randomized controlled study. Think. Skills Creat. 2016, 19, 188–197. [Google Scholar] [CrossRef]
- Conner, T.S.; Silvia, P.J. Creative days: A daily diary study of emotion, personality, and everyday creativity. Psychol. Aesthet. Create. Arts 2015, 9, 463–470. [Google Scholar] [CrossRef]
- Benson-Amram, S.; Holekamp, K.E. Innovative problem solving by wild spotted hyenas. Proc. R. Soc. Lond. B Biol. Sci. 2012, 279, 4087–4095. [Google Scholar] [CrossRef]
- Scarpuzzi, M.R.; Turner, T.N.; Tompkins, C.D.; Force, D.L.; Lacinak, C.T.; Kuczaj, S.A. The use of the least reinforcing scenario in a proactive training program. In Proceedings of the 27th Annual Conference of the International Marine Animal Trainers Association, Chicago, IL, USA, 6–10 December 1999. [Google Scholar]
- Jaakkola, K.; Brignac, S.; Erb, L.; Guarino, E.; Haddock, A.; Rodriguez, A. Trainer Interaction Can Improve Welfare Outcomes of Toy Enrichment for Isolated Animals: A Case Study. J. Zool. Bot. 2023, 4, 72–81. [Google Scholar] [CrossRef]
- Terrace, H.S. Errorless transfer of a discrimination across two continua. J. Exp. Anal. Behav. 1963, 6, 223–232. [Google Scholar] [CrossRef]
- Highfill, L.E.; Kuczaj, S.A. Do bottlenose dolphins (Tursiops truncatus) have distinct and stable personalities? Aquat. Mamm. 2007, 33, 380. [Google Scholar] [CrossRef]
- Kuczaj II, S.A.; Highfill, L.; Byerly, H. The importance of considering context in the assessment of personality characteristics: Evidence from ratings of dolphin personality. Int. J. Comp. Psychol. 2012, 25, 309–329. [Google Scholar] [CrossRef]
- Highfill, L.E.; Kuczaj, S.A., II. How studies of wild and captive dolphins contribute to our understanding of individual differences and personality. Int. J. Comp. Psychol. 2010, 23, 269–277. Available online: https://escholarship.org/uc/item/0xb2s7g3 (accessed on 20 March 2008). [CrossRef]
| Dolphin ID | Age Range during Tests (y) | Sex | SD Innovate Experience |
|---|---|---|---|
| Han * | ~30 | M | >5 y |
| Bill | 17 | M | >5 y |
| Ritchie ** | ~18 | M | >5 y |
| French | 14 | M | >5 y |
| Ronnie | 16 | M | >5 y |
| Champ | 6 | M | <5 y |
| Lenca | 6 | M | <5 y |
| Gracie ** | 27; 29 | F | >5 y |
| Maury | 16; 17 | F | >5 y |
| Bailey | 14; 16 | F | >5 y |
| Tilly | 8; 11 | F | <5 y |
| Poli | 8; 11 | F | <5 y |
| Constructs | Fluency | Flexibility | Originality | Elaboration |
|---|---|---|---|---|
| Operational Definitions | Correct—number of correct different behaviors presented in a session Percent correct | Energy—high (H), moderate (M), low (L), and combinations of same or different compound energy levels | Unique | Simple |
| Type— motor (M), vocal (V), bubbles (B), or combinations of same or different compound types | Single | Sequence | ||
| Repeat—number of consecutively reinforced behaviors (trials) before a repeat. | True original | Simultaneous | ||
| Invented | ||||
| Measures | Correct: reinforced N Percent Correct: Correct/total trials Repeat: consecutively reinforced new behaviors before a repeat (NR) | Energy: 1 = L 2 = M 3 = H 4 = L + L 5 = M + M 6 = H + H 7 = L + M 8 = L + H 9 = M + H 10 = L + M + H | Unique: performed by only 1 dolphin | Single action: 1 behavior performed |
| Type: 1 = M 2 = V 3 = B 4 = M + M 5 = V + V 6 = B + B 7 = M + V 8 = M + B 9 = V + B 10 = M + V + B | Single: behavior emitted a single time across sessions for one dolphin (may have been produced by other dolphins | Sequence: behaviors performed in sequence in a trial | ||
| True original: behavior emitted a single time across all sessions and all dolphins | Simultaneous: behaviors performed at the same time | |||
| Invented: behavior emitted that had never been seen in a dolphin’s stimulus control repertoire |
| Originality | Fluency | Flexibility | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Session # | Unique | Single | True Original | Total | F1 | F2 (%) | F3 | F4 | Energy | Type | Elaboration | ||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 1 | 2 | 3 | ||||||||||
| Bill | 2 | 0 | 3 | 1 | 4 | 9 | 75.0% | 12 | 4 | 2 | 0 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 5 | 0 |
| 3 | 0 | 8 | 6 | 14 | 18 | 52.9% | 34 | 5 | 5 | 4 | 4 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 10 | 2 | 1 | 3 | 0 | 0 | 2 | 0 | 0 | 0 | 11 | 7 | 0 | |
| 4 | 0 | 2 | 2 | 4 | 12 | 60.0% | 20 | 2 | 6 | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 8 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 8 | 4 | 0 | |
| 5 | 1 | 4 | 3 | 8 | 20 | 54.1% | 37 | 2 | 6 | 3 | 5 | 2 | 0 | 0 | 2 | 1 | 1 | 0 | 8 | 4 | 1 | 4 | 0 | 0 | 1 | 2 | 0 | 0 | 13 | 6 | 1 | |
| French | 2 | 0 | 3 | 5 | 8 | 15 | 88.2% | 17 | 7 | 4 | 4 | 3 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 8 | 3 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 6 | 0 |
| 3 | 0 | 0 | 0 | 0 | 5 | 38.5% | 13 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | |
| 4 | 0 | 1 | 4 | 5 | 10 | 45.5% | 22 | 4 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 2 | 0 | 3 | 0 | 0 | 5 | 0 | 0 | 1 | 1 | 0 | 0 | 4 | 6 | 0 | |
| 5 | 1 | 3 | 11 | 15 | 18 | 47.4% | 38 | 2 | 4 | 3 | 2 | 0 | 1 | 0 | 4 | 1 | 3 | 0 | 6 | 3 | 0 | 6 | 0 | 0 | 1 | 2 | 0 | 0 | 7 | 1 | 1 | |
| Ritchie | 2 | 0 | 2 | 0 | 2 | 9 | 50.0% | 18 | 4 | 2 | 3 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 6 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 6 | 3 | 0 |
| 3 | 0 | 3 | 1 | 4 | 9 | 33.3% | 27 | 2 | 2 | 3 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 7 | 2 | 0 | |
| 4 | 3 | 4 | 2 | 9 | 13 | 48.2% | 27 | 1 | 2 | 2 | 3 | 0 | 0 | 0 | 3 | 0 | 3 | 0 | 6 | 1 | 0 | 4 | 0 | 0 | 1 | 1 | 0 | 0 | 7 | 6 | 0 | |
| 5 | 1 | 2 | 3 | 6 | 8 | 53.3% | 15 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 4 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 3 | 0 | |
| Han | 2 | 0 | 4 | 1 | 5 | 13 | 61.9% | 21 | 2 | 3 | 4 | 3 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 9 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 6 | 7 | 0 |
| 3 | 1 | 8 | 11 | 20 | 24 | 63.2% | 38 | 6 | 7 | 3 | 4 | 4 | 0 | 0 | 4 | 0 | 2 | 0 | 9 | 3 | 1 | 5 | 0 | 0 | 2 | 4 | 0 | 0 | 10 | 12 | 2 | |
| 4 | 4 | 1 | 3 | 8 | 15 | 75.0% | 20 | 3 | 2 | 3 | 2 | 1 | 0 | 1 | 1 | 1 | 4 | 0 | 7 | 0 | 0 | 6 | 0 | 0 | 2 | 0 | 0 | 0 | 8 | 7 | 0 | |
| 5 | 1 | 3 | 2 | 6 | 15 | 53.6% | 28 | 1 | 4 | 1 | 3 | 2 | 0 | 0 | 4 | 1 | 0 | 0 | 6 | 2 | 0 | 3 | 0 | 0 | 1 | 3 | 0 | 0 | 8 | 7 | 0 | |
| Ronnie | 2 | 0 | 3 | 6 | 9 | 11 | 68.8% | 16 | 2 | 2 | 3 | 3 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 8 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 5 | 6 | 0 |
| 3 | 1 | 1 | 6 | 8 | 19 | 76.9% | 26 | 12 | 4 | 5 | 5 | 1 | 1 | 0 | 2 | 0 | 1 | 0 | 12 | 1 | 1 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 11 | 8 | 0 | |
| 4 | 1 | 8 | 7 | 16 | 20 | 46.5% | 43 | 2 | 6 | 5 | 5 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 13 | 3 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 11 | 9 | 0 | |
| 5 | 1 | 2 | 4 | 7 | 12 | 57.1% | 21 | 5 | 2 | 2 | 4 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 6 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 5 | 0 | |
| Champ | 2 | 0 | 4 | 1 | 5 | 12 | 57.1% | 21 | 8 | 8 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 1 | 0 |
| 3 | 1 | 4 | 5 | 10 | 15 | 42.9% | 35 | 6 | 3 | 3 | 1 | 6 | 0 | 0 | 1 | 1 | 0 | 0 | 7 | 1 | 0 | 3 | 0 | 0 | 2 | 2 | 0 | 0 | 6 | 9 | 0 | |
| 4 | 1 | 0 | 1 | 2 | 12 | 50.0% | 24 | 6 | 1 | 3 | 4 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 6 | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 5 | 4 | 3 | |
| 5 | 0 | 2 | 3 | 5 | 15 | 51.7% | 29 | 4 | 2 | 2 | 0 | 3 | 0 | 0 | 5 | 2 | 1 | 0 | 4 | 0 | 0 | 6 | 0 | 0 | 1 | 4 | 0 | 0 | 2 | 12 | 1 | |
| Lenca | 2 | 0 | 0 | 1 | 1 | 2 | 40.0% | 5 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| 3 | 0 | 4 | 5 | 9 | 9 | 20.9% | 43 | 3 | 5 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 1 | 0 | |
| 4 | 1 | 0 | 0 | 1 | 4 | 28.6% | 14 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | |
| 5 | 0 | 0 | 0 | 0 | 4.75 | 32.4% | 18 | 2.25 | 2.5 | 1 | 0.75 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 1.75 | 2.5 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 4.25 | 1 | 0 | |
| Originality | Fluency | Flexibility | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Session # | Unique | Single | True Original | Total | F1 | F2 | F3 | F4 | Energy | Type | Elaboration | ||||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 1 | 2 | 3 | ||||||||||
| Gracie | 2 | 0 | 0 | 0 | 0 | 5 | 100.0% | 5 | 5 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 4 | 44.4% | 9 | 2 | 1 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | |
| 4 | 1 | 1 | 0 | 2 | 9 | 48.0% | 25 | 6 | 3 | 1 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 2 | 0 | |
| 5 | 1 | 1 | 0 | 2 | 7 | 46.7% | 15 | 5 | 1 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 | 1 | 0 | |
| Maury | 2 | 0 | 1 | 0 | 1 | 5 | 55.6% | 9 | 2 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 3 | 2 | 0 |
| 3 | 1 | 3 | 5 | 9 | 13 | 48.2% | 27 | 3 | 5 | 2 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 6 | 2 | 0 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 8 | 5 | 0 | |
| 4 | 0 | 4 | 3 | 7 | 12 | 40.0% | 30 | 3 | 3 | 0 | 0 | 1 | 1 | 0 | 6 | 0 | 1 | 0 | 1 | 2 | 0 | 3 | 0 | 0 | 1 | 5 | 0 | 0 | 3 | 9 | 0 | |
| 5 | 1 | 3 | 2 | 6 | 7 | 43.8% | 16 | 4 | 5 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 5 | 2 | 0 | |
| Bailey | 2 | 0 | 1 | 0 | 1 | 4 | 18.2% | 22 | 2 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| 3 | 0 | 0 | 4 | 4 | 3 | 9.7% | 31 | 2 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | |
| 4 | 0 | 1 | 1 | 2 | 7 | 58.3% | 12 | 5 | 1 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 1 | 0 | |
| 5 | 0 | 1 | 0 | 1 | 8 | 57.1% | 14 | 5 | 1 | 2 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 4 | 3 | 1 | |
| Tilly | 2 | 0 | 1 | 2 | 3 | 5 | 35.7% | 14 | 4 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 |
| 3 | 0 | 1 | 0 | 1 | 2 | 25.0% | 8 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | |
| 4 | 2 | 4 | 1 | 7 | 7 | 70.0% | 10 | 6 | 2 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 5 | 1 | 1 | |
| 5 | 0 | 0 | 0 | 0 | 1 | 8.3% | 12 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Poli | 2 | 0 | 2 | 1 | 3 | 4 | 44.4% | 9 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 |
| 3 | 1 | 3 | 1 | 5 | 10 | 43.5% | 23 | 4 | 5 | 2 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 4 | 0 | |
| 4 | 1 | 1 | 2 | 4 | 5 | 41.7% | 12 | 2 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | |
| 5 | 1 | 2 | 3 | 6 | 4 | 28.6% | 14 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | |
| Fluency | Flexibility | Originality | ||||||
|---|---|---|---|---|---|---|---|---|
| Ranking | Dolphin | Sum z-Scores | Ranking | Dolphin | Sum z-Scores | Ranking | Dolphin | Sum z-Scores |
| 1st | Champ | 4.2608 | 1st | Han | 2.5334 | 1st | Han | 4.7476 |
| 2nd | Ronnie | 4.2186 | 2nd | French | 1.6584 | 2nd | Ronnie | 3.3314 |
| 3rd | Bill | 3.4854 | 3rd | Champ | 1.5830 | 3rd | Bill | 1.1134 |
| 4th | Han | 3.1532 | 4th | Maury | 1.5713 | 4th | Ritchie | 0.9176 |
| 5th | French | 0.5512 | 5th | Bill | 1.5628 | 5th | French | 0.2435 |
| 6th | Maury | 0.3063 | 6th | Ritchie | −0.1823 | 6th | Maury | 0.2421 |
| 7th | Gracie | −1.3096 | 7th | Ronnie | −0.2818 | 7th | Champ | 0.0406 |
| 8th | Ritchie | −1.8720 | 8th | Bailey | −0.7510 | 8th | Poli | −0.1678 |
| 9th | Poli | −2.2056 | 9th | Tilly | −0.8508 | 9th | Tilly | −1.7673 |
| 10th | Bailey | −3.0629 | 10th | Poli | −1.8686 | 10th | Lenca | −2.3649 |
| 11th | Lenca | −3.1954 | 11th | Gracie | −2.3190 | 11th | Gracie | −3.0027 |
| 12th | Tilly | −4.3299 | 12th | Lenca | −2.6555 | 12th | Bailey | −3.3336 |
| Ranking | Dolphin | Summed z-Scores |
|---|---|---|
| 1st | Han | 3.6375 |
| 2nd | Ronnie | 2.0242 |
| 3rd | Bill | 2.0239 |
| 4th | Champ | 1.8703 |
| 5th | French | 1.0482 |
| 6th | Maury | 0.9429 |
| 7th | Ritchie | −0.2532 |
| 8th | Poli | −1.5416 |
| 9th | Tilly | −2.0970 |
| 10th | Bailey | −2.2524 |
| 11th | Gracie | −2.4878 |
| 12th | Lenca | −2.9149 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeater, D.B.; Dudzinski, K.M.; Melzer, D.; Magee, A.R.; Robinett, M.; Guerra, G.; Salazar, K.; Bolton, T.; Hill, H.M. Training the Concept of Innovate in Dolphins (Tursiops truncatus) Is Both Creative and Cognitively Stimulating. Animals 2024, 14, 896. https://doi.org/10.3390/ani14060896
Yeater DB, Dudzinski KM, Melzer D, Magee AR, Robinett M, Guerra G, Salazar K, Bolton T, Hill HM. Training the Concept of Innovate in Dolphins (Tursiops truncatus) Is Both Creative and Cognitively Stimulating. Animals. 2024; 14(6):896. https://doi.org/10.3390/ani14060896
Chicago/Turabian StyleYeater, Deirdre B., Kathleen M. Dudzinski, Dawn Melzer, Andrew R. Magee, Michaela Robinett, Gonzalo Guerra, Kimberly Salazar, Teri Bolton, and Heather Manitzas Hill. 2024. "Training the Concept of Innovate in Dolphins (Tursiops truncatus) Is Both Creative and Cognitively Stimulating" Animals 14, no. 6: 896. https://doi.org/10.3390/ani14060896
APA StyleYeater, D. B., Dudzinski, K. M., Melzer, D., Magee, A. R., Robinett, M., Guerra, G., Salazar, K., Bolton, T., & Hill, H. M. (2024). Training the Concept of Innovate in Dolphins (Tursiops truncatus) Is Both Creative and Cognitively Stimulating. Animals, 14(6), 896. https://doi.org/10.3390/ani14060896







