A Cadaveric Study Using Anatomical Cross-Section and Computed Tomography for the Coelomic Cavity in Juvenile Cory’s Shearwater (Aves, Procellariidae, Calonectris borealis)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. CT Study
2.3. Anatomical Sections
2.4. Anatomic Evaluation
3. Results
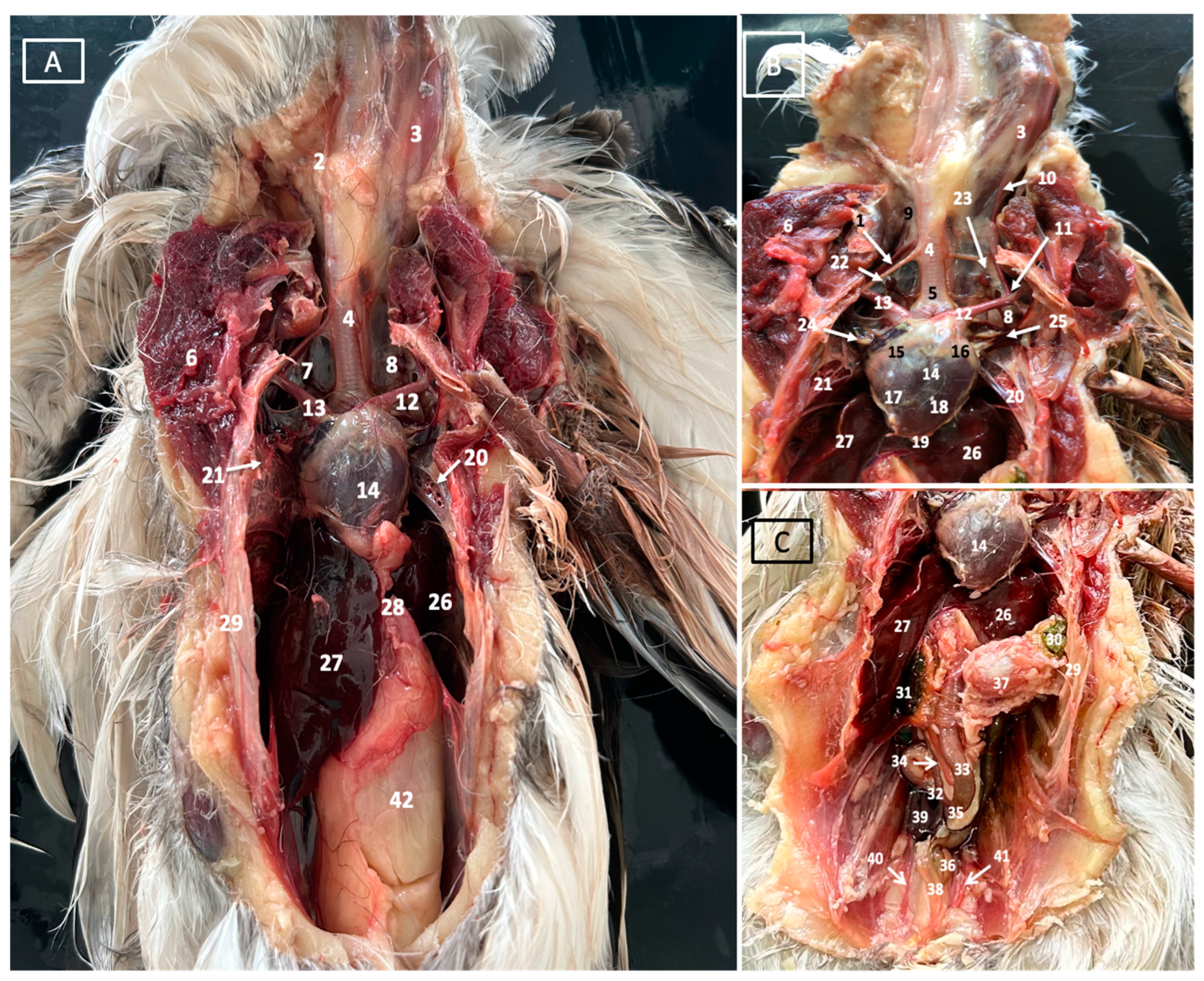
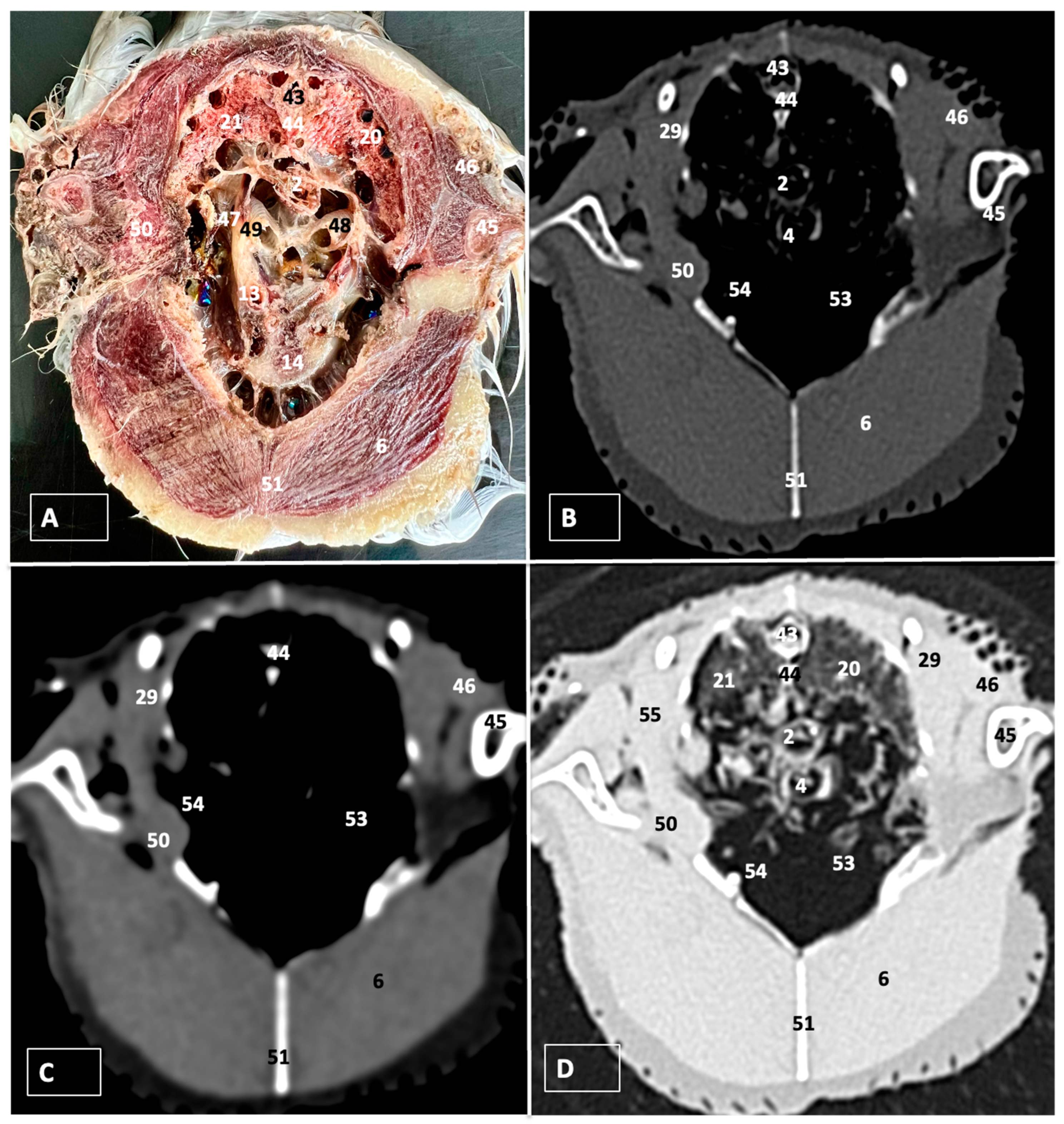
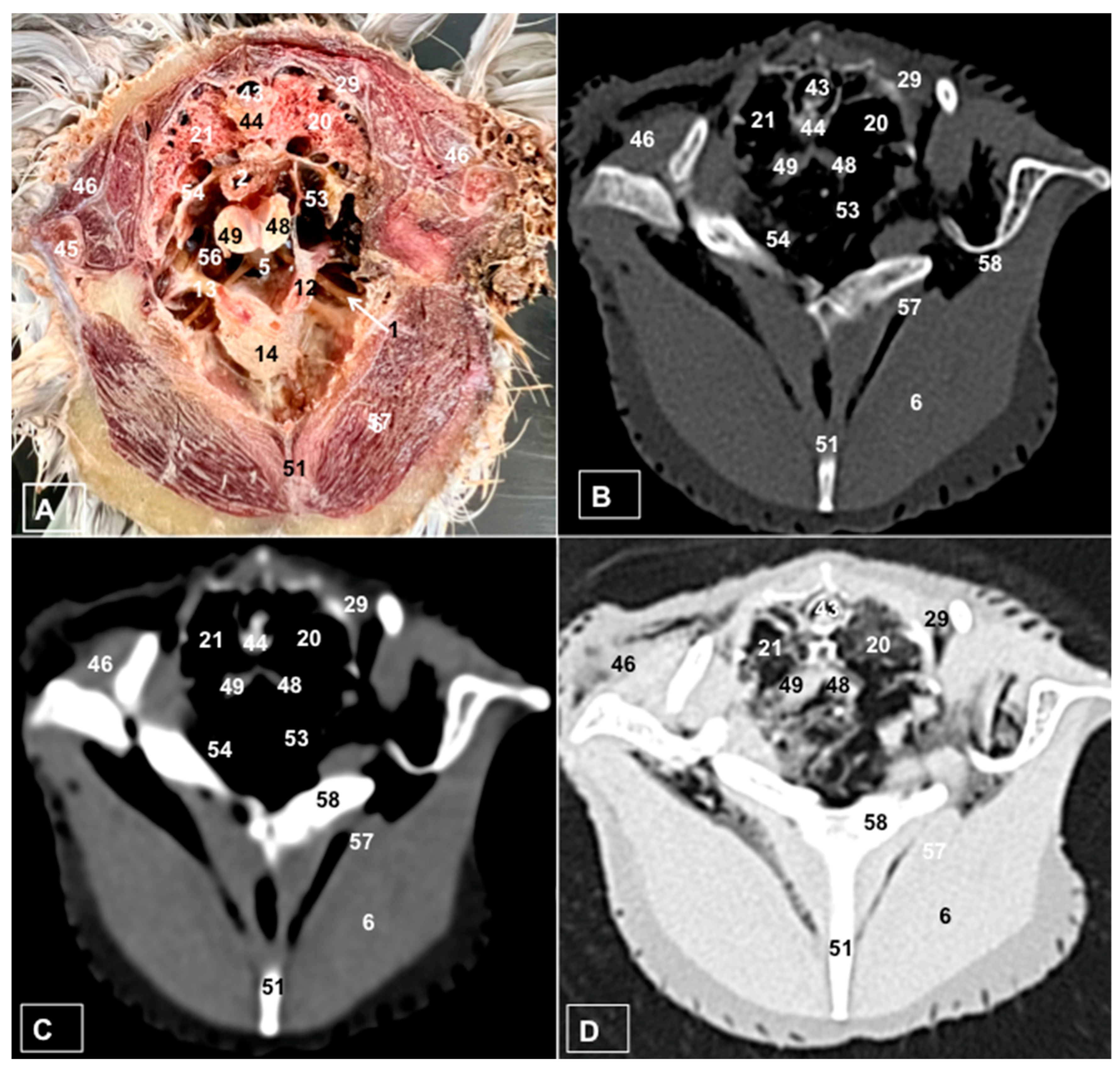
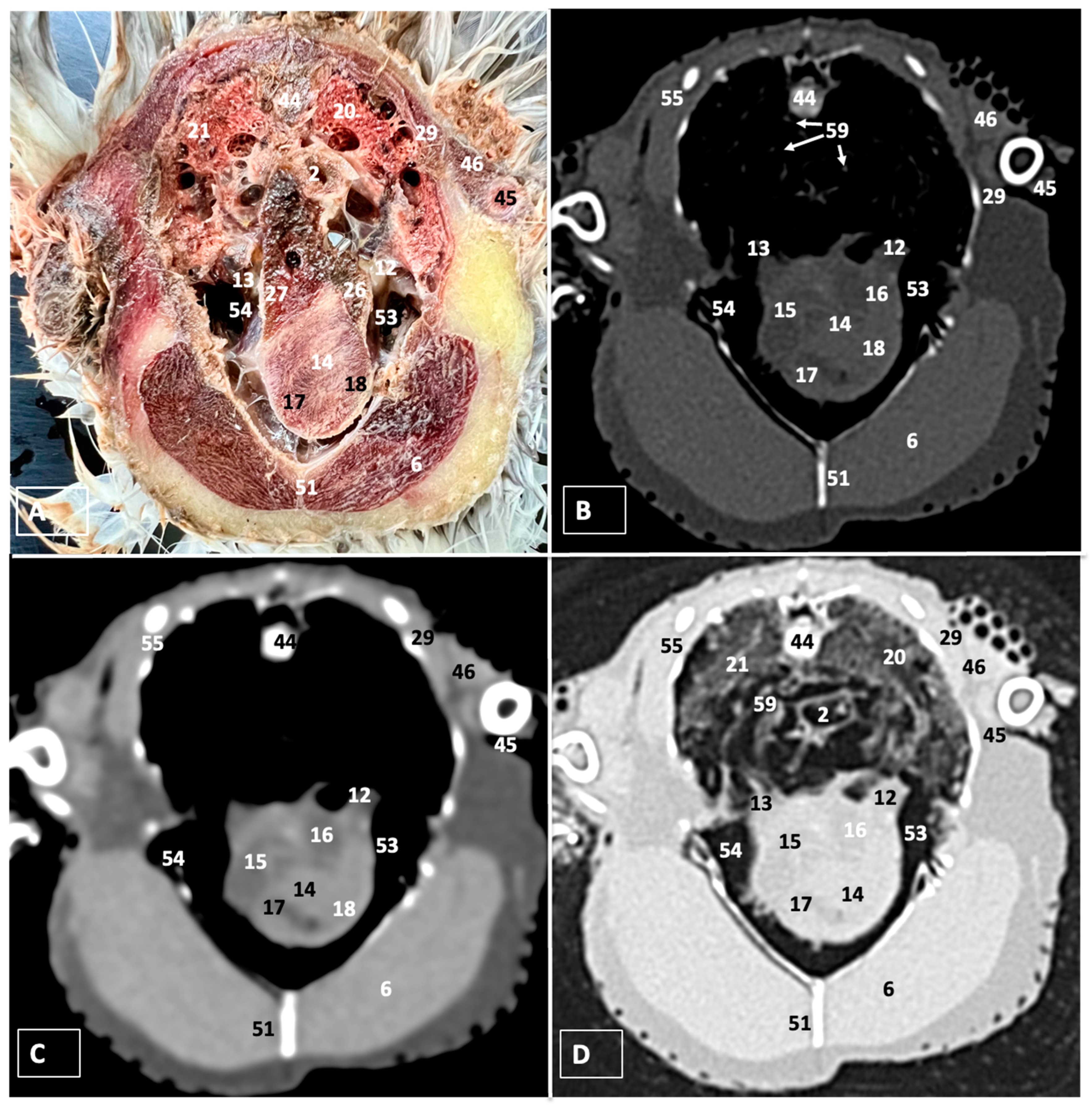
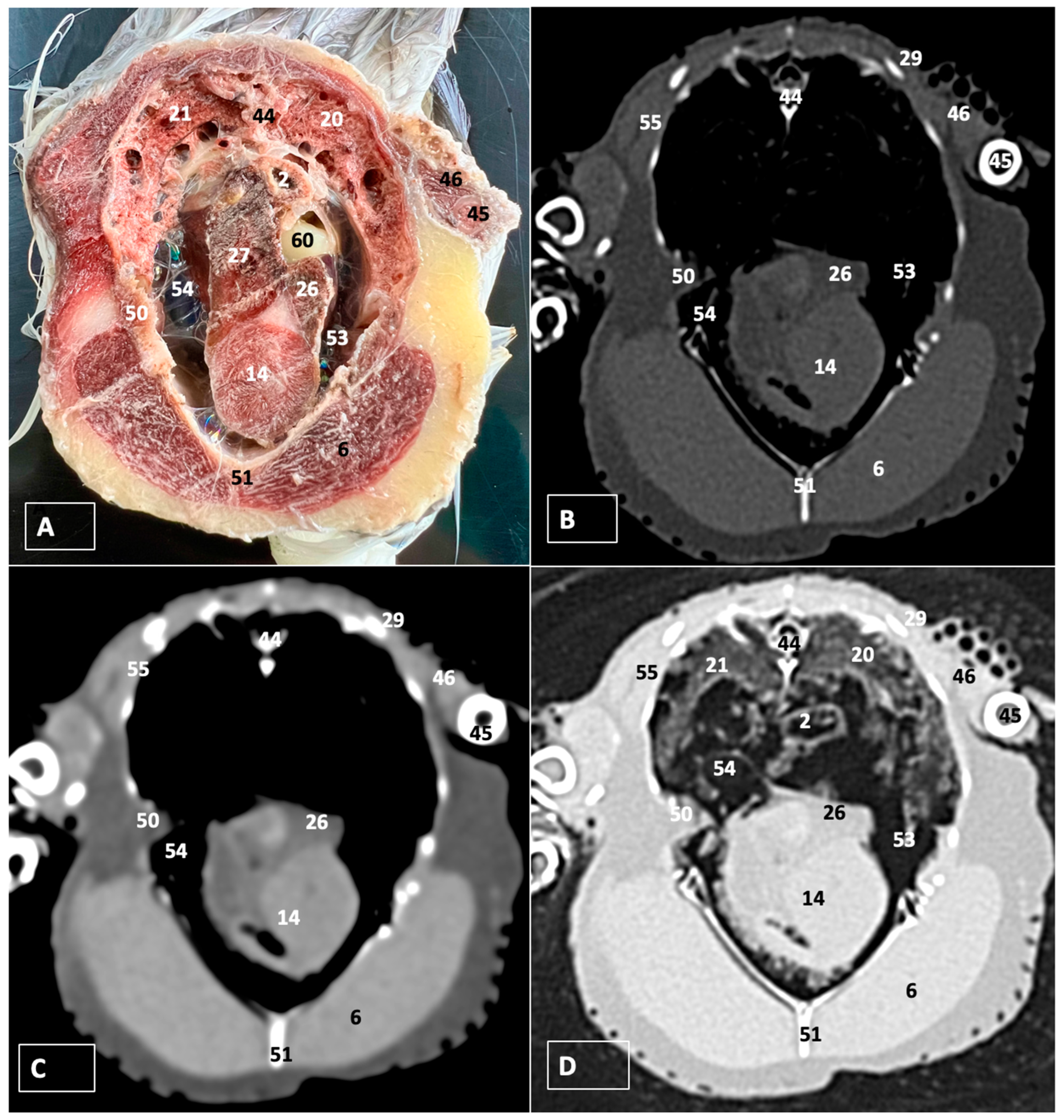
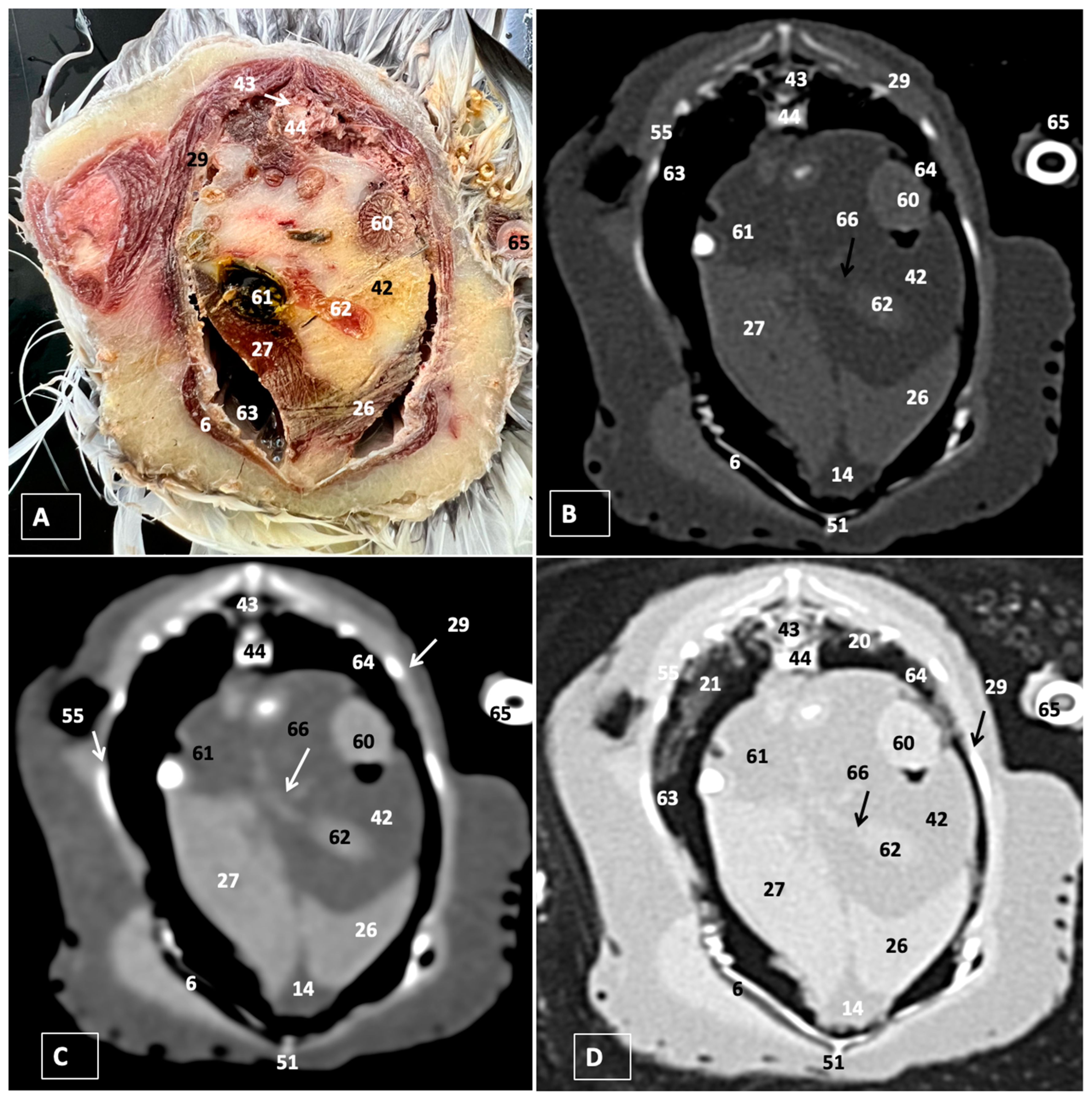
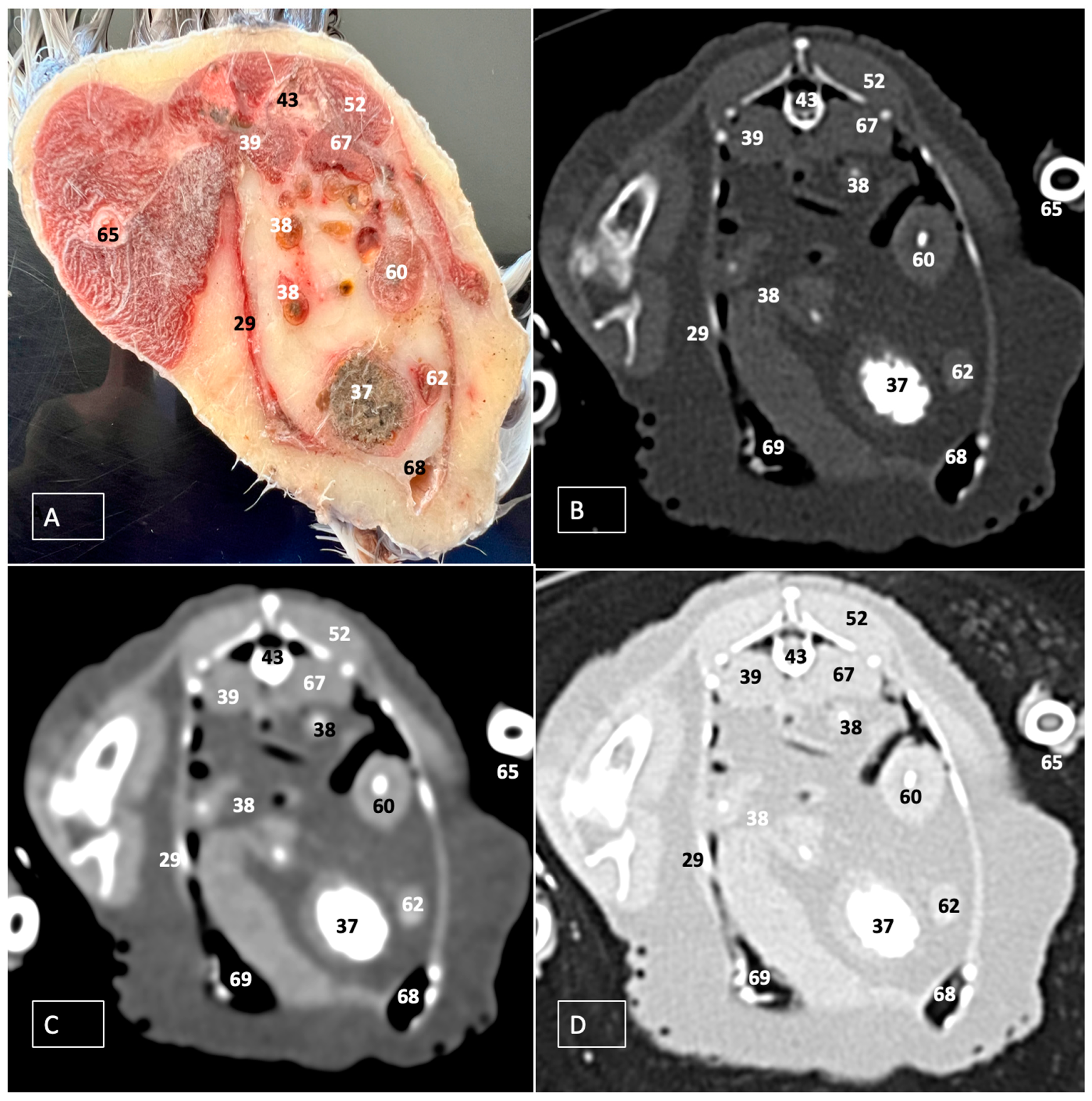
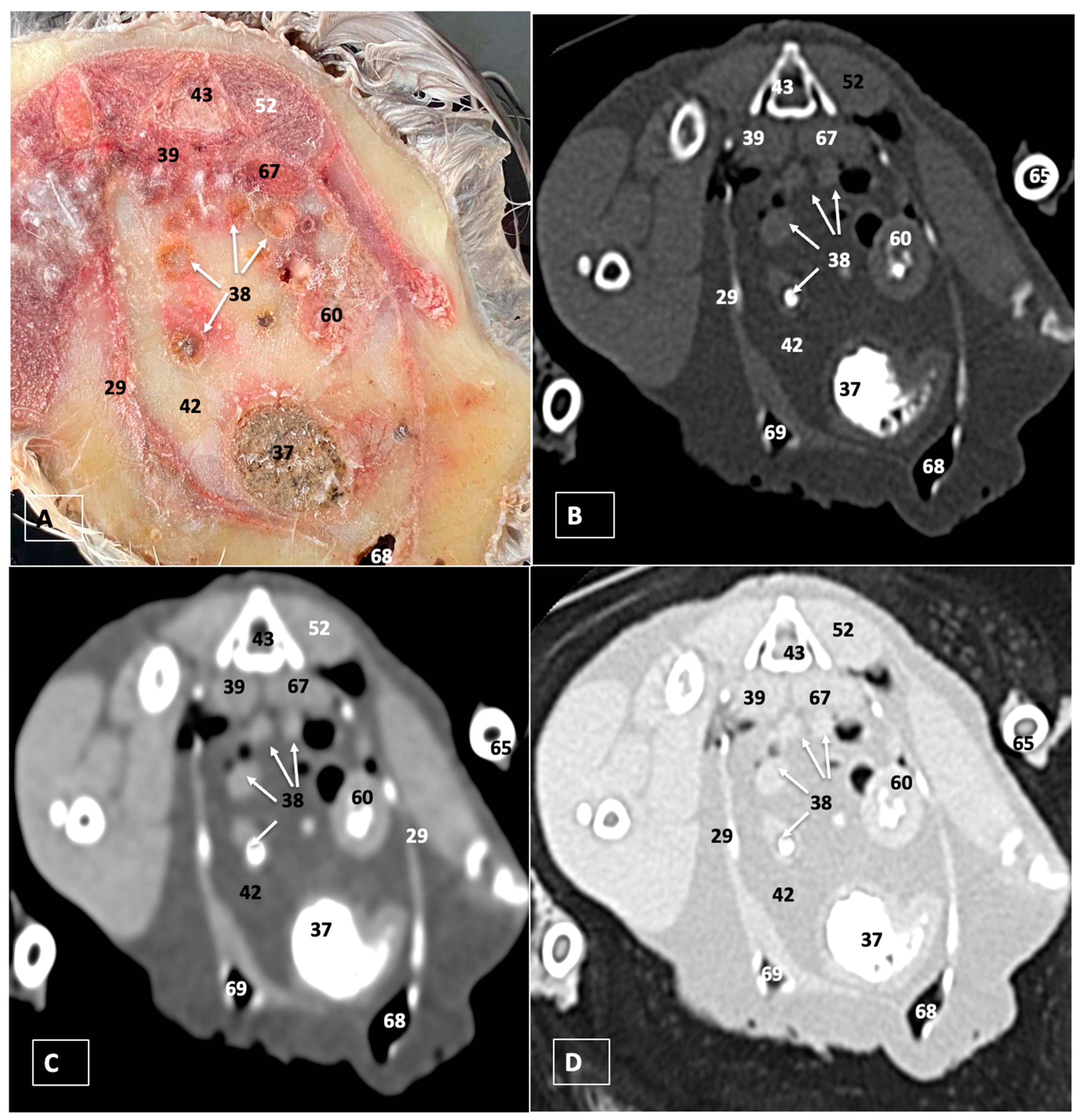
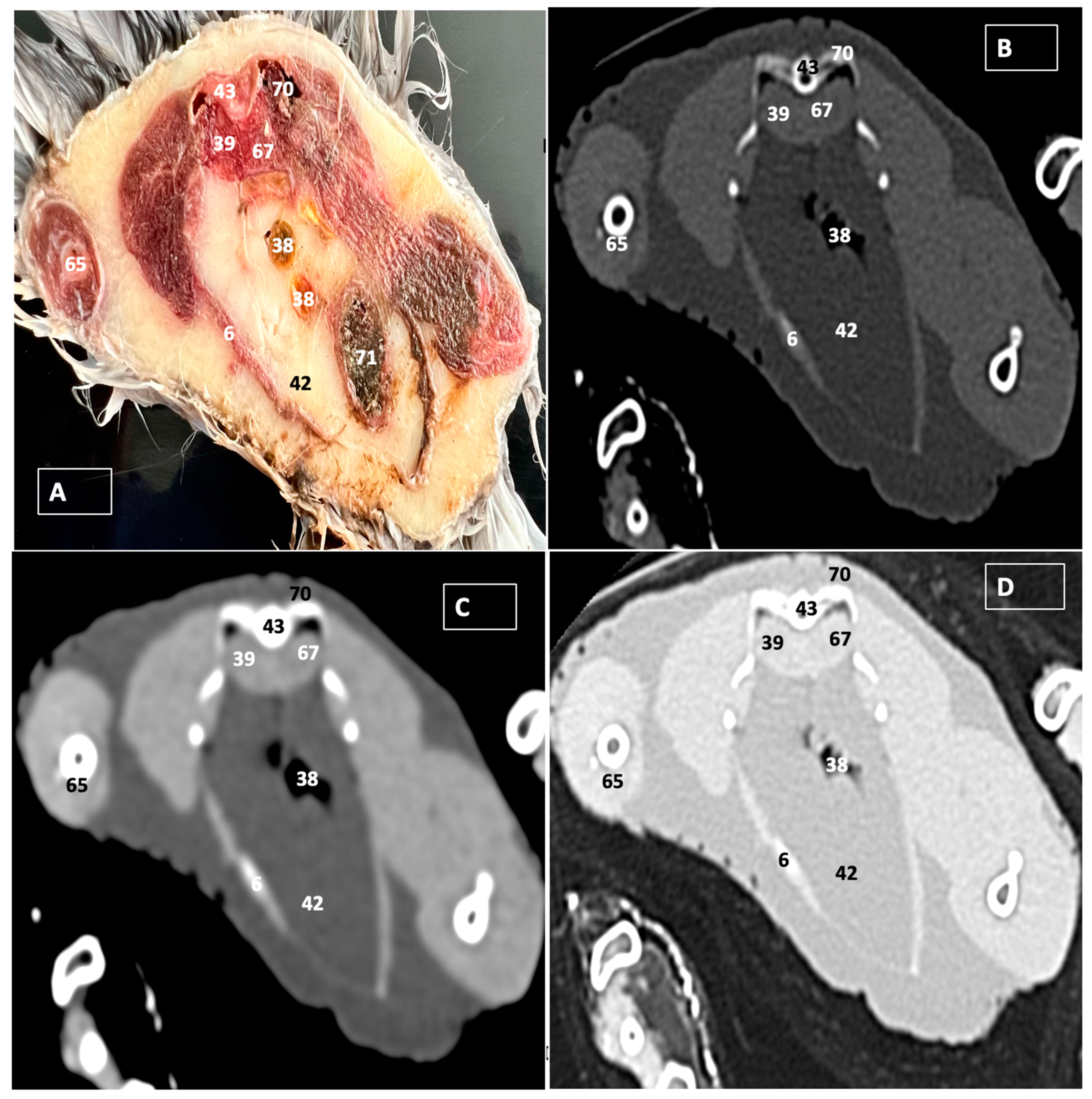
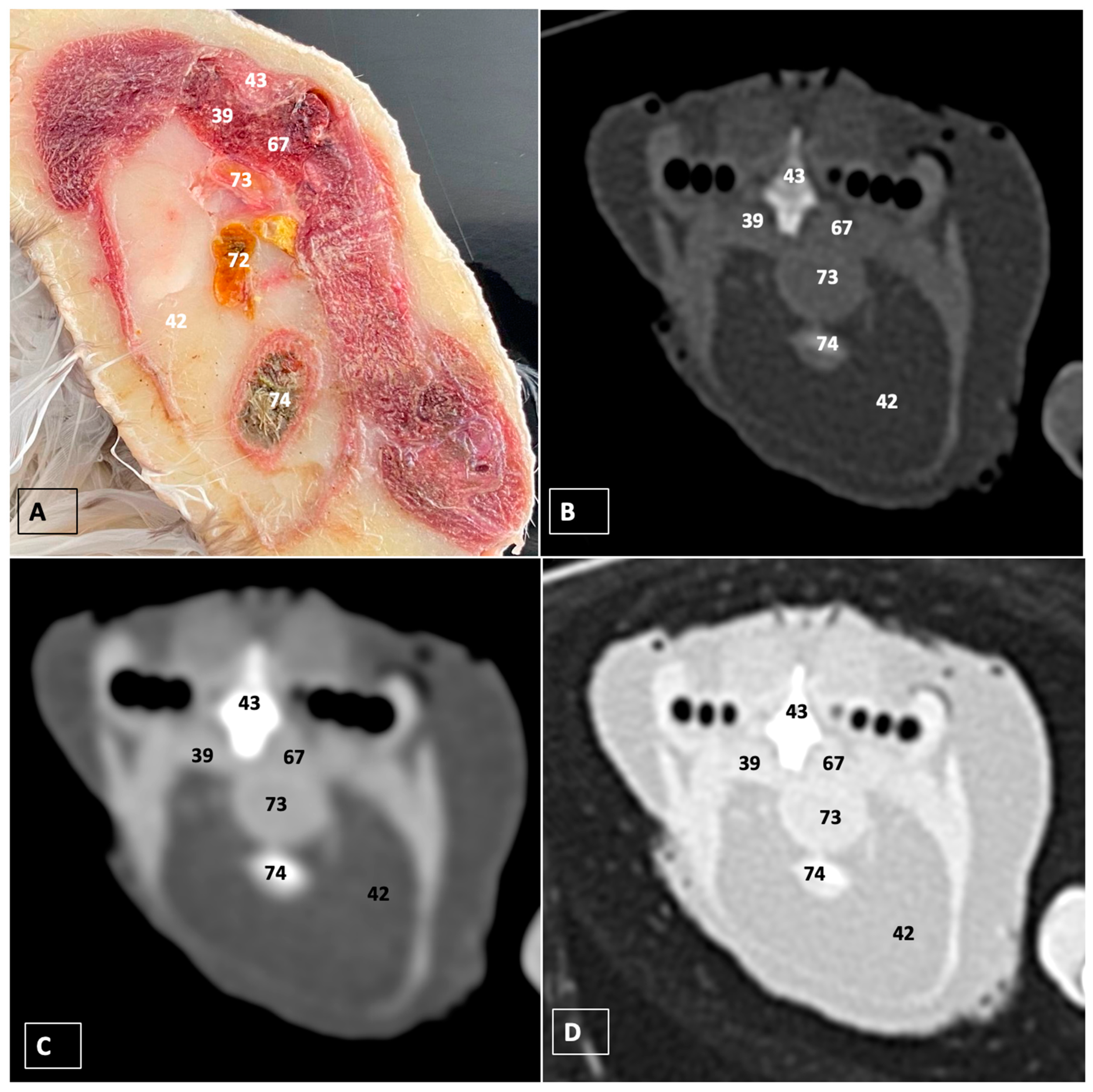
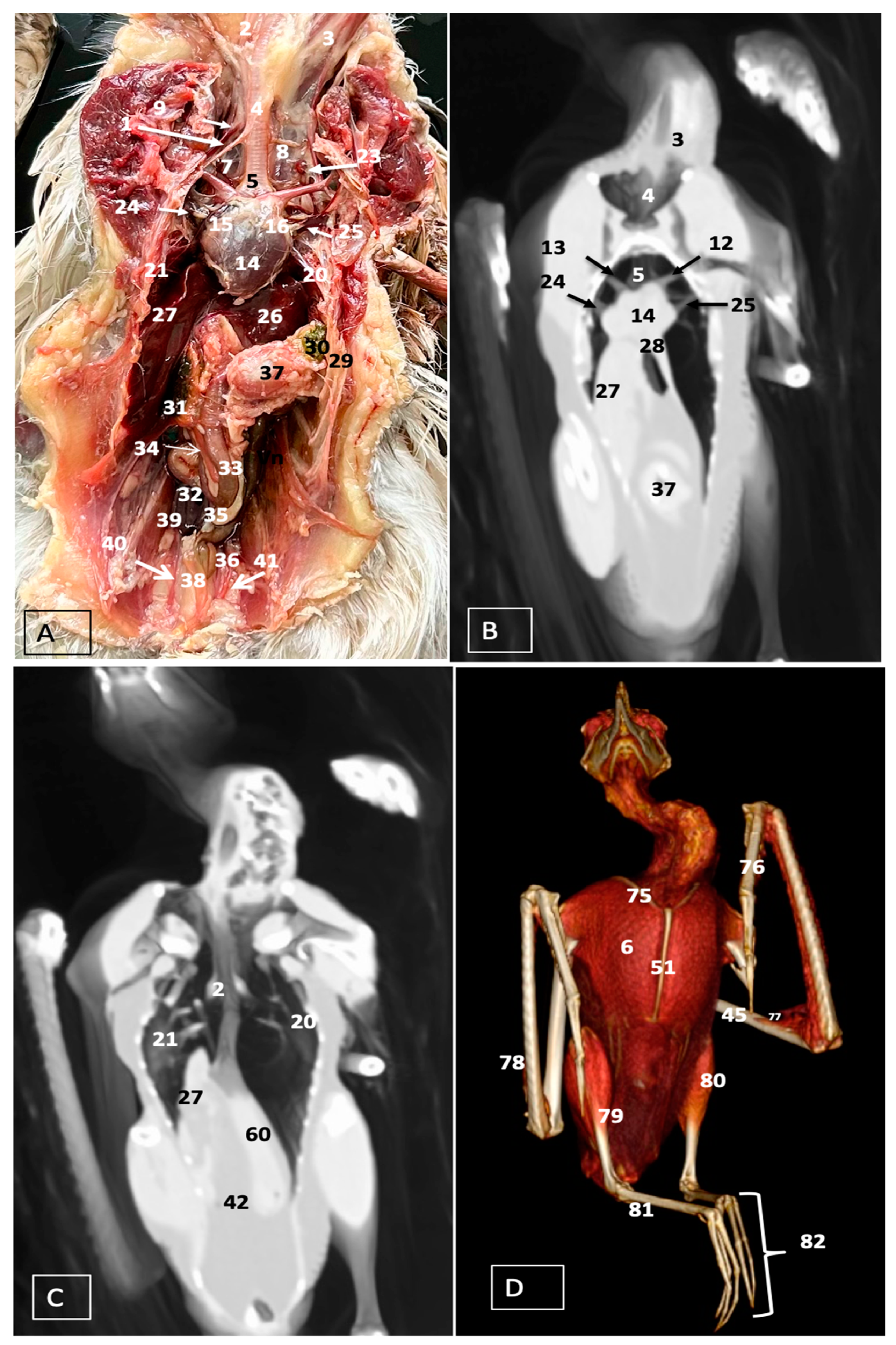
3.1. Anatomical Dissections and Cross-Sections
3.2. Computed Tomography Images
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pardela Cenicienta, Especie Clave para la Conservación de los Mares—SEO/BirdLife. Available online: https://seo.org/pardela-cenicienta-especie-clave-para-la-conservacion-de-los-mares/ (accessed on 1 January 2024).
- Pardela Cenicienta Mediterránea—SEO/BirdLife. Available online: https://seo.org/ave/pardela-cenicienta-mediterranea/ (accessed on 1 January 2024).
- (PDF) Pardela Cenicienta_Calonectris borealis borealis_. Available online: https://www.researchgate.net/publication/271271786_Pardela_Cenicienta_Calonectris_borealis_borealis (accessed on 1 January 2024).
- MIGRACIÓN Y ECOLOGÍA ESPACIAL DE LAS POBLACIONES ESPAÑOLAS DE PARDELA CENICIENTA. Available online: https://doi.org/10.31170/0056 (accessed on 1 January 2024).
- IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org/ (accessed on 1 February 2024).
- Konishi, M.; Emlen, S.T.; Ricklefs, R.E.; Wingfield, J.C. Contributions of Bird Studies to Biology. Science 1989, 246, 465–472. Available online: https://www.science.org/doi/10.1126/science.2683069 (accessed on 13 January 2024). [CrossRef] [PubMed]
- Current Therapy in Avian Medicine and Surgery|PDF|Veterinary Medicine|Pathology. Available online: https://es.scribd.com/document/384071318/Current-Therapy-in-Avian-Medicine-and-Surgery (accessed on 13 January 2024).
- Diagnostic Imaging of Exotic Pets: Birds, Small Mammals, Reptiles|VetBooks. Available online: https://vetbooks.ir/diagnostic-imaging-of-exotic-pets-birds-small-mammals-reptiles/ (accessed on 13 January 2024).
- Morales-Bordon, D.; Encinoso, M.; Arencibia, A.; Jaber, J.R. Cranial Investigations of Crested Porcupine (Hystrix cristata) by Anatomical Cross-Sections and Magnetic Resonance Imaging. Animals 2023, 13, 2551. Available online: https://www.mdpi.com/2076-2615/13/16/2551/htm (accessed on 13 January 2024). [CrossRef] [PubMed]
- Fumero-Hernández, M.; Quintana, M.E.; Ramírez, A.S.; Fariña, I.M.; Calabuig, P.; Jaber, J.R. Morphometric Study of the Eyeball of the Loggerhead Turtle (Caretta caretta) Using Computed Tomography (CT). Animals 2023, 13, 1016. Available online: https://sciprofiles.com/publication/view/9aad60d6772e5b8f24e899e325335034 (accessed on 13 January 2024). [CrossRef] [PubMed]
- Jaber, J.R.; Fumero-Hernández, M.; Corbera, J.A.; Morales, I.; Amador, M.; Ramírez Zarzosa, G.; Encinoso, M. Cross-Sectional Anatomy and Computed Tomography of the Coelomic Cavity in Juvenile Atlantic Puffins (Aves, Alcidae, Fratercula arctica). Animals 2023, 13, 2933. Available online: https://www.mdpi.com/2076-2615/13/18/2933/htm (accessed on 13 January 2024). [CrossRef] [PubMed]
- Abraham, M.E.; Wakamatsu, N.; Lossie, G.A.; Karcher, D.M.; Heng, H.G.; Murakami, M. Computed tomographic and magnetic resonance imaging anatomy of the coelomic cavity in market-age commercial Pekin Ducks (Anas platyrhynchos domesticus). Anat. Histol. Embryol. 2023, 52, 843–861. Available online: https://pubmed.ncbi.nlm.nih.gov/37357377/ (accessed on 11 January 2024). [CrossRef]
- Alonso-Farré, J.M.; Gonzalo-Orden, M.; Barreiro-Vázquez, J.D.; Ajenjo, J.M.; Barreiro-Lois, A.; Llarena-Reino, M.; Degollada, E. Cross-sectional anatomy, computed tomography and magnetic resonance imaging of the thoracic region of common dolphin (Delphinus delphis) and striped dolphin (Stenella coeruleoalba). J. Vet. Med. Ser. C Anat. Histol. Embryol. 2014, 43, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Tanaka, T.; Kiyatake, I.; Izawa, T.; Furuya, M.; Sasai, K. Contrast-enhanced computed tomography and cross-sectional anatomy of the trunk in the brownbanded bamboo shark (Chiloscyllium punctatum). J. Vet. Med. Ser. C Anat. Histol. Embryol. 2023, 52, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Banzato, T.; Selleri, P.; Veladiano, I.A.; Martin, A.; Zanetti, E.; Zotti, A. Comparative evaluation of the cadaveric, radiographic and computed tomographic anatomy of the heads of green iguana (Iguana iguana), common tegu (Tupinambis merianae) and bearded dragon (Pogona vitticeps). BMC Vet. Res. 2012, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Banzato, T.; Selleri, P.; Veladiano, I.A.; Zotti, A. Comparative evaluation of the cadaveric and computed tomographic features of the coelomic cavity in the green iguana (Iguana iguana), black and white tegu (Tupinambis merianae) and bearded dragon (Pogona vitticeps). J. Vet. Med. Ser. C Anat. Histol. Embryol. 2013, 42, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Veladiano, I.A.; Banzato, T.; Bellini, L.; Montani, A.; Catania, S.; Zotti, A. Computed tomographic anatomy of the heads of blue-and-gold macaws (Ara ararauna), African grey parrots (Psittacus erithacus), and monk parakeets (Myiopsitta monachus). Am. J. Vet. Res. 2016, 77, 1346–1356. [Google Scholar] [CrossRef] [PubMed]
- Veladiano, I.A.; Banzato, T.; Bellini, L.; Montani, A.; Catania, S.; Zotti, A. Normal computed tomographic features and reference values for the coelomic cavity in pet parrots. BMC Vet. Res. 2016, 12, 182. Available online: https://pubmed.ncbi.nlm.nih.gov/27596377/ (accessed on 11 January 2024). [CrossRef] [PubMed]
- dos Santos, G.J.; da Silva, J.P.; Hippólito, A.G.; Ferro, B.S.; Oliveira, E.L.R.; Okamoto, P.T.C.G.; Lourenço, M.L.G.; de Vasconcelos Machado, V.M.; Rahal, S.C.; Teixeira, C.R.; et al. Computed tomographic and radiographic morphometric study of cardiac and coelomic dimensions in captive blue-fronted Amazon parrots (Amazona aestiva, Linnaeus, 1758) with varying body condition scores. Anat. Histol. Embryol. 2020, 49, 299–306. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/ahe.12528 (accessed on 11 January 2024). [CrossRef] [PubMed]
- da Silva, J.P.; Rahal, S.C.; Castiglioni, M.C.R.; Baldissera Gonçalves, R.A.; Doiche, D.P.; Moresco, A.; Mamprim, M.J.; Vulcano, L.C. Radiography and computed tomography of the heart and lower respiratory tract in toco toucans (Ramphastos toco). Anat. Histol. Embryol. 2020, 49, 541–549. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/ahe.12559 (accessed on 11 January 2024). [CrossRef] [PubMed]
- Sisson: Anatomía de los Animales Domésticos v....—Google Académico. Available online: https://scholar.google.com/scholar_lookup?title=Anatom%C3%ADa+de+los+Animales+Dom%C3%A9sticos&author=Sisson,+S.&author=Getty,+R.&author=Grossman,+J.D.&publication_year=1982 (accessed on 13 January 2024).
- König, H.; Liebich, H.; Bragulla, H. Anatomie und Propädeutik des Geflügels: Lehrbuch und Farbatlas für Studium und Praxis. Available online: https://cir.nii.ac.jp/crid/1130000798308564608 (accessed on 13 January 2024).
- Pepperberg, I.M.; Howell, K.S.; Banta, P.A.; Patterson, D.K.; Meister, M. Measurement of Grey Parrot (Psittacus erithacus) Trachea via Magnetic Resonance Imaging, Dissection, and Electron Beam Computed Tomography. J. Morphol. 1998, 238, 81–91. Available online: https://onlinelibrary.wiley.com/doi/10.1002/ (accessed on 13 January 2024). [CrossRef]
- Romagnano, A.; Shiroma, J.T.; Heard, D.J.; Johnson, R.D.; Schiering, M.R.; Mladinich, C.R.J. MAGNETIC RESONANCE IMAGING OF THE BRAIN AND COELOMIC CAVITY OF THE DOMESTIC PIGEON (Columba livia domestica). Vet. Radiol. Ultrasound 1996, 37, 431–440. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/j.1740-8261.1996.tb01256.x (accessed on 13 January 2024). [CrossRef]
- Tomographic Anatomy of the Golden Eagle (Aquila chrysaetos) on JSTOR. Available online: https://www.jstor.org/stable/20460266?casa_token=7aagwF4LiQ0AAAAA%3AtUlvpojf3tSoTAhSYAv_2dZYtJfM93_YoeG0-lHGvqc6FXdqyb41AuSL427u5pj1Fjt4EfF2xldnJ7GYD7ikHoQFcP3mpC5V4xJzT4p478Z9mntXPjA (accessed on 13 January 2024).
- Sandoval, J. Anatomía Veterinaria; Imprenta Moderna: Logroño, Spain, 2003. [Google Scholar]
- Dyce, K.M.; Aja Guardiola, S.; Morales Saavedra, J.L.; Palacios Martínez, J.R.; Wensing, C.J.G.; Sack, W.O. Anatomía Veterinaria. Available online: https://books.google.com/books/about/Anatom%C3%ADa_veterinaria.html?hl=es&id=0AwwCgAAQBAJ (accessed on 10 December 2023).
- König, H.E.; Korbel, R.; Liebich, H.G.; Bragulla, H.; Klupiec, C. Avian Anatomy : Textbook and Colour Atlas; 5m Publishing: Sheffield, UK, 2016; 340p. [Google Scholar]
- MacLelland, J. Atlas en Color de Anatomía de las Aves. 1992. Available online: https://dialnet.unirioja.es/servlet/libro?codigo=157364 (accessed on 7 January 2024).
- Arencibia, A.; Corbera, J.A.; Ramírez, G.; Díaz-bertrana, M.L.; Pitti, L.; Morales, M.; Jaber, J.R. Anatomical Assessment of the Thorax in the Neonatal Foal Using Computed Tomography Angiography, Sectional Anatomy, and Gross Dissections. Animals 2020, 10, 1045. Available online: https://pubmed.ncbi.nlm.nih.gov/32560487/ (accessed on 1 February 2024). [CrossRef] [PubMed]
- Paré, J.A.; Veterinarian, S. BSAVA Manual of Psittacine Birds, 2nd ed. Can. Vet. J. 2008, 49, 268. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2249719/ (accessed on 1 February 2024).
- Fumero-Hernández, M.; Encinoso, M.; Melian, A.; Nuez, H.A.; Salman, D.; Jaber, J.R. Cross Sectional Anatomy and Magnetic Resonance Imaging of the Juvenile Atlantic Puffin Head (Aves, Alcidae, Fratercula arctica). Animals 2023, 13, 3434. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Rodríguez, B.; Negro, J. GPS tracking for mapping seabird mortality induced by light pollution. Sci. Rep. 2015, 5, 10670. [Google Scholar] [CrossRef] [PubMed]
- Imber, M.J. Behaviour of petrels in relation to the moon and artificial lights. Notornis 1975, 22, 302–306. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales Espino, A.; Déniz, S.; Fumero-Hernández, M.; Encinoso, M.; Calabuig, P.; Conde-Felipe, M.; Jaber, J.R. A Cadaveric Study Using Anatomical Cross-Section and Computed Tomography for the Coelomic Cavity in Juvenile Cory’s Shearwater (Aves, Procellariidae, Calonectris borealis). Animals 2024, 14, 858. https://doi.org/10.3390/ani14060858
Morales Espino A, Déniz S, Fumero-Hernández M, Encinoso M, Calabuig P, Conde-Felipe M, Jaber JR. A Cadaveric Study Using Anatomical Cross-Section and Computed Tomography for the Coelomic Cavity in Juvenile Cory’s Shearwater (Aves, Procellariidae, Calonectris borealis). Animals. 2024; 14(6):858. https://doi.org/10.3390/ani14060858
Chicago/Turabian StyleMorales Espino, Alejandro, Soraya Déniz, Marcos Fumero-Hernández, Mario Encinoso, Pascual Calabuig, Magnolia Conde-Felipe, and José Raduan Jaber. 2024. "A Cadaveric Study Using Anatomical Cross-Section and Computed Tomography for the Coelomic Cavity in Juvenile Cory’s Shearwater (Aves, Procellariidae, Calonectris borealis)" Animals 14, no. 6: 858. https://doi.org/10.3390/ani14060858
APA StyleMorales Espino, A., Déniz, S., Fumero-Hernández, M., Encinoso, M., Calabuig, P., Conde-Felipe, M., & Jaber, J. R. (2024). A Cadaveric Study Using Anatomical Cross-Section and Computed Tomography for the Coelomic Cavity in Juvenile Cory’s Shearwater (Aves, Procellariidae, Calonectris borealis). Animals, 14(6), 858. https://doi.org/10.3390/ani14060858






