Effect of Fixatives and Fixation Period on Morphology and Immunohistochemistry of Feline Ovarian Tissue
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Ovary Transport and Preparation of Ovarian Fragments
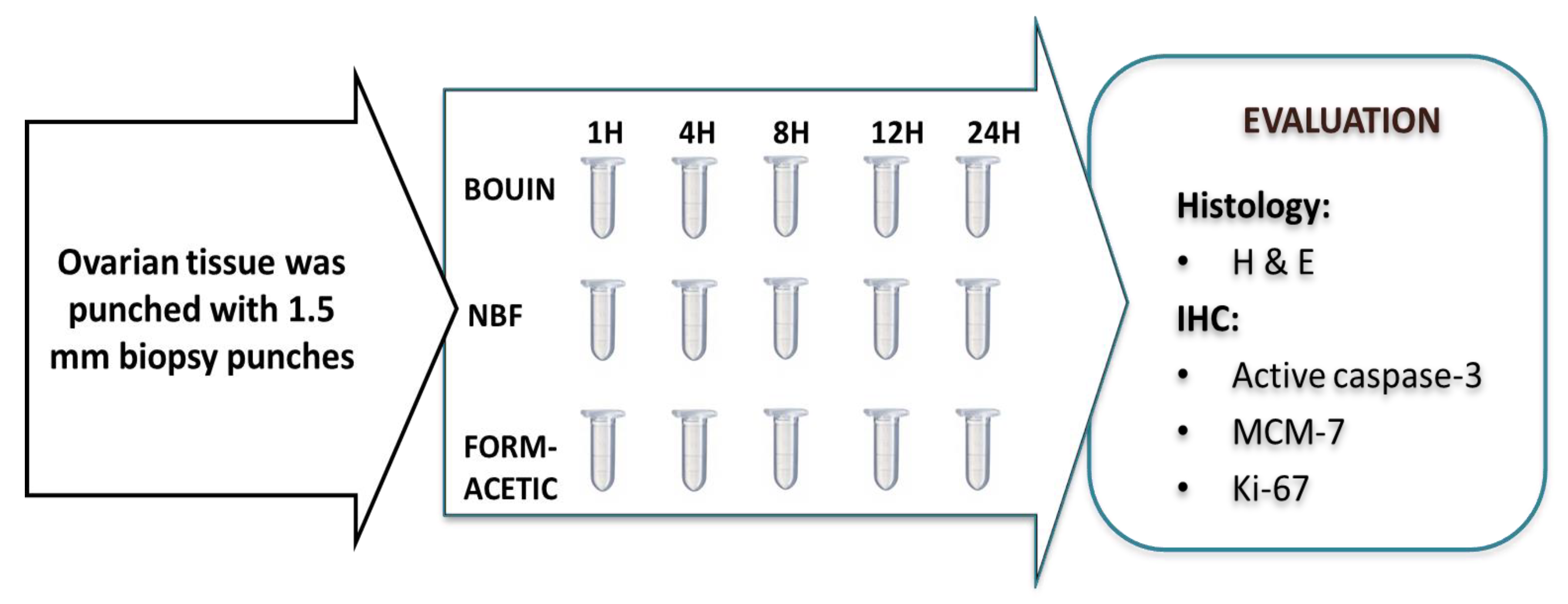
2.3. Experimental Design
2.4. Histology
Image Analysis (Hematoxylin and Eosin)
2.5. Immunohistochemistry
Image Analysis (Immunohistochemistry)
2.6. Statistical Analysis
3. Results
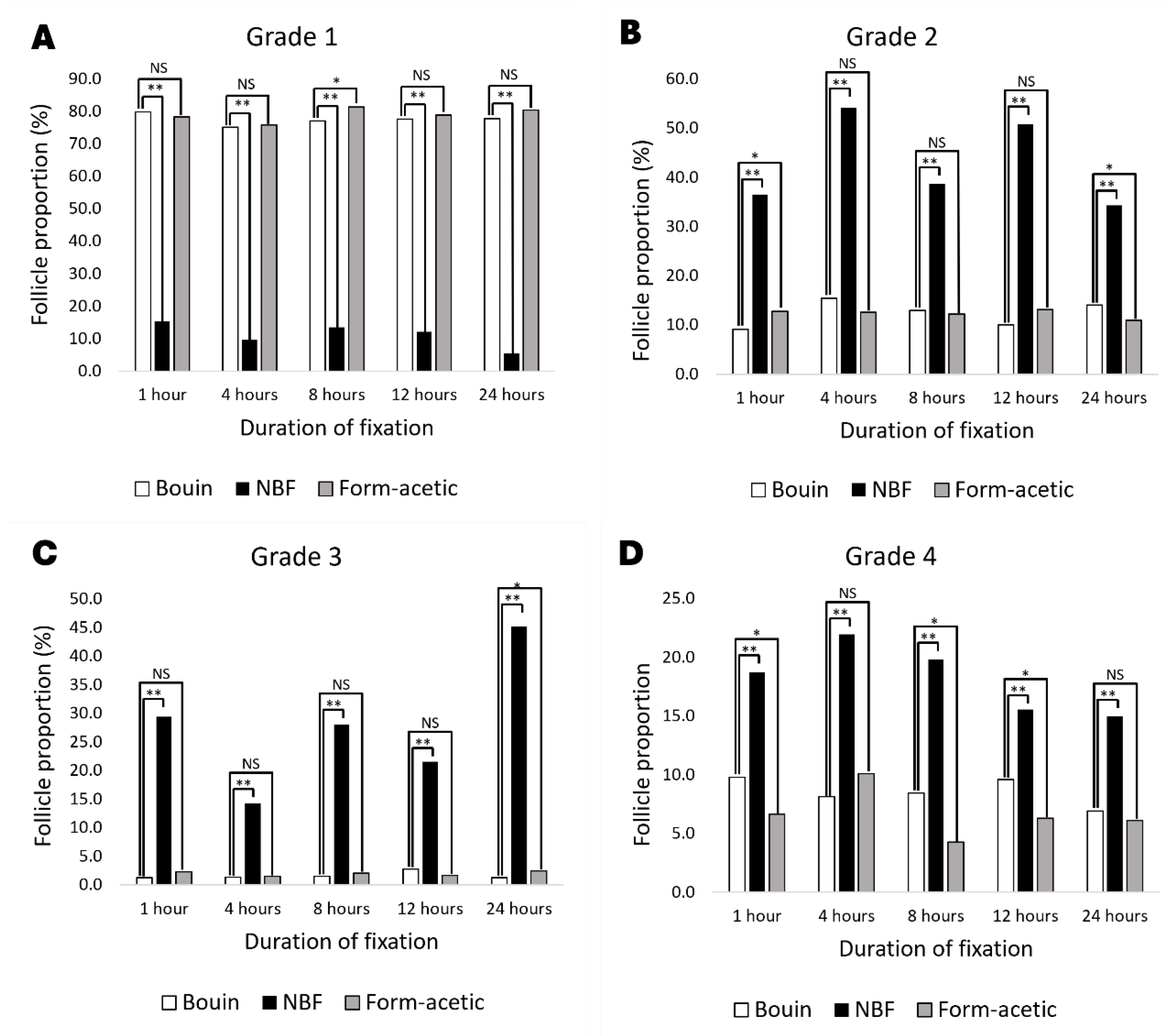
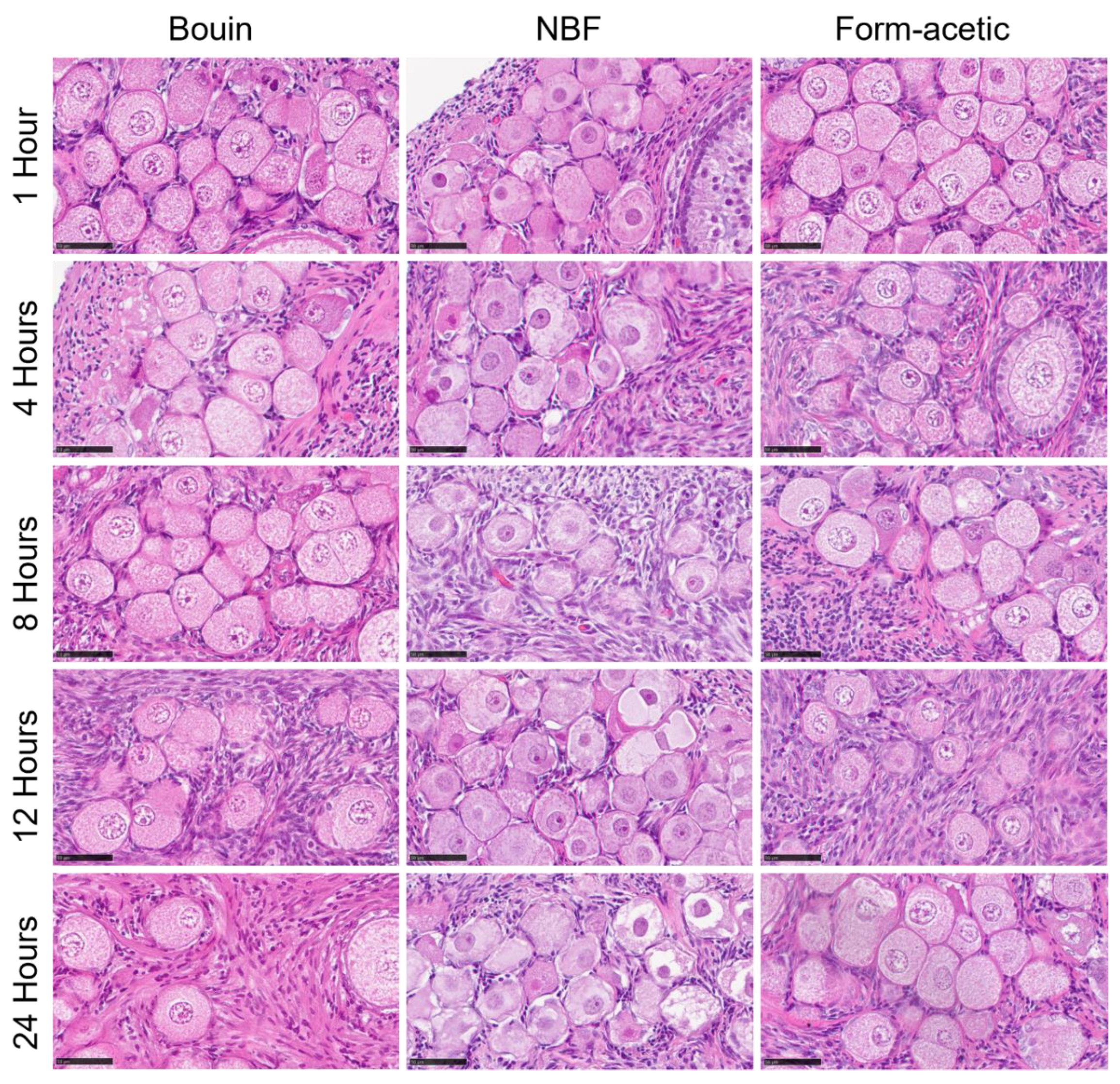
3.1. Histology
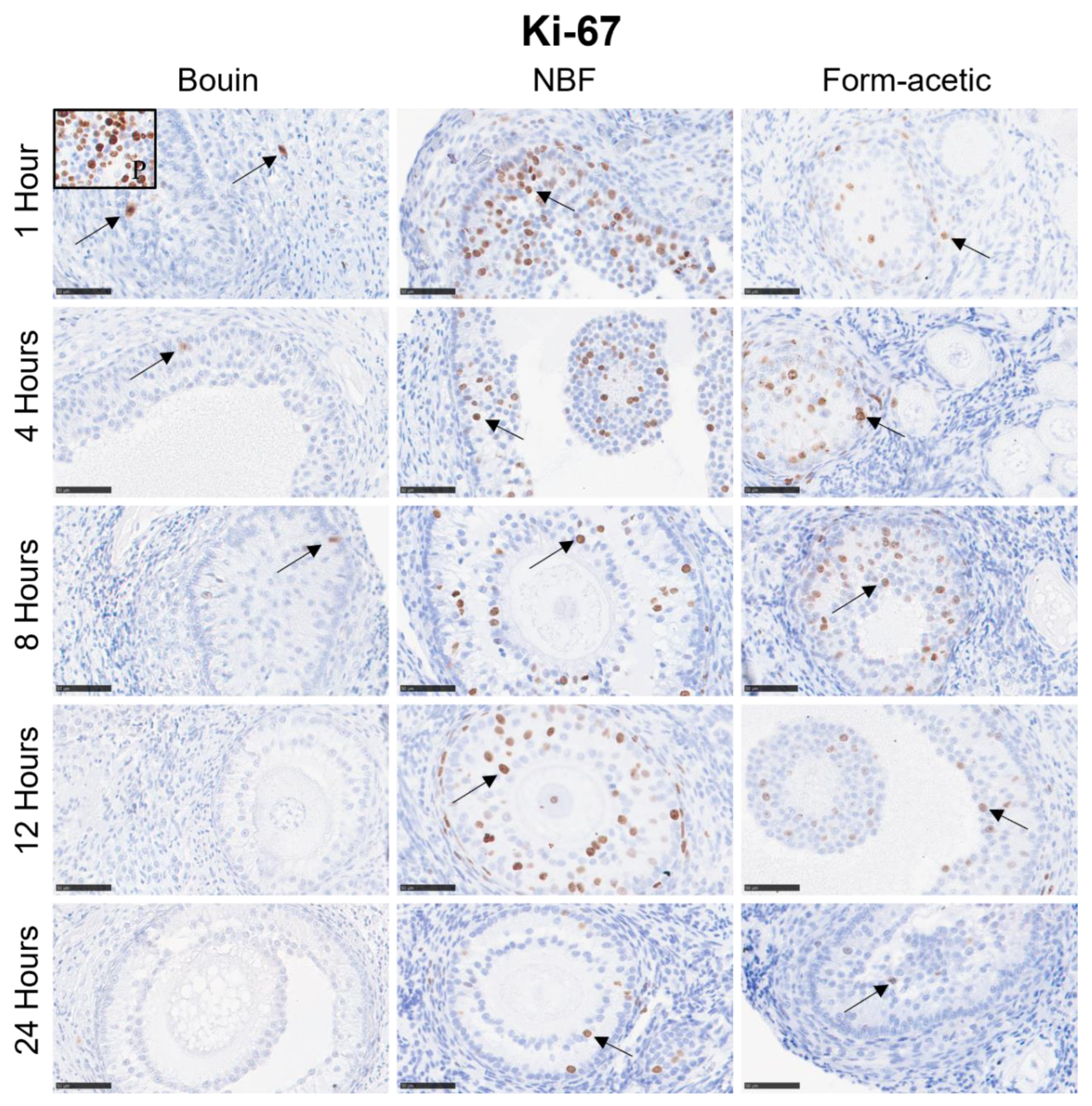
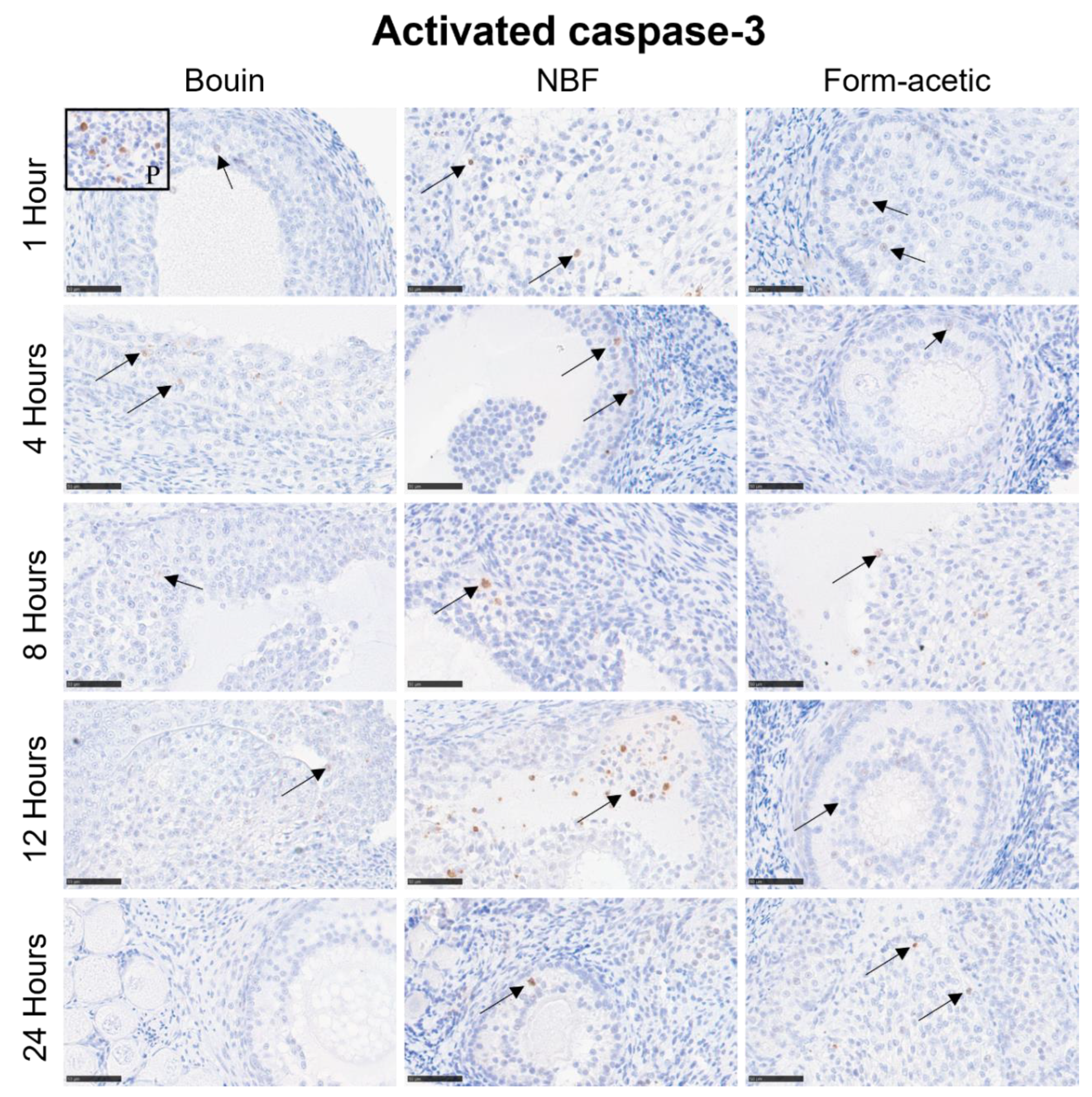
3.2. Immunohistochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macklon, K.T. Cryopreservation of Ovarian Tissue Works, but Challenges Remain. Fertil. Steril. 2020, 114, 281–282. [Google Scholar] [CrossRef]
- Duffin, K.; Howie, R.; Kelsey, T.W.; Wallace, H.B.; Anderson, R.A. Long-Term Follow-up to Assess Criteria for Ovarian Tissue Cryopreservation for Fertility Preservation in Young Women and Girls with Cancer. Hum. Reprod. 2023, 38, 1076–1085. [Google Scholar] [CrossRef]
- Comizzoli, P. Biobanking Efforts and New Advances in Male Fertility Preservation for Rare and Endangered Species. Asian J. Androl. 2015, 17, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Comizzoli, P. Integrating Fertility Preservation and Cryo-Banking into the Conservation of Rare and Endangered Deer Species. Anim. Prod. Sci. 2020, 60, 1227–1232. [Google Scholar] [CrossRef]
- Jewgenow, K.; Stolte, M. Isolation of Preantral Follicles from Nondomestic Cats—Viability and Ultrastructural Investigations. Anim. Reprod. Sci. 1996, 44, 183–193. [Google Scholar] [CrossRef]
- Jewgenow, K.; Zahmel, J. Preservation of Female Genetic Resources in Feline Species. Theriogenology 2020, 156, 124–129. [Google Scholar] [CrossRef]
- Singh, H.; Bishen, K.A.; Garg, D.; Sukhija, H.; Sharma, D.; Tomar, U. Fixation and Fixatives: Roles and Functions—A Short Review. Dent. J. Adv. Stud. 2019, 7, 51–55. [Google Scholar] [CrossRef]
- Howat, W.J.; Wilson, B.A. Tissue Fixation and the Effect of Molecular Fixatives on Downstream Staining Procedures. Methods 2014, 70, 12–19. [Google Scholar] [CrossRef]
- Brito, D.C.C.; Ñaupas, L.V.S.; Souza, S.S.; Alcântara, G.L.H.; Figueiredo, J.R.; Santos, R.R.; Rodrigues, A.P.R. Interference of Fixatives and Fixation Period on the Morphologic Analysis of Ovarian Preantral Follicles. Zygote 2022, 30, 144–147. [Google Scholar] [CrossRef]
- Groelz, D.; Sobin, L.; Branton, P.; Compton, C.; Wyrich, R.; Rainen, L. Non-Formalin Fixative Versus Formalin-Fixed Tissue: A Comparison of Histology and Rna Quality. Exp. Mol. Pathol. 2013, 94, 188–194. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; pp. 53–766. [Google Scholar]
- Paavilainen, L.; Edvinsson, Å.; Asplund, A.; Hober, S.; Kampf, C.; Pontén, F.; Wester, K. The Impact of Tissue Fixatives on Morphology and Antibody-Based Protein Profiling in Tissues and Cells. J. Histochem. Cytochem. 2010, 58, 237–246. [Google Scholar] [CrossRef]
- Fox, C.H.; Johnson, F.B.; Whiting, J.; Roller, P.P. Formaldehyde Fixation. J. Histochem. Cytochem. 1985, 33, 845–853. [Google Scholar] [CrossRef]
- Eltoum, I.; Fredenburgh, J.; Myers, R.B.; Grizzle, W.E. Introduction to the Theory and Practice of Fixation of Tissues. J. Histotechnol. 2001, 24, 173–190. [Google Scholar] [CrossRef]
- Buesa, R.J. Histology without Formalin? Ann. Diagn. Pathol. 2008, 12, 387–396. [Google Scholar] [CrossRef]
- Haque, Z.; Rahman, M.A.; Khan, M.Z.I.; Hussan, M.T.; Alam, M.M. Alcohol-Based Fixatives Can Better Preserve Tissue Morphology Than Formalin. Int. J. Morphol. 2020, 38, 1371–1375. [Google Scholar] [CrossRef]
- Jalil, M.J.; Muhammad, A.S.; Salman, M.D. Histomorphometric Evaluation of Mice Testicular Tissue Fixed by Two Types of Fixatives. Iraqi J. Sci. 2017, 58, 1363–1370. [Google Scholar]
- Sompuram, S.R.; Vani, K.; Messana, E.; Bogen, S.A. A Molecular Mechanism of Formalin Fixation and Antigen Retrieval. Am. J. Clin. Pathol. 2004, 121, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Alkali, I.M.; Colombo, M.; Luciano, A.M.; Nizanski, W.; Ali Hassan, H.; Dziegiel, P.; Luvoni, G.C. Culture of Vitrified Bovine Ovarian Tissue on Agarose Gel Inserts Maintains Follicle Integrity. Reproduction 2023, 166, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Adeniran, B.V.; Bjarkadottir, B.D.; Appeltant, R.; Lane, S.; Williams, S.A. Improved Preservation of Ovarian Tissue Morphology That Is Compatible with Antigen Detection Using a Fixative Mixture of Formalin and Acetic Acid. Hum. Reprod. 2021, 36, 1871–1890. [Google Scholar] [CrossRef] [PubMed]
- Spencer, L.; Bancroft, J.; Bancroft, J.; Gamble, M. Tissue Processing. Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012; pp. 105–123. [Google Scholar]
- Golberg, M.; Wysiadecki, G.; Kobos, J.; Brzeziński, P.; Polguj, M.; Clarke, E.; Barszcz, K.; Balawender, K.; Radek, M.; Żytkowski, A. Application of Automated Immunohistochemistry in Anatomical Research: A Brief Review of the Method. Transl. Res. Anat. 2022, 28, 100211. [Google Scholar] [CrossRef]
- Juríková, M.; Danihel, Ľ.; Polák, Š.; Varga, I. Ki67, Pcna, and Mcm Proteins: Markers of Proliferation in the Diagnosis of Breast Cancer. Acta Histochem. 2016, 118, 544–552. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; Mcart, D.G.; Dunne, P.D.; Mcquaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. Qupath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Sarma, U.C.; Winship, A.L.; Hutt, K.J. Comparison of Methods for Quantifying Primordial Follicles in the Mouse Ovary. J. Ovarian Res. 2020, 13, 121. [Google Scholar] [CrossRef]
- Rahman, M.A.; Sultana, N.; Ayman, U.; Bhakta, S.; Afrose, M.; Afrin, M.; Haque, Z. Alcoholic Fixation over Formalin Fixation: A New, Safer Option for Morphologic and Molecular Analysis of Tissues. Saudi J. Biol. Sci. 2022, 29, 175–182. [Google Scholar] [CrossRef]
- Ananthanarayanan, V.; Pins, M.R.; Meyer, R.E.; Gann, P.H. Immunohistochemical Assays in Prostatic Biopsies Processed in Bouin’s Fixative. J. Clin. Pathol. 2005, 58, 322–324. [Google Scholar] [CrossRef]
- Dos Santos, J.T.; Silva-Santos, K.C.; Andrade, E.R.; Lisboa, L.A.; Schneider, C.L.; Ciquini, A.; Ferreira, R.; Da Nobrega Junior, J.E.; Seneda, M.M. Effect of Fixative Type and Fixation Time on the Morphology of Bovine Preantral Ovarian Follicles. Semin. Ciências Agrárias 2012, 33, 297–304. [Google Scholar]
- Howroyd, P.; Hoyle-Thacker, R.; Lyght, O.; Williams, D.; Kleymenova, E. Morphology of the Fetal Rat Testis Preserved in Different Fixatives. Toxicol. Pathol. 2005, 33, 300–304. [Google Scholar] [CrossRef]
- Latendresse, J.R.; Warbrittion, A.R.; Jonassen, H.; Creasy, D.M. Fixation of Testes and Eyes Using a Modified Davidson’s Fluid: Comparison with Bouin’s Fluid and Conventional Davidson’s Fluid. Toxicol. Pathol. 2002, 30, 524–533. [Google Scholar] [CrossRef]
- Akkoyunlu, G.; Tepekoy, F. Immunohistochemistry of Paraffin Sections from Mouse Ovaries. In Oogenesis: Methods Protocols; Humana Press: New York, NY, USA, 2016; pp. 269–274. [Google Scholar]
- Groelz, D.; Viertler, C.; Pabst, D.; Dettmann, N.; Zatloukal, K. Impact of Storage Conditions on the Quality of Nucleic Acids in Paraffin Embedded Tissues. PLoS ONE 2018, 13, e0203608. [Google Scholar] [CrossRef]
- Zanini, C.; Gerbaudo, E.; Ercole, E.; Vendramin, A.; Forni, M. Evaluation of Two Commercial and Three Home-Made Fixatives for the Substitution of Formalin: A Formaldehyde-Free Laboratory Is Possible. Environ. Health 2012, 11, 59. [Google Scholar] [CrossRef]
- Goldstein, N.S.; Ferkowicz, M.; Odish, E.; Mani, A.; Hastah, F. Minimum Formalin Fixation Time for Consistent Estrogen Receptor Immunohistochemical Staining of Invasive Breast Carcinoma. Am. J. Clin. Pathol. 2003, 120, 86–92. [Google Scholar] [CrossRef]
- Webster, J.D.; Miller, M.A.; Dusold, D.; Ramos-Vara, J. Effects of Prolonged Formalin Fixation on Diagnostic Immunohistochemistry in Domestic Animals. J. Histochem. Cytochem. 2009, 57, 753–761. [Google Scholar] [CrossRef]
- Van Seijen, M.; Brcic, L.; Gonzales, A.N.; Sansano, I.; Bendek, M.; Brcic, I.; Lissenberg-Witte, B.; Korkmaz, H.I.; Geiger, T.; Kammler, R.; et al. Impact of Delayed and Prolonged Fixation on the Evaluation of Immunohistochemical Staining on Lung Carcinoma Resection Specimen. Virchows Arch. 2019, 475, 191–199. [Google Scholar] [CrossRef]
- Werner, M.; Chott, A.; Fabiano, A.; Battifora, H. Effect of Formalin Tissue Fixation and Processing on Immunohistochemistry. Am. J. Surg. Pathol. 2000, 24, 1016–1019. [Google Scholar] [CrossRef]
- Grigorev, I.; Korzhevskii, D. Current Technologies for Fixation of Biological Material for Immunohistochemical Analysis. Coвpeмeнныe Texнoлoгии в Мeдицинe 2018, 10, 156–164. [Google Scholar] [CrossRef]
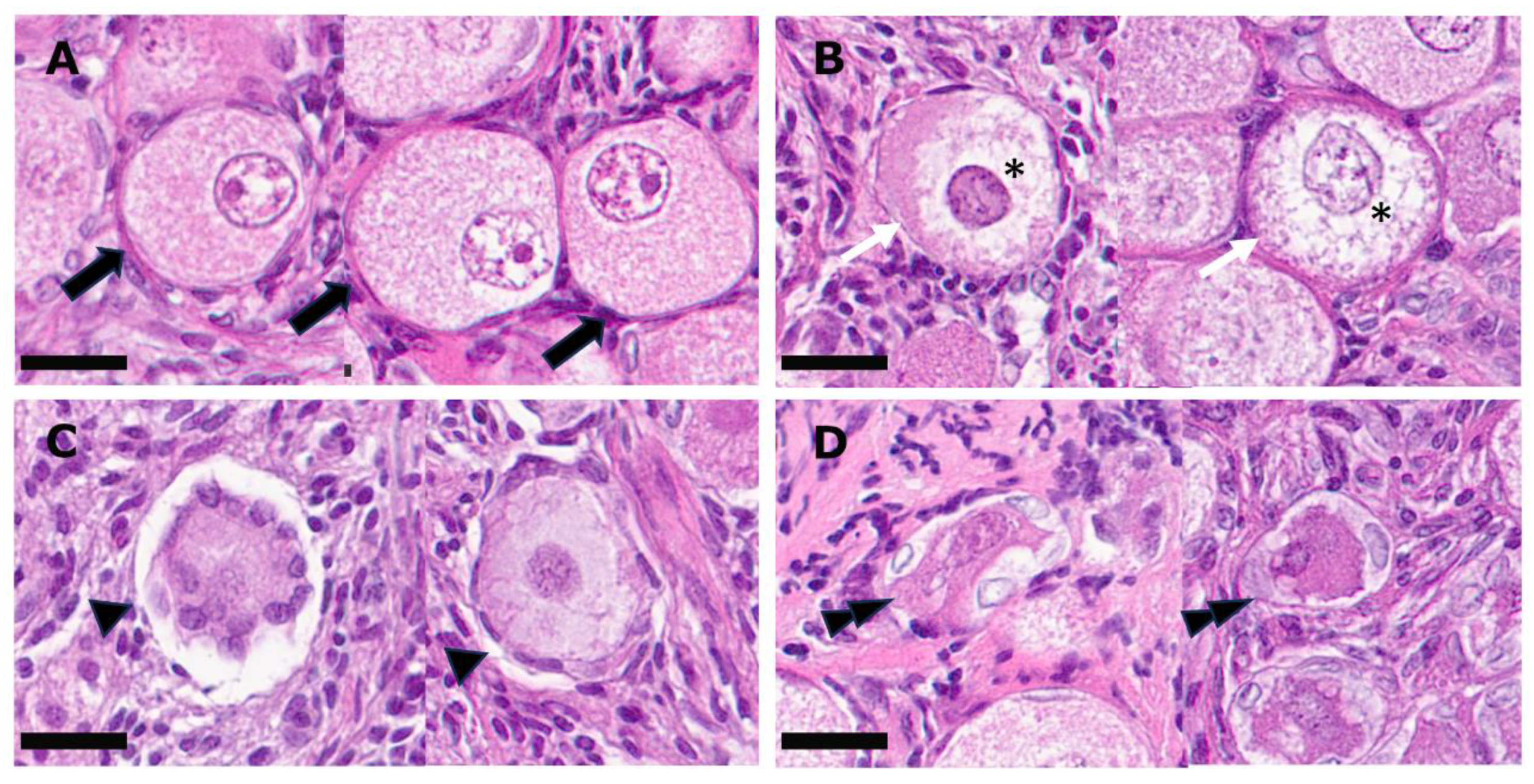


| Follicle | Description |
|---|---|
| Grade 1 | Follicle is spherical in shape, evenly distributed follicular cells, intact stroma, spherical oocyte, intact nucleus and nucleolus with homogenous cytoplasm. |
| Grade 2 | Follicle is spherical in shape, evenly distributed follicular cells, intact stroma and spherical oocyte, misshapen nucleus and/or not homogenous cytoplasm. |
| Grade 3 | Follicular cells pulled away from the stroma but oocyte spherical. |
| Grade 4 | Follicular cells pulled away from the stroma and/or oocyte misshapen, vacuolated, pyknotic nucleus and/or disorganized granulosa cells. |
| Primordial | Oocyte surrounded by flattened follicular cells. |
| Transitional | Some flattened follicular cells have been converted to cuboidal cells. |
| Primary | Oocyte surrounded by cuboidal follicular cells. |
| Secondary | Antral space appears. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkali, I.M.; Colombo, M.; Rodak, O.; Nizanski, W.; Luvoni, G.C. Effect of Fixatives and Fixation Period on Morphology and Immunohistochemistry of Feline Ovarian Tissue. Animals 2024, 14, 825. https://doi.org/10.3390/ani14060825
Alkali IM, Colombo M, Rodak O, Nizanski W, Luvoni GC. Effect of Fixatives and Fixation Period on Morphology and Immunohistochemistry of Feline Ovarian Tissue. Animals. 2024; 14(6):825. https://doi.org/10.3390/ani14060825
Chicago/Turabian StyleAlkali, Isa Mohammed, Martina Colombo, Olga Rodak, Wojciech Nizanski, and Gaia Cecilia Luvoni. 2024. "Effect of Fixatives and Fixation Period on Morphology and Immunohistochemistry of Feline Ovarian Tissue" Animals 14, no. 6: 825. https://doi.org/10.3390/ani14060825
APA StyleAlkali, I. M., Colombo, M., Rodak, O., Nizanski, W., & Luvoni, G. C. (2024). Effect of Fixatives and Fixation Period on Morphology and Immunohistochemistry of Feline Ovarian Tissue. Animals, 14(6), 825. https://doi.org/10.3390/ani14060825







