Pre-Harvest Food Safety Challenges in Food-Animal Production in Low- and Middle-Income Countries
Abstract
Simple Summary
Abstract
1. Introduction
2. Overview of Food Safety Legislative and Regulatory Frameworks and Implementation Challenges in LMIC
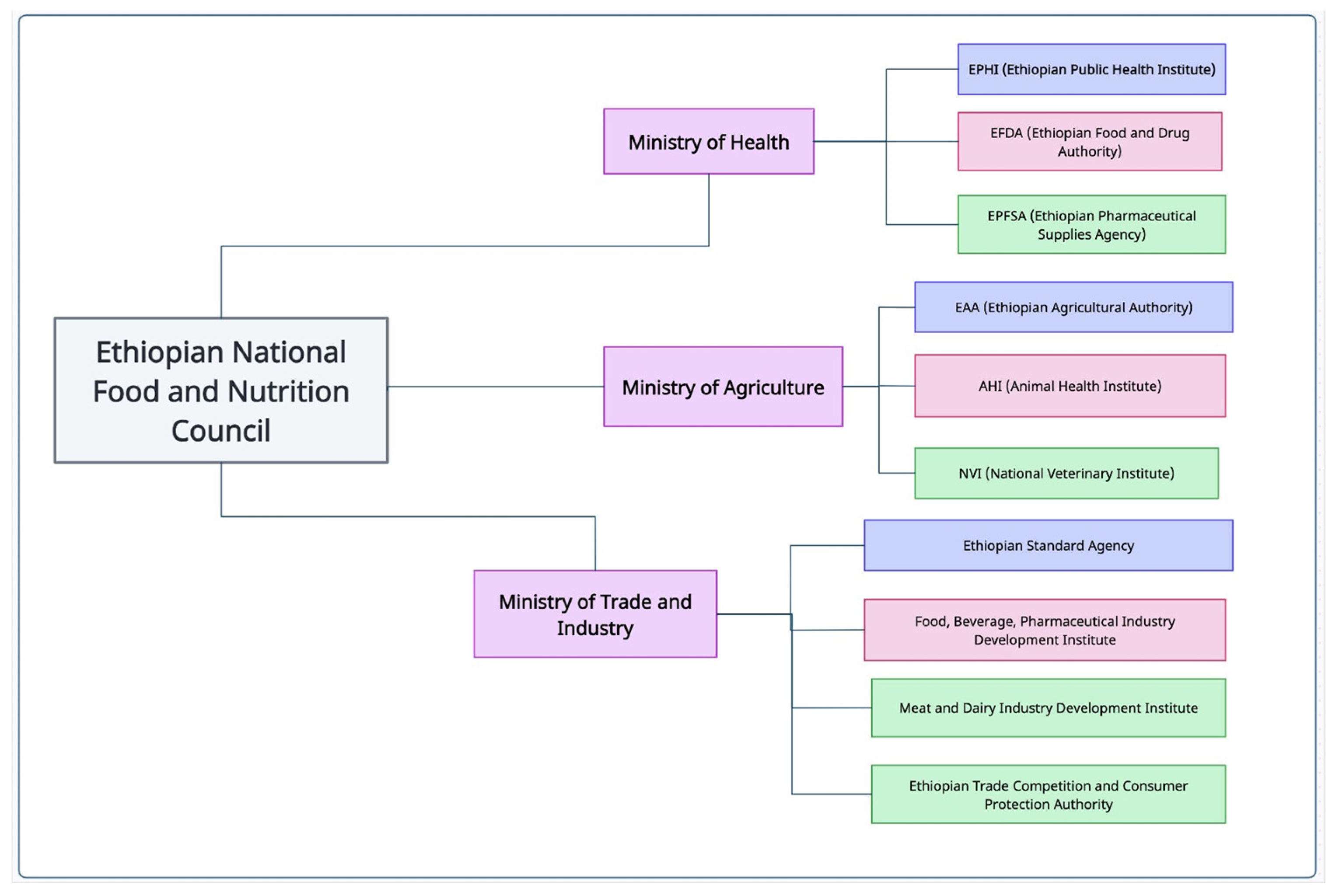
3. Critical Food Safety Issues at Farm Level in LMIC
3.1. Animal Health: Implication on Food Safety
3.2. Antibiotic Resistance as a Food Safety Issue at Farm Level
3.3. Implication of Knowledge, Attitude, and Practice of Farmers on Food Safety
4. Intervention Aiming Food Safety Problems at LMIC and Their Outcomes
5. One Health Approach: A Key Strategy for Mitigating Pre-Harvest Food Safety Risks
6. Conclusions
7. Way Forward
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fred, F.; Huei-Shyong, W.; Suresh, M. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef]
- World Health Organization. Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/199350/9789241565165_eng.pdf (accessed on 4 August 2023).
- World Health Organization. Global Strategy for Food Safety 2022–2030: Towards Stronger Food Safety Systems and Global Cooperation. Geneva: World Health Organization; 2021. License: CC BY-NC-SA 3.0 IGO. Available online: https://www.who.int/publications/i/item/9789240057685 (accessed on 7 March 2023).
- Newman, K.L.; Leon, J.S.; Rebolledo, P.A.; Scallan, E. The impact of socioeconomic status on foodborne illness in high-income countries: A systematic review. Epidemiol. Infect. 2015, 143, 2473–2485. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pires, S.M.; Liu, Z.; Ma, X.; Guo, Y. Surveillance of foodborne disease outbreaks in china, 2003–2017. Food Control 2020, 118, 107359. [Google Scholar] [CrossRef]
- Wang, S.K.; Fu, L.M.; Chen, G.W.; Xiao, H.M.; Pan, D.; Shi, R.F.; Yang, L.G.; Sun, G.J. Multisite survey of bacterial contamination in ready-to-eat meat products throughout the cooking and selling processes in urban supermarket, Nanjing, China. Food Sci. Nutr. 2020, 8, 2427–2435. [Google Scholar] [CrossRef]
- Grace, D. Burden of foodborne disease in low-income and middle-income countries and opportunities for scaling food safety interventions. Food Sec. 2023, 15, 1475–1488. [Google Scholar] [CrossRef]
- Grace, D. Food Safety in Low-and Middle-Income Countries. Int. J. Environ. Res. Public Health 2015, 12, 10490–10507. [Google Scholar] [CrossRef]
- World Health Organization (WHO) Food Safety. Key Facts. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 18 November 2023).
- Herrero, M.; Grace, D.; Njuki, J.; Johnson, N.; Enahoro, D.; Silvestri, S.; Rufino, M.C. The Roles of livestock in developing countries. Animals 2013, 7, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Fanzo, J.; Rudie, C.; Sigman, I.; Grinspoon, S.; Benton, T.G.; Brown, M.E.; Covic, N.; Fitch, K.; Golden, C.D.; Grace, D.; et al. Sustainable food systems and nutrition in the 21st century: A report from the 22nd annual Harvard Nutrition Obesity Symposium. Am. J. Clin. Nutr. 2022, 115, 18–33. [Google Scholar] [CrossRef]
- Alders, R.G.; Campbell, A.; Costa, R.; Guèye, E.F.; Ahasanul Hoque, M.; Perezgrovas-Garza, R.; Rota, A.; Wingett, K. Livestock across the world: Diverse animal species with complex roles in human societies and ecosystem services. Anim. Front. 2021, 11, 20–29. [Google Scholar] [CrossRef]
- Maria, D.D.; Ram, M.H. Leveraging Livestock Production Systems for Human Nutrition in Developing Countries. Animal Husbandry. 2022. Available online: https://www.intechopen.com/chapters/79464 (accessed on 4 December 2023). [CrossRef]
- Steinfeld, H.; Wassenaar, T.; Jutzi, S. Livestock production systems in developing countries: Status, drivers, trends. Rev. Sci. Tech. Int. Epiz. 2006, 25, 505–516. [Google Scholar] [CrossRef]
- Jouve, J.L. Principles of food safety legislation. Food Control 1998, 9, 75–81. [Google Scholar] [CrossRef]
- Nayak, R.; Jespersen, L. Development of a framework to capture the maturity of food safety regulatory and enforcement agencies: Insights from a Delphi study. Food Control 2022, 142, 109220. [Google Scholar] [CrossRef]
- FAO. Food Safety and Quality; Food Control System. Available online: https://www.fao.org/food-safety/food-control-systems/en/ (accessed on 15 August 2023).
- Si, R.S.; Zhang, X.Q.; Yao, Y.; Zhang, S.X.; Heng, W. Unpacking the myth between increased government initiatives and reduced selling of dead live stocks in China: An approach towards exploring hidden danger of zoonotic diseases. One Health 2021, 13, 100344. [Google Scholar] [CrossRef]
- Food Safety Law of the People’s Republic of China (2021 Amendment). Available online: https://www.doc88.com/p-34761794779398.html (accessed on 24 November 2023).
- Animal Epidemic Prevention Law of the People’s Republic of China (2021 Revision). Available online: https://www.doc88.com/p-05729290833001.html (accessed on 24 November 2023).
- Regulations on the Management of Pig Slaughtering. Available online: https://www.docin.com/p-2145148441.html (accessed on 24 November 2023).
- Three Year Action Plan for Strict Standards to Promote and Improve Safety in Livestock and Poultry Slaughtering. Available online: http://www.moa.gov.cn/govpublic/xmsyj/202304/t20230407_6424887.htm?eqid=ffb474d9000052e30000000664361d06 (accessed on 24 November 2023). (In Chinese)
- Tajkarimi, M.; Salam, A.I.; Angela, M.F. Food safety challenges associated with traditional foods in Arabic speaking countries of the Middle East. Trends Food Sci. Technol. 2013, 29, 116–123. [Google Scholar] [CrossRef]
- World Health Organization. Strengthening Food Safety Systems in the WHO Eastern Mediterranean Region. 2019. Available online: https://www.who.int/westernpacific/activities/strengthening-food-safety-systems (accessed on 10 December 2023).
- Grace, D.; Kang’ethe, E.; Bonfoh, B.; Roesel, K.; Makita, K. Food safety policy in 9 African countries. In Proceedings of the 4th Annual Leverhulme Centre for Integrative Research on Agriculture and Health (LCIRAH) Conference, London, UK, 3–4 June 2014. [Google Scholar]
- Federal Democratic Republic of Ethiopia. Food and Nutrition Policy. Available online: https://www.nipn.ephi.gov.et/sites/default/files/2020-05/Food%20and%20Nutrition%20Policy.pdf (accessed on 20 November 2023).
- Zhang, J.; Cai, Z.; Cheng, M.; Zhang, H.; Zhang, H.; Zhu, Z. Association of Internet Use with Attitudes Toward Food Safety in China: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2019, 16, 4162. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Liang, M.; Gao, J.; Wang, J.; Chen, S.; Cheng, J. Chinese Consumers’ Trust in Food Safety Surveillance Sampling of Commonly Consumed Foods. Foods 2022, 11, 1971. [Google Scholar] [CrossRef] [PubMed]
- Blekking, J.; Waldman, K.; Tuholske, C.; Evans, T. Formal/informal employment and urban food security in Sub-Saharan Africa. Appl. Geogr. 2020, 114, 102131. [Google Scholar] [CrossRef]
- Oloo, B.; Daisy, L.; Oniang’o, R. Food Safety Legislation in Some Developing Countries. Food Safety-Some Global Trends. 2018. Available online: https://www.intechopen.com/chapters/61873 (accessed on 6 September 2023). [CrossRef]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Feed The Future. EatSafe: Evidence and Action Towards Safe, Nutritious Food. Review of Food Safety Policy and Legislation in Ethiopia. 2022. Available online: https://www.gainhealth.org/sites/default/files/publications/documents/Review%20of%20Food%20Safety%20Policy%20in%20Ethiopia.pdf (accessed on 12 August 2023).
- Mutua, E.N.; Bett, B.K.; Bukachi, S.A.; Estambale, B.A.; Nyamongo, I.K. From policy to practice: An assessment of biosecurity practices in cattle, sheep and goats production, marketing and slaughter in Baringo County, Kenya. PLoS ONE 2022, 17, e0266449. [Google Scholar] [CrossRef] [PubMed]
- OIE. Animal Production Food Safety Working Group. Guide to good farming practices for animal production food safety. Rev. Sci. Tech. Int. Epiz. 2006, 25, 823–836. [Google Scholar]
- Kasimanickam, V.; Kasimanickam, M.; Kasimanickam, R. Antibiotics Use in Food Animal Production: Escalation of Antimicrobial Resistance: Where Are We Now in Combating AMR? Med. Sci. 2021, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Haileselassie, M.; Redae, G.; Berhe, G.; Henry, C.J.; Nickerson, M.T.; Tyler, B.; Mulugeta, A. Why are animal source foods rarely consumed by 6-23 months old children in rural communities of Northern Ethiopia? A qualitative study. PLoS ONE 2020, 15, e0225707. [Google Scholar] [CrossRef] [PubMed]
- He, Y.N.; Yang, X.G.; Xia, J.; Zhao, L.Y.; Yang, Y.X. Consumption of meat and dairy products in China: A review. Proc. Nutr. Soc. 2016, 75, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Wang, Z.W.; Zhu, X.H. New reflections on food security and land use strategies based on the evolution of Chinese dietary patterns. Land Use Policy 2023, 126, 106520. [Google Scholar] [CrossRef]
- Lichang, S.; Zhengkai, Y.; Xingning, X.; Jiele, M.; Haoqi, H.; Chenggang, X.; Wen, W.; Xiaoyun, Q. Investigation of microbial communities across swine slaughter stages and disinfection efficacy assessment in a pig slaughterhouse. LWT 2023, 187, 115334. [Google Scholar] [CrossRef]
- Stärk, K.D. Lebensmittelsicherheit durch Herdenmanagement [Food safety achieved through herd management]. Schweiz. Arch. Tierheilkd. 2000, 142, 673–678. [Google Scholar]
- Mesele, F.; Leta, S.; Amenu, K.; Abunna, F. Occurrence of Escherichia coli O157:H7 in lactating cows and dairy farm environment and the antimicrobial susceptibility pattern at Adami Tulu Jido Kombolcha District, Ethiopia. BMC Vet. Res. 2023, 19, 6. [Google Scholar] [CrossRef]
- Sarba, E.J.; Wirtu, W.; Gebremedhin, E.Z.; Borena, B.M.; Marami, L.M. Occurrence and antimicrobial susceptibility patterns of Escherichia coli and Escherichia coli O157 isolated from cow milk and milk products, Ethiopia. Sci. Rep. 2023, 13, 16018. [Google Scholar] [CrossRef]
- Abebe, R.; Markos, A.; Abera, M.; Mekbib, B. Incidence rate, risk factors, and bacterial causes of clinical mastitis on dairy farms in Hawassa City, southern Ethiopia. Sci. Rep. 2023, 13, 10945. [Google Scholar] [CrossRef]
- Dejene, H.; Abunna, F.; Tuffa, A.C.; Gebresenbet, G. Epidemiology and Antimicrobial Susceptibility Pattern of E. coli O157:H7 Along Dairy Milk Supply Chain in Central Ethiopia. Vet. Med. 2022, 13, 131–142. [Google Scholar] [CrossRef]
- Su, Z.; Tong, P.; Zhang, L.; Zhang, M.; Wang, D.; Ma, K.; Zhang, Y.; Liu, Y.; Xia, L.; Xie, J. First Isolation and Molecular Characterization of blaCTX-M-121 -Producing Escherichia coli O157:H7 From Cattle in Xinjiang, China. Front. Vet. Sci. 2021, 8, 574801. [Google Scholar] [CrossRef] [PubMed]
- Monique, L.S.; Ludmilla, S.S.B.; Isabella, M.M.S.; Marcos, V.A.D.V.L. Prevalence of pathogens and microbiological quality of milk marketed in the region of the Recôncavo from Bahia, Brazil. Int. J. Environ. Agric. Res. 2016, 2, 75–81. [Google Scholar]
- Kang’ethe, E.K.; Onono, J.O.; McDermott, B.; Arimi, M. Isolation of E. coli O157:H7 from milk and cattle faeces from urban dairy farming and non dairy farming neighbour households in Dagoretti Division, Nairobi, Kenya: Prevalence and risk factors. East Afr. Med. J. 2007, 84 (Suppl. S11), S65–S75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kou, X.; Cai, H.; Huang, S.; Ni, Y.; Luo, B.; Qian, H.; Ji, H.; Wang, X. Prevalence and Characteristics of Staphylococcus aureus Isolated From Retail Raw Milk in Northern Xinjiang, China. Front. Microbiol. 2021, 12, 705947. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhin, E.Z.; Ararso, A.B.; Borana, B.M.; Kelbesa, K.A.; Tadese, N.D.; Marami, L.M.; Sarba, E.J. Isolation and Identification of Staphylococcus aureus from Milk and Milk Products, Associated Factors for Contamination, and Their Antibiogram in Holeta, Central Ethiopia. Vet. Med. Int. 2022, 6, 6544705. [Google Scholar] [CrossRef]
- Dittmann, K.K.; Chaul, L.T.; Lee, S.H.I.; Corassin, C.H.; Fernandes de Oliveira, C.A.; Pereira De Martinis, E.C.; Alves, V.F.; Gram, L.; Oxaran, V. Staphylococcus aureus in Some Brazilian Dairy Industries: Changes of Contamination and Diversity. Front. Microbiol. 2017, 8, 2049. [Google Scholar] [CrossRef]
- Gume, B.; Berhanu, L.; Kassa, T.; Bediru, H.; Fikre, A.G.; Dadi, L.S.; Mereta, S.T. Bacterial hazard identification and exposure assessment of raw milk consumption in Jimma zone, South West Ethiopia. BMC Microbiol. 2023, 23, 166. [Google Scholar] [CrossRef]
- Seyoum, E.T.; Woldetsadik, D.A.; Mekonen, T.K.; Gezahegn, H.A.; Gebreyes, W.A. Prevalence of Listeria monocytogenes in raw bovine milk and milk products from central highlands of Ethiopia. J. Infect. Dev. Ctries 2015, 9, 1204–1209. [Google Scholar] [CrossRef]
- Ning, P.; Guo, K.; Cheng, L.; Xu, L.; Zhang, C.; Hongjie, C.; Cheng, Y.; Xu, R.; Liu, W.; Lv, Q.; et al. Pilot survey of raw whole milk in China for Listeria monocytogenes using PCR. Food Control 2013, 31, 176–179. [Google Scholar] [CrossRef]
- Alemayehu, G.; Mestawet, T.; Rahmeto, A. Isolation, molecular detection and antimicrobial susceptibility profile of Salmonella from raw cow milk collected from dairy farms and households in southern Ethiopia. BMC Microbiol. 2022, 22, 84. [Google Scholar] [CrossRef]
- Castañeda-Salazar, R.; Pulido, A.; Ángel-Rodríguez, G.; Zafra, C.; Oliver-Espinosa, O. Isolation and identification of Salmonella spp. in raw milk from dairy herds in Colombia. Braz. J. Vet. Res. Anim. Sci. 2021, 58, e172805. [Google Scholar] [CrossRef]
- Eguale, T.; Engidawork, E.; Gebreyes, W.A.; Asrat, D.; Alemayehu, H.; Medhin, G.; Johnson, R.P.; Gunn, J.S. Fecal prevalence, serotype distribution and antimicrobial resistance of Salmonellae in dairy cattle in central Ethiopia. BMC Microbiol. 2016, 16, 20. [Google Scholar] [CrossRef]
- Ketema, L.; Ketema, Z.; Kiflu, B.; Alemayehu, H.; Terefe, Y.; Ibrahim, M.; Eguale, T. Prevalence and Antimicrobial Susceptibility Profile of Salmonella Serovars Isolated from Slaughtered Cattle in Addis Ababa, Ethiopia. Biomed. Res. Int. 2018, 2018, 9794869. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.S.; Cox, L.A.; Dickson, J.S.; Hurd, H.S.; Phillips, I.; Miller, G.Y. Modeling the relationship between food animal health and human foodborne illness. Prev. Vet. Med. 2007, 79, 186–203. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Xu, S.; Tang, Z.; Li, Z.; Zhang, L. Use of Antimicrobials in Food Animals and Impact of Transmission of Antimicrobial Resistance on Humans. Biosaf. Health 2021, 3, 32–38. [Google Scholar] [CrossRef]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Monte, D.F.; Lincopan, N.; Fedorka-Cray, P.J.; Landgraf, M. Current insights on high priority antibiotic-resistant Salmonella enterica in food and foodstuffs: A review. Curr. Opin. Food Sci. 2019, 26, 35–46. [Google Scholar] [CrossRef]
- Fuentes-Castillo, D.; Castro-Tardón, D.; Esposito, F.; Neves, I.; Rodrigues, L.; Fontana, H.; Fuga, B.; Catão-Dias, J.L.; Lincopan, N. Genomic evidences of gulls as reservoirs of critical priority CTX-M-producing Escherichia coli in Corcovado Gulf, Patagonia. Sci. Total Environ. 2023, 874, 162564. [Google Scholar] [CrossRef] [PubMed]
- Kahn, L.H.; Bergeron, G.; Bourassa, M.W.; De Vegt, B.; Gill, J.; Gomes, F.; Malouin, F.; Opengart, K.; Ritter, G.D.; Singer, R.S.; et al. From farm management to bacteriophage therapy: Strategies to reduce antibiotic use in animal agriculture. Ann. N. Y. Acad. Sci. 2019, 41, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.E.; Hyun, D.; Jezek, A.; Samore, M.H. Mortality, Length of Stay, and Healthcare Costs Associated With Multidrug-Resistant Bacterial Infections Among Elderly Hospitalized Patients in the United States. Clin. Infect. Dis. 2022, 74, 1070–1080. [Google Scholar] [CrossRef]
- Tao, Q.; Wu, Q.; Zhang, Z.; Liu, J.; Tian, C.; Huang, Z.; Malakar, P.K.; Pan, Y.; Zhao, Y. Meta-Analysis for the Global Prevalence of Foodborne Pathogens Exhibiting Antibiotic Resistance and Biofilm Formation. Front. Microbiol. 2022, 13, 906490. [Google Scholar] [CrossRef]
- Eguale, T.; Asrat, D.; Alemayehu, H.; Nana, I.; Gebreyes, W.A.; Gunn, J.S.; Engidawork, E. Phenotypic and genotypic characterization of temporally related nontyphoidal Salmonella strains isolated from humans and food animals in central Ethiopia. Zoonoses Public Health 2018, 65, 766–776. [Google Scholar] [CrossRef]
- de Souza, Z.N.; de Moura, D.F.; de Almeida Campos, L.A.; Córdula, C.R.; Cavalcanti, I.M.F. Antibiotic resistance profiles on pathogenic bacteria in the Brazilian environments. Arch. Microbiol. 2023, 205, 185. [Google Scholar] [CrossRef]
- Esposito, F.; Cardoso, B.; Sellera, F.P.; Sano, E.; Fuentes-Castillo, D.; Fontana, H.; Fuga, B.; Moura, Q.; Sato, M.I.Z.; Brandão, C.J.; et al. Expansion of healthcare-associated hypervirulent KPC-2-producing Klebsiella pneumoniae ST11/KL64 beyond hospital settings. One Health 2023, 17, 100594. [Google Scholar] [CrossRef]
- Montenegro, K.; Flores, C.; Nascimento, A.P.A.; Farias, B.O.; Brito, A.S.G.; Magaldi, M.; Gimenez, A.; de Filippis, I.; Clementino, M.M.; Bianco, K.; et al. Occurrence of Klebsiella pneumoniae ST244 and ST11 extensively drug-resistant producing KPC, NDM, OXA-370 in wastewater, Brazil. J. Appl. Microbiol. 2023, 134, lxad130. [Google Scholar] [CrossRef]
- Ling, A.L.; Pace, N.R.; Hernandez, M.T.; LaPara, T.M. Tetracycline Resistance and Class 1 Integron Genes Associated with Indoor and Outdoor Aerosols. Environ. Sci. Technol. 2013, 47, 4046–4052. [Google Scholar] [CrossRef]
- Bai, H.; He, L.Y.; Wu, D.L.; Gao, F.Z.; Zhang, M.; Zou, H.Y.; Yao, M.S.; Ying, G.G. Spread of Airborne Antibiotic Resistance from Animal Farms to the Environment: Dispersal Pattern and Exposure Risk. Environ. Int. 2022, 158, 106927. [Google Scholar] [CrossRef] [PubMed]
- George, P.B.L.; Rossi, F.; St-Germain, M.-W.; Amato, P.; Badard, T.; Bergeron, M.G.; Boissinot, M.; Charette, S.J.; Coleman, B.L.; Corbeil, J.; et al. Antimicrobial Resistance in the Environment: Towards Elucidating the Roles of Bioaerosols in Transmission and Detection of Antibacterial Resistance Genes. Antibiotics 2022, 11, 974. [Google Scholar] [CrossRef] [PubMed]
- Faye, I.; Cindy, B.L.; David, K.J.M.; Cyril, G.G. Regulatory pathways to enable the licensing of alternatives to antibiotics. Biologicals 2018, 53, 72–75. [Google Scholar] [CrossRef]
- Gemeda, B.A.; Amenu, K.; Magnusson, U.; Dohoo, I.; Hallenberg, G.S.; Alemayehu, G.; Desta, H.; Wieland, B. Antimicrobial Use in Extensive Smallholder Livestock Farming Systems in Ethiopia: Knowledge, Attitudes, and Practices of Livestock Keepers. Front. Vet. Sci. 2020, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Kisoo, L.; Muloi, D.M.; Oguta, W.; Ronoh, D.; Kirwa, L.; Akoko, J.; Fèvre, E.M.; Moodley, A.; Wambua, L. Practices and drivers for antibiotic use in cattle production systems in Kenya. One Health 2023, 17, 100646. [Google Scholar] [CrossRef]
- Mogotu, M.W.; Abong, G.O.; Mburu, J.; Ndambi, O.A. Assessment of hygiene practices and microbial safety of milk supplied by smallholder farmers to processors in selected counties in Kenya. Trop. Anim. Health Prod. 2022, 54, 220. [Google Scholar] [CrossRef]
- Duguma, B. Farmers’ perceptions of major challenges to smallholder dairy farming in selected towns of Jimma Zone, Oromia Regional State, Ethiopia: Possible influences, impacts, coping strategies and support required. Heliyon 2022, 8, e09581. [Google Scholar] [CrossRef]
- Tigabu, E.; Asrat, D.; Kassa, T.; Sinmegn, T.; Molla, B.; Gebreyes, W. Assessment of risk factors in milk contamination with Staphylococcus aureus in urban and peri-urban small-holder dairy farming in central Ethiopia. J. Zoonosis Public Health 2015, 62, 637–643. [Google Scholar] [CrossRef]
- Donkor, E.S.; Aning, K.G.; Omore, A.; Nurah, G.K.; Osafo, E.L.K.; Staal, S. Risk Factors in the Hygienic Quality of Milk in Ghana. Open Food Sci. J. 2007, 1, 6–9. [Google Scholar] [CrossRef]
- Ombui, J.N.; Arimi, S.M.; McDermott, J.J.; Mbusua, S.K.; Githua, A.; Muthoni, J. Quality of raw milk collected and marketed by diary co-operative societies in Kiambu district, Kenya. Bull. Anim. Health Prod. Afr. 1995, 43, 277–284. [Google Scholar]
- Nyokabi, N.S.; Phelan, L.; Gemechu, G.; Berg, S.; Lindahl, J.F.; Mihret, A.; Wood, J.L.N.; Moore, H.L. From farm to table: Exploring food handling and hygiene practices of meat and milk value chain actors in Ethiopia. BMC Public Health 2023, 23, 899. [Google Scholar] [CrossRef] [PubMed]
- Gizaw, S.; Woldehanna, M.; Anteneh, H.; Ayledo, G.; Awol, F.; Gebreyohannes, G.; Gebremedhin, B.; Wieland, B. Animal Health Service Delivery in Crop-Livestock and Pastoral Systems in Ethiopia. Front. Vet. Sci. 2021, 8, 601878. [Google Scholar] [CrossRef] [PubMed]
- Amenu, K.; Agga, G.E.; Kumbe, A.; Shibiru, A.; Desta, H.; Tiki, W.; Dego, O.K.; Wieland, B.; Grace, D.; Alonso, S. MILK Symposium review: Community-tailored training to improve the knowledge, attitudes, and practices of women regarding hygienic milk production and handling in Borana pastoral area of southern Ethiopia. J. Dairy Sci. 2020, 103, 9748–9757. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J.; Action, O.B.O.T.R.C.; da Costa, M.R.; Nesbakken, T.; Meemken, D. Assessment of the Effectiveness of Pre-harvest Meat Safety Interventions to Control Foodborne Pathogens in Broilers: A Systematic Review. Curr. Clin. Microbiol. Rep. 2021, 8, 21–30. [Google Scholar] [CrossRef]
- Lourenco, J.M.; Broadway, P.R.; Callaway, T.R. Editorial: The Relationship of Animal Health and Management to Food Safety. Front. Anim. Sci. 2022, 3, 951316. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Panel on Biological Hazards (BIOHAZ); Scientific Opinion on Campylobacter in Broiler Meat Production: Control Options and Performance Objectives and/or Targets at Different Stages of the Food Chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- Wafula, W.N.; Matofari, W.J.; Nduko, M.J.; Lamuka, P. Effectiveness of the sanitation regimes used by dairy actors to control microbial contamination of plastic jerry cans’ surfaces. Food Contam. 2016, 3, 9. [Google Scholar] [CrossRef]
- Liu, J.S.; Li, X.C.; Zhang, Q.Y.; Han, L.F.; Xia, S.; Kassegne, K.; Zhu, Y.Z.; Yin, K.; Hu, Q.Q.; Xiu, L.S.; et al. China’s application of the One Health approach in addressing public health threats at the human-animal-environment interface: Advances and challenges. One Health 2023, 17, 100607. [Google Scholar] [CrossRef] [PubMed]
- Tambo, E.; Tang, S.; Ai, L.; Zhou, X.N. The value of China-Africa health development initiatives in strengthening “One Health” strategy. Glob. Health J. 2017, 1, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Erkyihun, G.A.; Gari, F.R.; Edao, B.M.; Kassa, G.M. A review on One Health approach in Ethiopia. One Health Outlook 2022, 4, 8. [Google Scholar] [CrossRef]
- Garcia, S.N.; Osburn, B.I.; Cullor, J.S. A one health perspective on dairy production and dairy food safety. One Health 2019, 7, 100086. [Google Scholar] [CrossRef]
- Moje, N.; Waktole, H.; Kassahun, R.; Megersa, B.; Chomen, M.T.; Leta, S.; Debela, M.; Amenu, K. Status of animal health biosecurity measures of dairy farms in urban and peri-urban areas of central Ethiopia. Front. Vet. Sci. 2023, 10, 1086702. [Google Scholar] [CrossRef]
| Bacterial Species | Sample Source | Prevalence (%) | References |
|---|---|---|---|
| E. coli O157:H7 | Milk | 4.5 | Mesele et al., 2023 [41] |
| Water | 8 | ||
| Manure | 4 | ||
| Feces | 3.9 | ||
| Milk and milk products | 0.2 | Sarba et al., 2023 [42] | |
| Cattle feces | 0.7 | Su et al., 2021 [45] | |
| Rectal swab | 1 | ||
| Carcass swab | 4 | ||
| Processed milk | 2.04 | Monique et al., 2016 [46] | |
| milk | 2.2 | Kang’ethe et al., 2007 [47] | |
| Cattle feces | 5.2 | ||
| S. aureus | Cows’ udder | 28.1 | Abebe et al., 2023 [43] |
| Cow milk | 61.7 | Kou et al., 2021 [48] | |
| Milk and milk products | 10.7 | Gebremedhin et al., 2022 [49] | |
| Milk | 10.9 | Dittmann et al., 2017 [50] | |
| Listeria monocytogenes | Milk | 2 | Gume et al., 2023 [51] |
| Milk and milk products | 5.6 | Seyoum et al., 2015 [52] | |
| milk | 0.36 | Ning et al., 2013 [53] | |
| Salmonella | Milk | 10.4 | Alemayehu et al., 2022 [54] |
| Milk | 20.5 | Castañeda-Salazar et al., 2021 [55] | |
| Cattle feces | 2.3 | Eguale et al., 2016 [56] | |
| Meat carcass | 2.5 | Ketema et al., 2018 [57] | |
| Cattle feces | 4.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyoum, E.T.; Eguale, T.; Habib, I.; Oliveira, C.J.B.; Monte, D.F.M.; Yang, B.; Gebreyes, W.A.; Alali, W.Q. Pre-Harvest Food Safety Challenges in Food-Animal Production in Low- and Middle-Income Countries. Animals 2024, 14, 786. https://doi.org/10.3390/ani14050786
Seyoum ET, Eguale T, Habib I, Oliveira CJB, Monte DFM, Yang B, Gebreyes WA, Alali WQ. Pre-Harvest Food Safety Challenges in Food-Animal Production in Low- and Middle-Income Countries. Animals. 2024; 14(5):786. https://doi.org/10.3390/ani14050786
Chicago/Turabian StyleSeyoum, Eyasu T., Tadesse Eguale, Ihab Habib, Celso J. B. Oliveira, Daniel F. M. Monte, Baowei Yang, Wondwossen A. Gebreyes, and Walid Q. Alali. 2024. "Pre-Harvest Food Safety Challenges in Food-Animal Production in Low- and Middle-Income Countries" Animals 14, no. 5: 786. https://doi.org/10.3390/ani14050786
APA StyleSeyoum, E. T., Eguale, T., Habib, I., Oliveira, C. J. B., Monte, D. F. M., Yang, B., Gebreyes, W. A., & Alali, W. Q. (2024). Pre-Harvest Food Safety Challenges in Food-Animal Production in Low- and Middle-Income Countries. Animals, 14(5), 786. https://doi.org/10.3390/ani14050786






