Effects of Restraint Stress on Circulating Corticosterone and Met Enkephalin in Chickens: Induction of Shifts in Insulin Secretion and Carbohydrate Metabolism
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Experimental Design
2.3. Hormone and Glucose Assays
2.4. PENK (Met-Enkephalin Gene) Expression
2.5. Statistical Analysis
3. Results
3.1. Basal (Pre-Treatment) Concentrations of Corticosterone, Met-Enkephalin, Glucose and Insulin
3.2. Effects of Restraint Stress and/or Naltrexone Administration
3.3. Effects of Recovery following Restraint Stress
3.4. Effect of Repeated Blood Sample Collection in the Controls and Following Naltrexone Administration
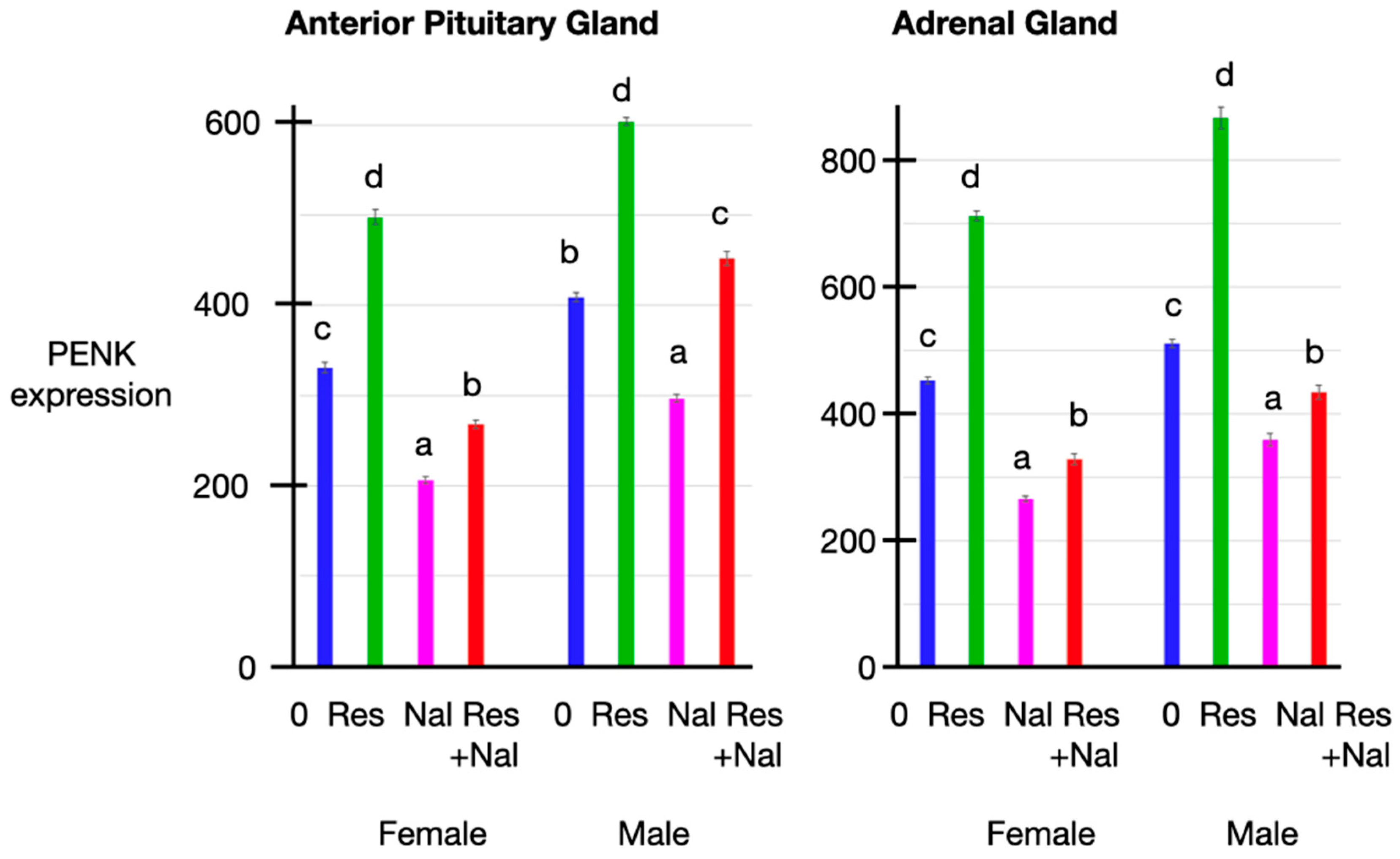
3.5. PENK Expression
3.6. Relationships between the Plasma Concentrations of Corticosterone, Met-Enkephalin, Insulin and Glucose Together with PENK Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDougall, S.J.; Paull, J.R.A.; Widdop, R.E.; Lawrence, A.J. Restraint stress: Differential cardiovascular responses in Wistar-Kyoto and Spontaneously Hypertensive Rats. Hypertension 2000, 35, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Olave, F.A.; Aguayo, F.I.; Román-Albasini, L.; Corrales, W.A.; Silva, J.P.; González, P.I.; Lagos, S.; García, M.A.; Alarcón-Mardones, M.; Rojas, P.S.; et al. Chronic restraint stress produces sex-specific behavioral and molecular outcomes in the dorsal and ventral rat hippocampus. Neurobiol. Stress 2022, 17, 100440. [Google Scholar] [CrossRef] [PubMed]
- Paré, W.P.; Glavin, G.B. Restraint stress in biomedical research: A review. Neurosci. Biobehav. Rev. 1986, 10, 339–370. [Google Scholar] [CrossRef] [PubMed]
- Selvam, R.; Jurkevich, A.; Kang, S.W.; Mikhailova, M.V.; Cornett, L.E.; Kuenzel, W.J. Distribution of the vasotocin subtype four receptor (VT4R) in the anterior pituitary gland of the chicken, Gallus gallus, and its possible role in the avian stress response. J. Neuroendocrinol. 2013, 25, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Kuenzel, W.J. Regulation of gene expression of vasotocin and corticotropin-releasing hormone receptors in the avian anterior pituitary by corticosterone. Gen. Comp. Endocrinol. 2014, 204, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kuenzel, W.J.; Kang, S.W.; Jurkevich, A. Neuroendocrine regulation of stress in birds with an emphasis on vasotocin receptors (VTRs). Gen. Comp. Endocrinol. 2013, 190, 18–23. [Google Scholar] [CrossRef]
- Nagarajan, G.; Tessaro, B.A.; Kang, S.W.; Kuenzel, W.J. Identification of arginine vasotocin (AVT) neurons activated by acute and chronic restraint stress in the avian septum and anterior diencephalon. Gen. Comp. Endocrinol. 2014, 202, 59–68. [Google Scholar] [CrossRef]
- Ericsson, M.; Jensen, P. Domestication and ontogeny effects on the stress response in young chickens (Gallus gallus). Sci. Rep. 2016, 6, 35818. [Google Scholar] [CrossRef]
- Ismail, S.N.; Awad, E.A.; Zulkifli, I.; Goh, Y.M.; Sazili, A.Q. Effects of method and duration of restraint on stress hormones and meat quality in broiler chickens with different body weights. Asian-Australas. J. Anim. Sci. 2019, 32, 865–873. [Google Scholar] [CrossRef]
- Peixoto, M.R.L.V.; Cooley, L.; Widowski, T.M. Maternal age and maternal environment affect stress reactivity and measures of social behaviour in laying hens. Sci. Rep. 2021, 11, 17499. [Google Scholar] [CrossRef]
- Kadhim, H.J.; Kuenzel, W.J. Interaction between the hypothalamo-pituitary-adrenal and thyroid axes during immobilization stress. Front. Physiol. 2022, 13, 972171. [Google Scholar] [CrossRef]
- Kvetnansky, R.; McCarty, R. Immobilization stress. In Encyclopedia of Stress, 2nd ed.; Fink, G., Ed.; Academic Press: San Diego, CA, USA, 2007; pp. 445–449. [Google Scholar]
- Walf, A.A.; Frye, C.A. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2007, 2, 322–328. [Google Scholar] [CrossRef]
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Richards, M.C.; Wakabayashi, C.; Kunugi, H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Shoji, H.; Miyakawa, T. Differential effects of stress exposure via two types of restraint apparatuses on behavior and plasma corticosterone level in inbred male BALB/cAJcl mice. Neuropsychopharmacol. Rep. 2020, 40, 73–84. [Google Scholar] [CrossRef]
- Vallès, A.; Martí, O.; García, A.; Armario, A. Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am. J. Physiol. 2000, 279, R1138–R1144. [Google Scholar] [CrossRef]
- Jaszczyk, A.; Juszczak, G.R. Glucocorticoids, metabolism and brain activity. Neurosci. Biobehav. Rev. 2021, 126, 113–145. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; de Guglielmo, G.; Hansson, A.C.; Ubaldi, M.; Kallupi, M.; Cruz, M.T.; Oleata, C.S.; Heilig, M.; Roberto, M. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: Significance for anxiety-like behaviors. J. Neurosci. 2014, 34, 363–372. [Google Scholar] [CrossRef]
- Schoenrock, S.A.; Tarantino, L.M. Animal models of environmental manipulations resulting in epigenetic modifications that increase risk for affective disorders. In Epigenetics in Psychiatry, 2nd ed.; Peedicayil, J., Grayson, D.R., Avramopoulos, D., Eds.; Academic Press: London, UK, 2014; pp. 181–205. [Google Scholar]
- Scanes, C.G. Animal well-being and behavior: Biology of stress in poultry with emphasis on glucocorticoids and the neutrophil to lymphocyte ratio. Poult. Sci. 2016, 95, 2208–2215. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, H.J.; Kidd, M.; Kang, S.W.; Kuenzel, W.J. Differential delayed responses of arginine vasotocin and its receptors in septo-hypothalamic brain structures and anterior pituitary that sustain hypothalamic-pituitary-adrenal (HPA) axis functions during acute stress. Gen. Comp. Endocrinol. 2020, 286, 113302. [Google Scholar] [CrossRef]
- Honda, K.; Kurachi, K.; Takagi, S.; Saneyasu, T.; Kamisoyama, H. Role of corticosterone in lipid metabolism in broiler chick white adipose tissue. J. Poult. Sci. 2022, 59, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Mitarai, Y.; Yoshioka, M.; Koizumi, N.; Shibahara, T.; Nakajima, Y. Serum levels of interleukin-6, alpha1-acid glycoprotein, and corticosterone in two-week-old chickens inoculated with Escherichia coli lipopolysaccharide. Poult. Sci. 1998, 77, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, F.; Song, Z.W.; Shao, H.T.; Bai, D.Y.; Ma, Y.B.; Kong, T.; Yang, F. The influence of immune stress induced by Escherichia coli lipopolysaccharide on the pharmacokinetics of danofloxacin in broilers. Poult. Sci. 2022, 101, 101629. [Google Scholar] [CrossRef]
- Scanes, C.G.; Hurst, K.; Thaxton, Y.; Archer, G.S.; Johnson, A. Effects of putative stressors and adrenocorticotropic hormone on plasma concentrations of corticosterone in market-weight male turkeys. Poult. Sci. 2020, 99, 1156–1162. [Google Scholar] [CrossRef]
- Huang, J.C.; Yang, J.; Huang, M.; Zhu, Z.S.; Sun, X.B.; Zhang, B.H.; Xu, X.L.; Meng, W.G.; Chen, K.J.; Xu, B.C. Effect of pre-slaughter shackling and wing flapping on plasma parameters, postmortem metabolism, AMPK, and meat quality of broilers. Poult. Sci. 2018, 97, 1841–1847. [Google Scholar] [CrossRef] [PubMed]
- Kannan, G.; Heath, J.L.; Wabeck, C.J.; Mench, J.A. Shackling of broilers: Effects on stress responses and breast meat quality. Br. Poult. Sci. 1997, 38, 323–332. [Google Scholar] [CrossRef]
- Scanes, C.G.; Hurst, K.; Thaxton, Y.; Archer, G.S.; Johnson, A. Effect of transportation and shackling on plasma concentrations of corticosterone and heterophil to lymphocyte ratios in market weight male turkeys in a commercial operation. Poult. Sci. 2020, 99, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ni, Y.; Guo, F.; Fu, W.; Grossmann, R.; Zhao, R. Effect of corticosterone on growth and welfare of broiler chickens showing long or short tonic immobility. Comp. Biochem. Physiol. A 2013, 164, 537–543. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, H.; Jiang, K.J.; Jiao, H.C.; Song, Z.G. Corticosterone administration and high-energy feed results in enhanced fat accumulation and insulin resistance in broiler chickens. Br. Poult. Sci. 2008, 49, 487–495. [Google Scholar] [CrossRef]
- Zhao, J.P.; Lin, H.; Jiao, H.C.; Song, Z.G. Corticosterone suppresses insulin- and NO-stimulated muscle glucose uptake in broiler chickens (Gallus gallus domesticus). Comp. Biochem. Physiol. 2009, 149, 448–454. [Google Scholar] [CrossRef]
- Zhao, J.P.; Jiao, H.C.; Song, Z.G.; Lin, H. Effects of L-arginine supplementation on glucose and nitric oxide (NO) levels and activity of NO synthase in corticosterone-challenged broiler chickens (Gallus gallus). Comp. Biochem. Physiol. C 2009, 150, 474–480. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, H.X.; Zhu, L.X.; Jiao, H.; Lin, H. Dexamethasone alters the expression of genes related to the growth of skeletal muscle in chickens (Gallus gallus domesticus). J. Mol. Endocrinol. 2011, 46, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.P.; Bao, J.; Wang, X.J.; Jiao, H.C.; Song, Z.G.; Lin, L. Altered gene and protein expression of glucose transporter1 underlies dexamethasone inhibition of insulin-stimulated glucose uptake in chicken muscles. J. Anim. Sci. 2012, 90, 4337–4345. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, S.; Wang, X.; Jiao, H.; Zhao, J.; Lin, H. Effect of dexamethasone on hypothalamic expression of appetite-related genes in chickens under different diet and feeding conditions. J. Anim. Sci. Biotechnol. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Pierzchala-Koziec, K.; Kępys, B.; Oeltgen, P.; Scanes, C.G. Developmental changes in the pituitary-adrenocortical axis and plasma met-enkephalin concentration in response to isolation stress in growing lambs. Folia Biol. 2018, 66, 53–61. [Google Scholar] [CrossRef]
- Scanes, C.G.; Pierzchala-Koziec, K. Perspectives on endogenous opioids in birds. Front. Physiol. 2018, 9, 1842. [Google Scholar] [CrossRef]
- Lightman, S.L.; Young, W.S. Changes in hypothalamic preproenkephalin A mRNA following stress and opiate withdrawal. Nature 1987, 328, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Vágási, C.I.; Tóth, Z.; Pénzes, J.; Pap, P.L.; Ouyang, J.Q.; Lendvai, Á.Z. The relationship between hormones, glucose, and oxidative damage is condition and stress dependent in a free-living passerine bird. Physiol. Biochem. Zool. 2020, 93, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Moore, I.M.; Jessop, T.S. Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm. Behav. 2003, 43, 39–47. [Google Scholar] [CrossRef]
- Pickering, A.D.; Pottinger, T.G.; Carragher, J.; Sumpter, J.P. The effects of acute and chronic stress on the levels of reproductive hormones in the plasma of mature male brown trout, Salmo trutta L. Gen. Comp. Endocrinol. 1987, 68, 249–259. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Skomal, G.B.; Mandelman, J.W. The physiological response to anthropogenic stressors in marine elasmobranch fishes: A review with a focus on the secondary response. Comp. Biochem. Physiol. A 2012, 162, 146–155. [Google Scholar] [CrossRef]
- Shaughnessy, C.A.; McCormick, S.D. 11-Deoxycortisol is a stress responsive and gluconeogenic hormone in a jawless vertebrate, the sea lamprey (Petromyzon marinus). J. Exp. Biol. 2021, 224, jeb241943. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Baker, B.I.; Bird, D.J.; Buckingham, J.C. Hypothalamo-pituitary-interrenal responses to opioid substances in the trout. II. Effects of morphine and D-Ala2, Met5-enkephalinamide on plasma cortisol titres in vivo. Gen. Comp. Endocrinol. 1987, 68, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Bird, D.J.; Buckingham, J.C.; Baker, B.I.; Mukherjee, S. Hypothalamo-pituitary-interrenal responses to opioid substances in the trout. I. Effects of morphine on the release in vitro of corticotrophin-releasing activity from the hypothalamus and corticotrophin from the pituitary gland. Gen. Comp. Endocrinol. 1987, 68, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Tsagarakis, S.; Navarra, P.; Rees, L.H.; Besser, M.; Grossman, A.; Navara, P. Morphine directly modulates the release of stimulated corticotrophin-releasing factor-41 from rat hypothalamus in vitro. Endocrinology 1989, 124, 2330–2335. [Google Scholar] [CrossRef] [PubMed]
- Vassoler, F.M.; Toorie, A.M.; Byrnes, E.M. Transgenerational blunting of morphine-induced corticosterone secretion is associated with dysregulated gene expression in male offspring. Brain Res. 2018, 1679, 19–25. [Google Scholar] [CrossRef]
- Gulati, R.; Agrawal, D.K.; Ali, R.; Srivastava, U.; Hossain, M.M. Effect of met-enkephalin on blood glucose level. Indian J. Physiol. Pharmacol. 1998, 42, 559–562. [Google Scholar]
- Leach, R.P.; Allan, E.H.; Titheradge, M.A. The stimulation of glycogenolysis in isolated hepatocytes by opioid peptides. Biochem. J. 1985, 227, 191–197. [Google Scholar] [CrossRef]
- Negri, M.; Tonnarini, G.; D’Alessandro, M.; Fallucca, F. Plasma met-enkephalin in type I diabetes. Metabolism 1992, 41, 460–461. [Google Scholar] [CrossRef]
- Fallucca, F.; Tonnarini, G.; Di Biase, N.; D’Alessandro, M.; Negri, M. Plasma met-enkephalin levels in diabetic patients: Influence of autonomic neuropathy. Metabolism 1996, 45, 1065–1068. [Google Scholar] [CrossRef]
- Giugliano, D.; Quatraro, A.; Console, G.; Ceriello, A.; Torella, R.; D’Onofrio, F. Inhibitory effect of enkephalin on insulin secretion in healthy subjects and in non-insulin-dependent diabetic subjects. Metabolism 1987, 36, 286–289. [Google Scholar] [CrossRef]
- Mátis, G.; Kulcsár, A.; Mackei, M.; Petrilla, J.; Neogrády, Z. Comparative study on the modulation of incretin and insulin homeostasis by butyrate in chicken and rabbit. PLoS ONE 2018, 13, e0205512. [Google Scholar] [CrossRef]
- Ji, J.; Tao, Y.; Zhang, X.; Pan, J.; Zhu, X.; Wang, H.; Du, P.; Zhu, Y.; Huang, Y.Q.; Chen, W. Dynamic changes of blood glucose, serum biochemical parameters and gene expression in response to exogenous insulin in Arbor Acres broilers and Silky fowls. Sci. Rep. 2020, 10, 6697. [Google Scholar] [CrossRef]
- Seki, Y.; Sato, K.; Kono, T.; Abe, H.; Akiba, Y. Broiler chickens (Ross strain) lack insulin-responsive glucose transporter GLUT4 and have GLUT8 cDNA. Gen. Comp. Endocrinol. 2003, 133, 80–87. [Google Scholar] [CrossRef]
- Freeman, B.M.; Manning, A.C. The effects of repeated injections of adrenaline on the response of the fowl to further alarm stimulation. Res. Vet. Sci. 1979, 27, 76–81. [Google Scholar] [CrossRef]
- Salimi, M.; Eskandari, F.; Binayi, F.; Eliassi, A.; Ghanbarian, H.; Hedayati, M.; Fahanik-Babaei, J.; Eftekhary, M.; Keyhanmanesh, R.; Zardooz, H. Maternal stress induced endoplasmic reticulum stress and impaired pancreatic islets’ insulin secretion via glucocorticoid receptor upregulation in adult male rat offspring. Sci. Rep. 2022, 12, 12552. [Google Scholar] [CrossRef]
- Ghalami, J.; Zardooz, H.; Rostamkhani, F.; Farrokhi, B.; Hedayati, M. Glucose-stimulated insulin secretion: Effects of high-fat diet and acute stress. J. Endocrinol. Investig. 2013, 36, 835–842. [Google Scholar]
- Byers, M.S.; Howard, C.; Wang, X. Avian and mammalian facilitative glucose transporters. Microarrays 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- King, D.L.; Hazelwood, R.L. Regulation of avian insulin secretion by isolated perfused chicken pancreas. Am. J. Physiol. 1976, 231, 1830–1839. [Google Scholar] [CrossRef] [PubMed]
- Rideau, N.; Simon, J.; Leclercq, B. Further characterization of insulin secretion from the perfused duodenum-pancreas of chicken: A comparison of insulin release in chickens selected for high and low abdominal fat content. Endocrinology 1986, 119, 2635–2641. [Google Scholar] [CrossRef]
- Wise, J.K.; Hendler, R.; Felig, P. Influence of glucocorticoids on glucagon secretion and plasma amino acid concentrations in man. J. Clin. Investig. 1973, 52, 2774–2782. [Google Scholar] [CrossRef]
- van Raalte, D.H.; Nofrate, V.; Bunck, M.C.; van Iersel, T.; Schaap, J.E.; Nassander, U.K.; Diamant, M. Acute and 2-week exposure to prednisolone impair different aspects of beta-cell function in healthy men. Eur. J. Endocrinol. 2010, 162, 729–735. [Google Scholar] [CrossRef]
- Wajngot, A.; Giacca, A.; Grill, V.; Vranic, M.; Efendic, S. The diabetogenic effects of glucocorticoids are more pronounced in low- than in high-insulin responders. Proc. Natl. Acad. Sci. USA 1992, 89, 6035–6039. [Google Scholar] [CrossRef]
- Rafacho, A.; Cestari, T.M.; Taboga, S.R.; Boschero, A.C.; Bosqueiro, J.R. High doses of dexamethasone induce increased beta-cell proliferation in pancreatic rat islets. Am. J. Physiol. 2009, 296, E681–E689. [Google Scholar]
- Malaisse, W.J.; Malaisse-Lagae, F.; McCraw, E.F.; Wright, P.H. Insulin secretion in vitro by pancreatic tissue from normal, adrenalectomized, and cortisol-treated rats. Proc. Soc. Exp. Biol. Med. 1967, 124, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Lambillotte, C.; Gilon, P.; Henquin, J.-C. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J. Clin. Investig. 1997, 99, 414–423. [Google Scholar] [CrossRef]
- Longano, C.A.; Fletcher, H.P. Insulin release after acute hydrocortisone treatment in mice. Metab. Clin. Exp. 1983, 32, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, F.; Khan, A.; Cintra, A.; Davani, B.; Ling, Z.C.; Andersson, A.; Ostenson, C.G.; Gustafsson, J.; Efendic, S.; Okret, S. Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J. Clin. Investig. 1997, 100, 2094–2098. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, R.L.; Lorenz, F.W. Effects of fasting and insulin metabolism in the domestic fowl. Am. J. Physiol. 1959, 197, 47–51. [Google Scholar] [CrossRef]
- Depke, M.; Fusch, G.; Domanska, G.; Geffers, R.; Volker, H. Hypermetabolic syndrome as a consequence of repeated psychological stress in mice. Endocrinology 2008, 149, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Holmes, W.N.; Broock, R.L.; Devlin, J. Tritiated corticosteroid metabolism in intact and adenohypophysectomized ducks (Anas platyrhynchos). Gen. Comp. Endocrinol. 1974, 22, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Birrenkott, G.P.; Wiggins, M.E. Determination of dexamethasone and corticosterone half-lives. Poult. Sci. 1984, 63, 1064–1068. [Google Scholar] [CrossRef] [PubMed]


| Female | Male | |
|---|---|---|
| Corticosterone nmoles L−1 | 15.0 ± 0.41 | 15.0 ± 0.34 |
| Met-enkephalin pmoles L−1 | 37.1 ± 0.58 a | 41.9 ± 0.73 b |
| Insulin pmoles L−1 | 0.696 ± 0.019 | 0.714 ± 0.023 |
| Glucose mmoles L−1 | 9.89 ± 0.094 a | 11.2 ± 0.109 b |
| Treatments | Pre-Treatment | Treatment 30 min | 10 min Recovery | Pre-Treatment | Treatment 30 min | 10 min Recovery |
|---|---|---|---|---|---|---|
| Plasma concentrations of corticosterone nmoles L−1 | Plasma concentrations of Met-enkephalin pmoles L−1 | |||||
| Controls | ||||||
| Female | 15.0 ± 0.71 | 14.0 ± 0.71 p | 13.4 ± 0.93 | 38.6 ± 0.81 | 36.0 ± 1.00 p | 35.0 ± 1.14 |
| Male | 13.6 ± 0.51 | 16.2 ± 0.58 p | 15.0 ± 0.71 | 41.6 ± 0.81 | 43.8 ± 1.07 pq | 44.8 ± 1.07 |
| Restraint | ||||||
| Female | 14.0 ± 0.71 a | 25.6 ± 1.03 bq | 13.0 ± 0.71 a | 37.0 ± 1.22 a | 63.6 ± 1.44 br | 46.2 ± 1.56 a |
| Male | 15.8 ± 0.86 a | 32.4 ± 1.21 cr | 23.5 ± 1.64 b | 37.8 ± 1.07 a | 96.4 ± 1.44 cs | 88.0 ± 1.14 b |
| Naltrexone | ||||||
| Female | 16.8 ± 0.61 b | 23.4 ± 0.51 cpq | 12.6 ± 0.51 a | 35.6 ± 1.36 b | 26.0 ± 0.71 ap | 31.6 ± 0.51 b |
| Male | 15.4 ± 0.51 a | 26.4 ± 0.51 bq | 17.8 ± 0.37 a | 43.2 ± 0.86 b | 33.0 ± 0.71 ap | 41.6 ± 1.50 b |
| Restraint + naltrexone | ||||||
| Female | 14.1 ± 0.71 | 18.0 ± 0.32 pq | 16.8 ± 0.37 | 37.4 ± 1.12 b | 31.4 ± 1.57 ap | 27.8 ± 0.86 a |
| Male | 15.4 ± 0.71 | 23.0 ± 0.71 pq | 18.6 ± 0.93 pq | 45.0 ± 0.71 a | 65.0 ± 1.58 cr | 59.2 ± 1.24 b |
| Effects | 3-way ANOVA F = (P</=) | 3-way ANOVA F = (P</=) | ||||
| Sex | 56.9 (<0.001) | 540 (<0.001) | ||||
| Restraint | 71.1 (<0.001) | 1132 (<0.001) | ||||
| Naltrexone | 1.33 (0.257) | 583 (<0.001) | ||||
| Sex × restraint interaction | 8.57 (<0.01) | 218 (<0.001) | ||||
| Sex × naltrexone interaction | 0.20 (=0.66) | 0 | ||||
| Restraint × naltrexone interaction | 263 (<0.001) | 150 (<0.001) | ||||
| Sex × restraint interaction × naltrexone interaction | 1.33 (=0.26) | 0.21 (=0.65) | ||||
| Plasma concentrations of insulin pmoles L−1 | Plasma concentrations of glucose mmoles L−1 | |||||
| Controls | ||||||
| Female | 0.68 ± 0.031 a | 0.74 ± 0.03 bp | 0.83 ± 0.03 b | 10.3 ± 0.10 a | 11.1 ± 0.06 bp | 11.6 ± 0.13 c |
| Male | 0.71 ± 0.052 a | 0.89 ± 0.05 ap | 1.22 ± 0.06 b | 11.5 ± 0.06 a | 11.7 ± 0.12 ap | 12.2 ± 0.13 b |
| Restraint | ||||||
| Female | 0.73 ± 0.040 a | 15.1 ± 0.35 bq | 14.0 ± 1.06 b | 9.7 ± 0.13 a | 18.1 ± 0.28 cr | 13.8 ± 1.16 b |
| Male | 0.68 ± 0.056 a | 12.6 ± 0.36 bq | 15.4 ± 1.90 b | 11.4 ± 0.07 a | 25.1 ± 0.20 cs | 13.8 ± 0.55 b |
| Naltrexone | ||||||
| Female | 0.71 ± 0.035 a | 1.66 ± 0.06 bp | 1.85 ± 0.08 c | 9.4 ± 0.06 a | 10.6 ± 0.17 bp | 11.7 ± 0.12 c |
| Male | 0.76 ± 0.039 a | 1.85 ± 0.04 bp | 2.38 ± 0.04 c | 11.0 ± 0.14 a | 20.6 ± 0.14 cr | 14.0 ± 0.14 b |
| Restraint + naltrexone | ||||||
| Female | 0.66 ± 0.022 a | 15.3 ± 0.67 q | 18.8 ± 0.67 c | 10.2 ± 0.14 a | 15.0 ± 0.17 bq | 14.0 ± 0.35 b |
| Male | 0.72 ± 0.042 a | 13.8 ± 0.41 bq | 17.6 ± 0.35 c | 11.0 ± 0.14 a | 20.6 ± 0.11 cq | 14.0 ± 0.35 b |
| Effects | 3-way ANOVA F= (P</=) | 3-way ANOVA F= (P</=) | ||||
| Sex | 14.0 (=0.001) | 14.5 (=0.001) | ||||
| Restraint | 3022 (<0.001) | 235 (<0.001) | ||||
| Naltrexone | 12.0 (<0.01) | 15.1 (<0.001) | ||||
| Sex × restraint interaction | 20.1 (<0.001) | 8.6 (< 0.01) | ||||
| Sex × naltrexone interaction | 1.34 (=0.25) | 0.80 (=0.38) | ||||
| Restraint × naltrexone interaction | 0.30 (=0.59) | F = 2.06 (=0.16) | ||||
| Sex × restraint interaction × naltrexone interaction | F = 1.07 (=0.31) | F = 7.4 (<0.05) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scanes, C.G.; Pierzchała-Koziec, K.; Gajewska, A. Effects of Restraint Stress on Circulating Corticosterone and Met Enkephalin in Chickens: Induction of Shifts in Insulin Secretion and Carbohydrate Metabolism. Animals 2024, 14, 752. https://doi.org/10.3390/ani14050752
Scanes CG, Pierzchała-Koziec K, Gajewska A. Effects of Restraint Stress on Circulating Corticosterone and Met Enkephalin in Chickens: Induction of Shifts in Insulin Secretion and Carbohydrate Metabolism. Animals. 2024; 14(5):752. https://doi.org/10.3390/ani14050752
Chicago/Turabian StyleScanes, Colin G., Krystyna Pierzchała-Koziec, and Alina Gajewska. 2024. "Effects of Restraint Stress on Circulating Corticosterone and Met Enkephalin in Chickens: Induction of Shifts in Insulin Secretion and Carbohydrate Metabolism" Animals 14, no. 5: 752. https://doi.org/10.3390/ani14050752
APA StyleScanes, C. G., Pierzchała-Koziec, K., & Gajewska, A. (2024). Effects of Restraint Stress on Circulating Corticosterone and Met Enkephalin in Chickens: Induction of Shifts in Insulin Secretion and Carbohydrate Metabolism. Animals, 14(5), 752. https://doi.org/10.3390/ani14050752








