Dietary Lactobacillus delbrueckii Affects Ileal Bacterial Composition and Circadian Rhythms in Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Bacterial Sequencing
2.3. Statistical Analysis
3. Results
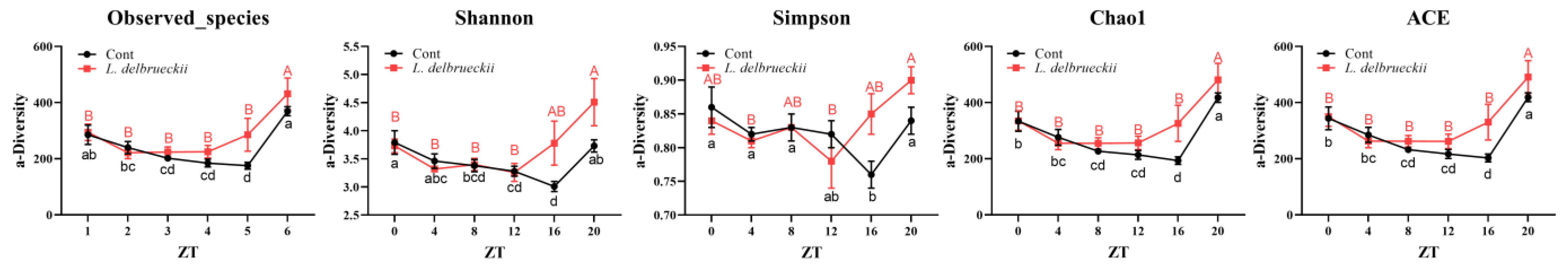
3.1. Effects of Dietary L. delbrueckii on Ileal Bacterial α-Diversity
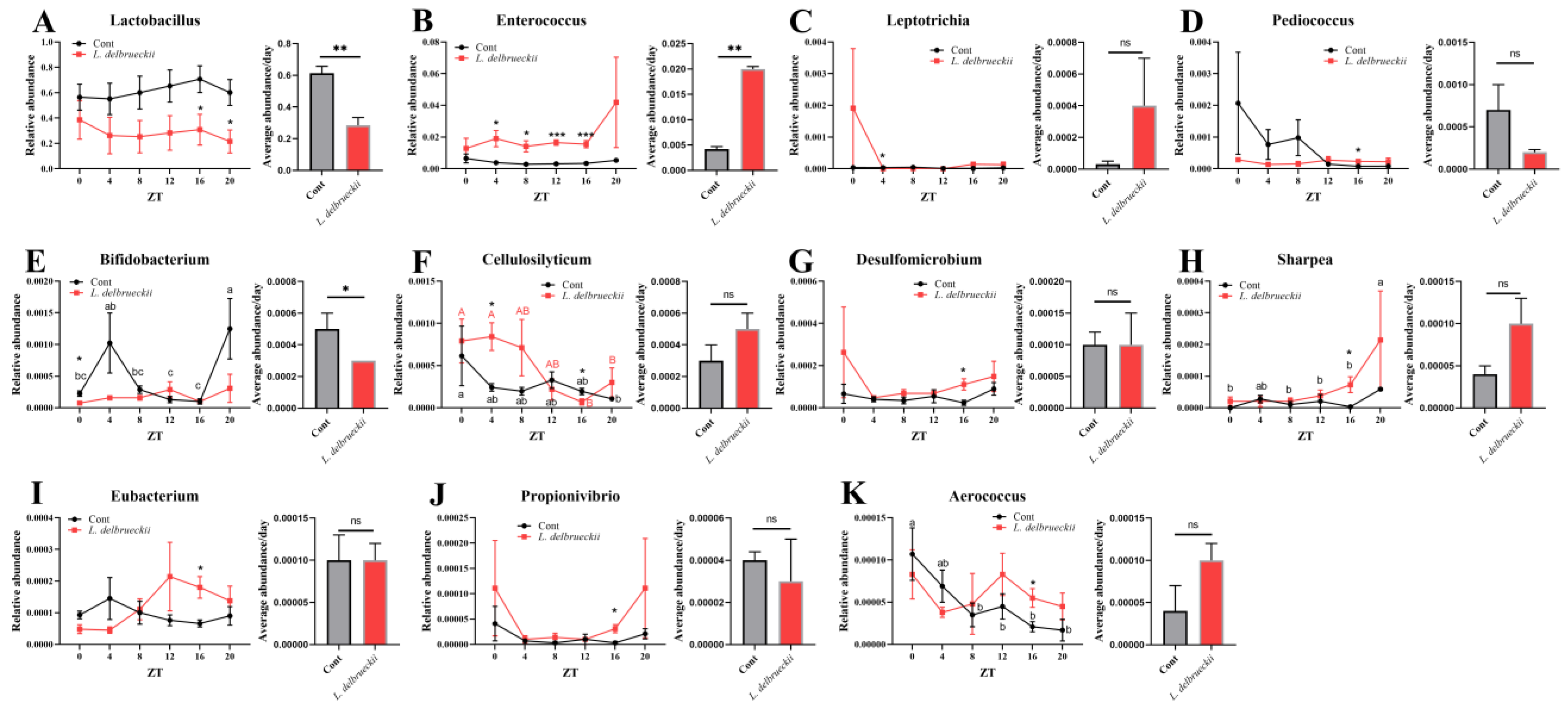
3.2. Effects of Dietary L. delbrueckii on Ileal Bacterial Composition
3.3. Effects of Dietary L. delbrueckii on Ileal Bacterial Functions
3.4. Effects of Dietary L. delbrueckii on Ileal Bacterial Circadian Rhythm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, J.; Duan, Y.; Li, R.; Liang, X.; Li, T.; Huang, X.; Yin, Y.; Yin, J. Gut microbial profiles and the role in lipid metabolism in Shaziling pigs. Anim. Nutr. 2022, 9, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhou, M.; Song, Z.; Deng, Y.; Xia, S.; Li, Y.; Huang, X.; Xiao, D.; Yin, Y.; Yin, J. Clec7a drives gut fungus-mediated host lipid deposition. Microbiome 2023, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Tian, Y.; Zhou, F.; Ma, J.; Xia, S.; Yang, T.; Ma, L.; Zeng, Q.; Liu, G.; et al. Obese Ningxiang pig-derived microbiota rewires carnitine metabolism to promote muscle fatty acid deposition in lean DLY pigs. Innovation 2023, 4, 100486. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.J.; Xia, Y.Y.; Wang, Y.X.; Han, D.D.; Liu, Y.L.; Li, J.H.; Fu, J.; Wang, L.L.; Gan, Z.D.; Liu, B.N.; et al. Gut microbiota bridges dietary nutrients and host immunity. Sci. China-Life Sci. 2023, 66, 2466–2514. [Google Scholar] [CrossRef]
- Li, Y.; Xia, S.; Jiang, X.; Feng, C.; Gong, S.; Ma, J.; Fang, Z.; Yin, J.; Yin, Y. Gut Microbiota and Diarrhea: An Updated Review. Front. Cell. Infect. Microbiol. 2021, 11, 625210. [Google Scholar] [CrossRef]
- Zhou, X.; He, Y.; Chen, J.; Xiong, X.; Yin, J.; Liang, J.; Peng, C.; Huang, C.; Guan, G.; Yin, Y. Colonic phosphocholine is correlated with Candida tropicalis and promotes diarrhea and pathogen clearance. NPJ Biofilms Microbiomes 2023, 9, 62. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.Y.; Hu, Y.C.; Huang, Z.Y.; Han, N.; Li, Y.; Zhuang, X.M.; Yin, J.Y.; Peng, H.; Gao, Q.S.; Zhang, W.P.; et al. Alterations in gut microbiota and metabolites associated with altitude-induced cardiac hypertrophy in rats during hypobaric hypoxia challenge. Sci. China-Life Sci. 2022, 65, 2093–2113. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Li, T.; Yin, J. Probiotic Species in the Modulation of Gut Microbiota: An Overview. BioMed Res. Int. 2018, 2018, 9478630. [Google Scholar] [CrossRef]
- Huang, W.Q.; Ma, T.; Liu, Y.Q.; Kwok, L.Y.; Li, Y.L.; Jin, H.; Zhao, F.Y.; Shen, X.; Shi, X.; Sun, Z.H.; et al. Spraying compound probiotics improves growth performance and immunity and modulates gut microbiota and blood metabolites of suckling piglets. Sci. China-Life Sci. 2022, 66, 1092–1107. [Google Scholar] [CrossRef]
- Brooks, J.F., 2nd; Behrendt, C.L.; Ruhn, K.A.; Lee, S.; Raj, P.; Takahashi, J.S.; Hooper, L.V. The microbiota coordinates diurnal rhythms in innate immunity with the circadian clock. Cell 2021, 184, 4154–4167.e4112. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Barber, A.F.; Noya, S.B.; Williams, J.A.; Li, F.; Daniel, S.G.; Bittinger, K.; Fang, J.; Sehgal, A. The microbiome stabilizes circadian rhythms in the gut. Proc. Natl. Acad. Sci. USA 2023, 120, e2217532120. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e1412. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, X.; Huang, X.; Fang, R.; Baba, K.; Bin, P.; et al. Administration of Exogenous Melatonin Improves the Diurnal Rhythms of the Gut Microbiota in Mice Fed a High-Fat Diet. mSystems 2020, 5, e00002-20. [Google Scholar] [CrossRef]
- Bishehsari, F.; Voigt, R.M.; Keshavarzian, A. Circadian rhythms and the gut microbiota: From the metabolic syndrome to cancer. Nat. Rev. Endocrinol. 2020, 16, 731–739. [Google Scholar] [CrossRef]
- Choi, H.; Rao, M.C.; Chang, E.B. Gut microbiota as a transducer of dietary cues to regulate host circadian rhythms and metabolism. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Wang, Y.; Li, Y.; Ye, C.; Ruhn, K.A.; Behrendt, C.L.; Olson, E.N.; Hooper, L.V. The intestinal microbiota programs diurnal rhythms in host metabolism through histone deacetylase 3. Science 2019, 365, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kuang, Z.; Yu, X.; Ruhn, K.A.; Kubo, M.; Hooper, L.V. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science 2017, 357, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Fawad, J.A.; Luzader, D.H.; Hanson, G.F.; Moutinho, T.J., Jr.; McKinney, C.A.; Mitchell, P.G.; Brown-Steinke, K.; Kumar, A.; Park, M.; Lee, S.; et al. Histone Deacetylase Inhibition by Gut Microbe-Generated Short-Chain Fatty Acids Entrains Intestinal Epithelial Circadian Rhythms. Gastroenterology 2022, 163, 1377–1390.e1311. [Google Scholar] [CrossRef]
- Zhen, Y.; Wang, Y.; He, F.; Chen, Y.; Hu, L.; Ge, L.; Wang, Y.; Wei, W.; Rahmat, A.; Loor, J.J.; et al. Homeostatic crosstalk among gut microbiome, hypothalamic and hepatic circadian clock oscillations, immunity and metabolism in response to different light-dark cycles: A multiomics study. J. Pineal Res. 2023, 75, e12892. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, H.; Chen, J.; Liu, Y.; Wen, W.; Li, Y.; Huang, X. Lactobacillus delbrueckii Ameliorates Intestinal Integrity and Antioxidant Ability in Weaned Piglets after a Lipopolysaccharide Challenge. Oxid. Med. Cell. Longev. 2020, 2020, 6028606. [Google Scholar] [CrossRef]
- Hou, G.; Peng, W.; Wei, L.; Li, R.; Yuan, Y.; Huang, X.; Yin, Y. Lactobacillus delbrueckii Interfere with Bile Acid Enterohepatic Circulation to Regulate Cholesterol Metabolism of Growing-Finishing Pigs via Its Bile Salt Hydrolase Activity. Front. Nutr. 2020, 7, 617676. [Google Scholar] [CrossRef]
- Kanmani, P.; Albarracin, L.; Kobayashi, H.; Iida, H.; Komatsu, R.; Humayun Kober, A.K.M.; Ikeda-Ohtsubo, W.; Suda, Y.; Aso, H.; Makino, S.; et al. Exopolysaccharides from Lactobacillus delbrueckii OLL1073R-1 modulate innate antiviral immune response in porcine intestinal epithelial cells. Mol. Immunol. 2018, 93, 253–265. [Google Scholar] [CrossRef]
- Mo, J.; Lu, Y.; Jiang, S.; Yan, G.; Xing, T.; Xu, D.; He, Y.; Xie, B.; Lan, G.; Chen, B.; et al. Effects of the Probiotic, Lactobacillus delbrueckii subsp. bulgaricus, as a Substitute for Antibiotics on the Gastrointestinal Tract Microbiota and Metabolomics Profile of Female Growing-Finishing Pigs. Animals 2022, 12, 1778. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Li, Y.H.; Yang, G.; Duan, J.L.; Yang, L.Y.; Chen, L.X.; Hou, S.L.; Huang, X.G. Oral administration of Lactobacillus delbrueckii enhances intestinal immunity through inducing dendritic cell activation in suckling piglets. Food Funct. 2022, 13, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Aßhauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef] [PubMed]
- Valeriano, V.D.; Balolong, M.P.; Kang, D.K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef]
- Li, Y.; Hou, S.; Peng, W.; Lin, Q.; Chen, F.; Yang, L.; Li, F.; Huang, X. Oral Administration of Lactobacillus delbrueckii during the Suckling Phase Improves Antioxidant Activities and Immune Responses after the Weaning Event in a Piglet Model. Oxid. Med. Cell. Longev. 2019, 2019, 6919803. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, M.; Yang, S.; Shen, C.; Wang, X.; Liu, W.; Guo, Y. Fermented milk of cheese-derived Lactobacillus delbrueckiisubsp.bulgaricus displays potentials in alleviating alcohol-induced hepatic injury and gut dysbiosis in mice. Food Res. Int. 2022, 157, 111283. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Li, J.; Hu, F.Z.; Zhang, K.; Luo, Y.; Janto, B.; Boissy, R.; Ehrlich, G.; Dong, X. Cellulosilyticum ruminicola, a newly described rumen bacterium that possesses redundant fibrolytic-protein-encoding genes and degrades lignocellulose with multiple carbohydrate-borne fibrolytic enzymes. Appl. Environ. Microbiol. 2010, 76, 3818–3824. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.E.; Kwon, M.S.; Whon, T.W.; Kim, D.W.; Yun, M.; Lee, J.; Shin, M.Y.; Kim, S.H.; Choi, H.J. Alteration of Gut Microbiota After Antibiotic Exposure in Finishing Swine. Front. Microbiol. 2021, 12, 596002. [Google Scholar] [CrossRef]
- Hirayama, J.; Hattori, A.; Takahashi, A.; Furusawa, Y.; Tabuchi, Y.; Shibata, M.; Nagamatsu, A.; Yano, S.; Maruyama, Y.; Matsubara, H.; et al. Physiological consequences of space flight, including abnormal bone metabolism, space radiation injury, and circadian clock dysregulation: Implications of melatonin use and regulation as a countermeasure. J. Pineal Res. 2023, 74, e12834. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin improves skin barrier damage caused by sleep restriction through gut microbiota. J. Pineal Res. 2023, 75, e12874. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J. Pineal Res. 2018, 65, e12524. [Google Scholar] [CrossRef]
- Santos, J.A.; Rempel, S.; Mous, S.T.; Pereira, C.T.; Ter Beek, J.; de Gier, J.W.; Guskov, A.; Slotboom, D.J. Functional and structural characterization of an ECF-type ABC transporter for vitamin B12. eLife 2018, 7, e35828. [Google Scholar] [CrossRef] [PubMed]
- De Jesus, L.C.L.; Aburjaile, F.F.; Sousa, T.J.; Felice, A.G.; Soares, S.C.; Alcantara, L.C.J.; Azevedo, V.A.C. Genomic Characterization of Lactobacillus delbrueckii Strains with Probiotics Properties. Front. Bioinform. 2022, 2, 912795. [Google Scholar] [CrossRef]
- Han, H.; Yi, B.; Zhong, R.; Wang, M.; Zhang, S.; Ma, J.; Yin, Y.; Yin, J.; Chen, L.; Zhang, H. From gut microbiota to host appetite: Gut microbiota-derived metabolites as key regulators. Microbiome 2021, 9, 162. [Google Scholar] [CrossRef]
- Murakami, M.; Tognini, P.; Liu, Y.; Eckel-Mahan, K.L.; Baldi, P.; Sassone-Corsi, P. Gut microbiota directs PPARγ-driven reprogramming of the liver circadian clock by nutritional challenge. EMBO Rep. 2016, 17, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.J.; Barrangou, R.; Klaenhammer, T.R. In Vivo Transcriptome of Lactobacillus acidophilus and Colonization Impact on Murine Host Intestinal Gene Expression. mBio 2021, 12, e03399-20. [Google Scholar] [CrossRef]

| Item | ZT0 | ZT4 | ZT8 | ZT12 | ZT16 | ZT20 | Average |
|---|---|---|---|---|---|---|---|
| Membrane transport | |||||||
| Cont | 0.1185 ± 0.0011 b | 0.1196 ± 0.0006 b | 0.1196 ± 0.0003 b | 0.1197 ± 0.0007 b | 0.1197 ± 0.0006 b | 0.1120 ± 0.0016 a | 0.1182 ± 0.0006 |
| L. delbrueckii | 0.1120 ± 0.0046 ab | 0.1180 ± 0.0009 a | 0.1181 ± 0.0006 a * | 0.1181 ± 0.0009 a | 0.1169 ± 0.0021 a | 0.1045 ± 0.0071 b | 0.1146 ± 0.0016 * |
| Metabolism of cofactors and vitamins | |||||||
| Cont | 0.0299 ± 0.0005 b | 0.0303 ± 0.0004 ab | 0.0306 ± 0.0003 ab | 0.0304 ± 0.0004 ab | 0.0298 ± 0.0003 b | 0.0313 ± 0.0003 a | 0.0304 ± 0.0002 |
| L. delbrueckii | 0.0310 ± 0.0004 | 0.0310 ± 0.0003 | 0.0311 ± 0.0003 | 0.0309 ± 0.0002 | 0.0309 ± 0.0003 * | 0.0319 ± 0.0004 | 0.0311 ± 0.0001 ** |
| Cell motility | |||||||
| Cont | 0.0137 ± 0.0018 b | 0.0152 ± 0.0015 b | 0.0161 ± 0.0007 b | 0.0158 ± 0.0013 b | 0.0141 ± 0.0011 b | 0.0199 ± 0.0004 a | 0.0158 ± 0.0006 |
| L. delbrueckii | 0.0153 ± 0.0014 | 0.0175 ± 0.0010 | 0.0183 ± 0.0009 | 0.0173 ± 0.0008 | 0.0181 ± 0.0009 * | 0.0186 ± 0.0022 | 0.0175 ± 0.0005 * |
| Endocrine system | |||||||
| Cont | 0.0067 ± 0.0001 b | 0.0066 ± 0.0001 b | 0.0067 ± 0.0001 b | 0.0067 ± 0.0001 b | 0.0065 ± 0.0001 b | 0.0071 ± 0.0001 a | 0.0067 ± 0.0001 |
| L. delbrueckii | 0.0071 ± 0.0003 ab | 0.0068 ± 0.0001 ab | 0.0068 ± 0.0001 ab | 0.0068 ± 0.0001 b | 0.0069 ± 0.0001 ab * | 0.0076 ± 0.0005 a | 0.0070 ± 0.0001 * |
| Signaling molecules and interaction | |||||||
| Cont | 0.0024 ± 0.0001 a | 0.0024 ± 0.0001 a | 0.0024 ± 0.0000 a | 0.0025 ± 0.0001 a | 0.0024 ± 0.0001 a | 0.0021 ± 0.0001 b | 0.0024 ± 0.0000 |
| L. delbrueckii | 0.0025 ± 0.0000 a | 0.0027 ± 0.0001 a | 0.0027 ± 0.0001 a * | 0.0025 ± 0.0001 a | 0.0024 ± 0.0001 ab | 0.0021 ± 0.0001 b | 0.0025 ± 0.0001 |
| Nervous system | |||||||
| Cont | 0.0018 ± 0.0001 b | 0.0018 ± 0.0001 b | 0.0018 ± 0.0000 b | 0.0018 ± 0.0001 b | 0.0017 ± 0.0001 b | 0.0022 ± 0.0001 a | 0.0018 ± 0.0000 |
| L. delbrueckii | 0.0020 ± 0.0002 b | 0.0019 ± 0.0001 b | 0.0019 ± 0.0000 b | 0.0019 ± 0.0000 b | 0.0020 ± 0.0001 b * | 0.0024 ± 0.0002 a | 0.0020 ± 0.0001 |
| Item | Mesor | Amplitude | Acrophase (h) | p Value |
|---|---|---|---|---|
| Lactobacillus | ||||
| Cont | 0.61 | 0.07 | 14.62 | 0.03 |
| L. delbrueckii | 0.28 | 0.02 | 23.55 | 0.89 |
| Romboutsia | ||||
| Cont | 0.04 | 0.01 | 5.07 | 0.06 |
| L. delbrueckii | 0.08 | 0.02 | 8.33 | 0.05 |
| Terrisporobacter | ||||
| Cont | 0.02 | 0.01 | 2.65 | 0.01 |
| L. delbrueckii | 0.03 | 0.01 | 3.36 | 0.26 |
| Erysipelatoclostridium | ||||
| Cont | 0.00 | 0.00 | 18.52 | 0.46 |
| L. delbrueckii | 0.00 | 0.00 | 16.29 | 0.00 |
| Cellulosilyticum | ||||
| Cont | 0.00 | 0.00 | 1.61 | 0.75 |
| L. delbrueckii | 0.00 | 0.00 | 3.58 | 0.00 |
| Eubacterium | ||||
| Cont | 0.00 | 0.00 | 3.76 | 0.11 |
| L. delbrueckii | 0.00 | 0.00 | 14.18 | 0.01 |
| Weissella | ||||
| Cont | 0.00 | 0.00 | 3.70 | 0.02 |
| L. delbrueckii | 0.00 | 0.00 | 12.18 | 0.72 |
| Item | Mesor | Amplitude | Acrophase (h) | p Value |
|---|---|---|---|---|
| Enzyme families | ||||
| Cont | 0.03 | 0.00 | 8.29 | 0.04 |
| L. delbrueckii | 0.03 | 0.00 | 6.62 | 0.02 |
| Lipid metabolism | ||||
| Cont | 0.03 | 0.00 | 8.49 | 0.03 |
| L. delbrueckii | 0.03 | 0.00 | 7.81 | 0.06 |
| Folding, sorting and degradation | ||||
| Cont | 0.02 | 0.00 | 20.87 | 0.01 |
| L. delbrueckii | 0.02 | 0.00 | 20.50 | 0.09 |
| Transport and catabolism | ||||
| Cont | 0.02 | 0.00 | 20.82 | 0.04 |
| L. delbrueckii | 0.02 | 0.00 | 20.63 | 0.09 |
| Cellular community prokaryotes | ||||
| Cont | 0.02 | 0.00 | 8.64 | 0.01 |
| L. delbrueckii | 0.02 | 0.00 | 8.54 | 0.10 |
| Cellular processes and signaling | ||||
| Cont | 0.02 | 0.00 | 8.53 | 0.15 |
| L. delbrueckii | 0.02 | 0.00 | 7.64 | 0.04 |
| Xenobiotics biodegradation and metabolism | ||||
| Cont | 0.01 | 0.00 | 9.01 | 0.01 |
| L. delbrueckii | 0.01 | 0.00 | 8.53 | 0.07 |
| Metabolism of terpenoids and polyketides | ||||
| Cont | 0.01 | 0.00 | 8.65 | 0.02 |
| L. delbrueckii | 0.01 | 0.00 | 7.48 | 0.04 |
| Endocrine and metabolic diseases | ||||
| Cont | 0.00 | 0.00 | 20.45 | 0.04 |
| L. delbrueckii | 0.00 | 0.00 | 19.72 | 0.09 |
| Signaling molecules and interaction | ||||
| Cont | 0.00 | 0.00 | 8.41 | 0.25 |
| L. delbrueckii | 0.00 | 0.00 | 6.86 | 0.03 |
| Viral protein family | ||||
| Cont | 0.00 | 0.00 | 8.36 | 0.03 |
| L. delbrueckii | 0.00 | 0.00 | 7.74 | 0.06 |
| Neurodegenerative diseases | ||||
| Cont | 0.00 | 0.00 | 8.53 | 0.01 |
| L. delbrueckii | 0.00 | 0.00 | 8.11 | 0.06 |
| Digestive system | ||||
| Cont | 0.00 | 0.00 | 22.27 | 0.05 |
| L. delbrueckii | 0.00 | 0.00 | 21.63 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Yin, Z.; Zhang, M.; Huang, X.; Yin, J. Dietary Lactobacillus delbrueckii Affects Ileal Bacterial Composition and Circadian Rhythms in Pigs. Animals 2024, 14, 412. https://doi.org/10.3390/ani14030412
Luo W, Yin Z, Zhang M, Huang X, Yin J. Dietary Lactobacillus delbrueckii Affects Ileal Bacterial Composition and Circadian Rhythms in Pigs. Animals. 2024; 14(3):412. https://doi.org/10.3390/ani14030412
Chicago/Turabian StyleLuo, Wenxin, Zhangzheng Yin, Mingliang Zhang, Xingguo Huang, and Jie Yin. 2024. "Dietary Lactobacillus delbrueckii Affects Ileal Bacterial Composition and Circadian Rhythms in Pigs" Animals 14, no. 3: 412. https://doi.org/10.3390/ani14030412
APA StyleLuo, W., Yin, Z., Zhang, M., Huang, X., & Yin, J. (2024). Dietary Lactobacillus delbrueckii Affects Ileal Bacterial Composition and Circadian Rhythms in Pigs. Animals, 14(3), 412. https://doi.org/10.3390/ani14030412






