1. Introduction
In recent years, the European Union (EU) has witnessed a significant shift in animal welfare standards, with an increasing emphasis on free-range housing systems for laying hens. In Austria, about 20% of egg production is being generated from free-range production systems [
1]. This transition from conventional cages to more spacious and humane environments has raised questions about potential changes in the dietary composition of these birds. Understanding and quantifying the intake of outdoor plant material by free-range laying hens is critical, as it can provide valuable insight for producers seeking to implement effective feeding strategies that align with the birds’ nutritional requirements. However, this attempt is challenging due to the inherent heterogeneity of outdoor environments and variations in the decomposition of plant material among different animal and plant species [
2,
3]. Identifying key plant species consumed outdoors and applying knowledge of asynchronous fluctuations in animal nutritional requirements arising from these outdoor foraging activities can significantly impact the management of these flocks [
3,
4]. Former studies [
5] estimated that approximately 10% to 15% and 20% to 25% of total feed intake in broilers and laying hens, respectively, may come from pasture, highlighting the need for accurate assessment methods. Unlike feed, free-range birds’ plant intake cannot be controlled under practical circumstances and may be subject to variation [
6,
7].
Early attempts at free-range nutritional contribution evaluation involved field observations and direct monitoring of animals, but these methods proved time-consuming, required expertise in plant identification, and were often limited to observing one animal at a time [
8]. Surgical techniques like esophageal surgeries and rumen fistulation were developed in ruminant nutrition, but similar procedures proved intricate and unsuitable for small animals, particularly non-ruminant species [
3] and poultry [
5]. Additionally, physiological processes sometimes rendered plant material unrecognizable [
2]. As an alternative, non-invasive post-digestive approaches were explored, including micro-histological analysis of fecal cuticles. While offering advantages, this method demanded substantial labor, time, specialized field observers, and faced the challenge of non-proportional representation of plant material in feces [
2,
3,
9].
The use of markers in the diet has been confirmed as a successful alternative, among which are n-alkanes, which are naturally found in the epicuticular waxes of plants as mixtures of differing carbon chain lengths. The n-alkanes have received considerable attention as fecal markers for estimating the diet composition of housed [
10] and grazing animals [
11], considering the unique and different alkanes patterns of each plant species and special plant organs [
12,
13,
14,
15]. While n-alkanes have proven valuable in estimating herbage intake in grazing ruminants, their application in poultry remains limited [
16,
17,
18,
19,
20]. The main issue at hand is the lack of knowledge about recovery rates of n-alkanes in non-ruminant species, especially poultry, and the various factors influencing this parameter.
The current study aimed to assess the accuracy of the determination method for plant intake by laying hens using n-alkanes as markers, while evaluating the recovery rates of the various n-alkanes in feces. Estimations of plant inclusion into the diet were conducted through a linear equation-solving method that leveraged the non-negative least squares procedure. We hypothesized that changes in the dietary composition, resulting from external plant consumption, would affect the n-alkane profile in feces and consequently its recovery rate when compared to a diet consisting exclusively of commercial feed.
3. Results
The inclusion of 1% alfalfa in the diets had no discernible impact on the analyzed chemical composition of the diets and consequently did not affect the daily feed intake, which remained at approximately 70 g/day per animal, as shown in
Table 2.
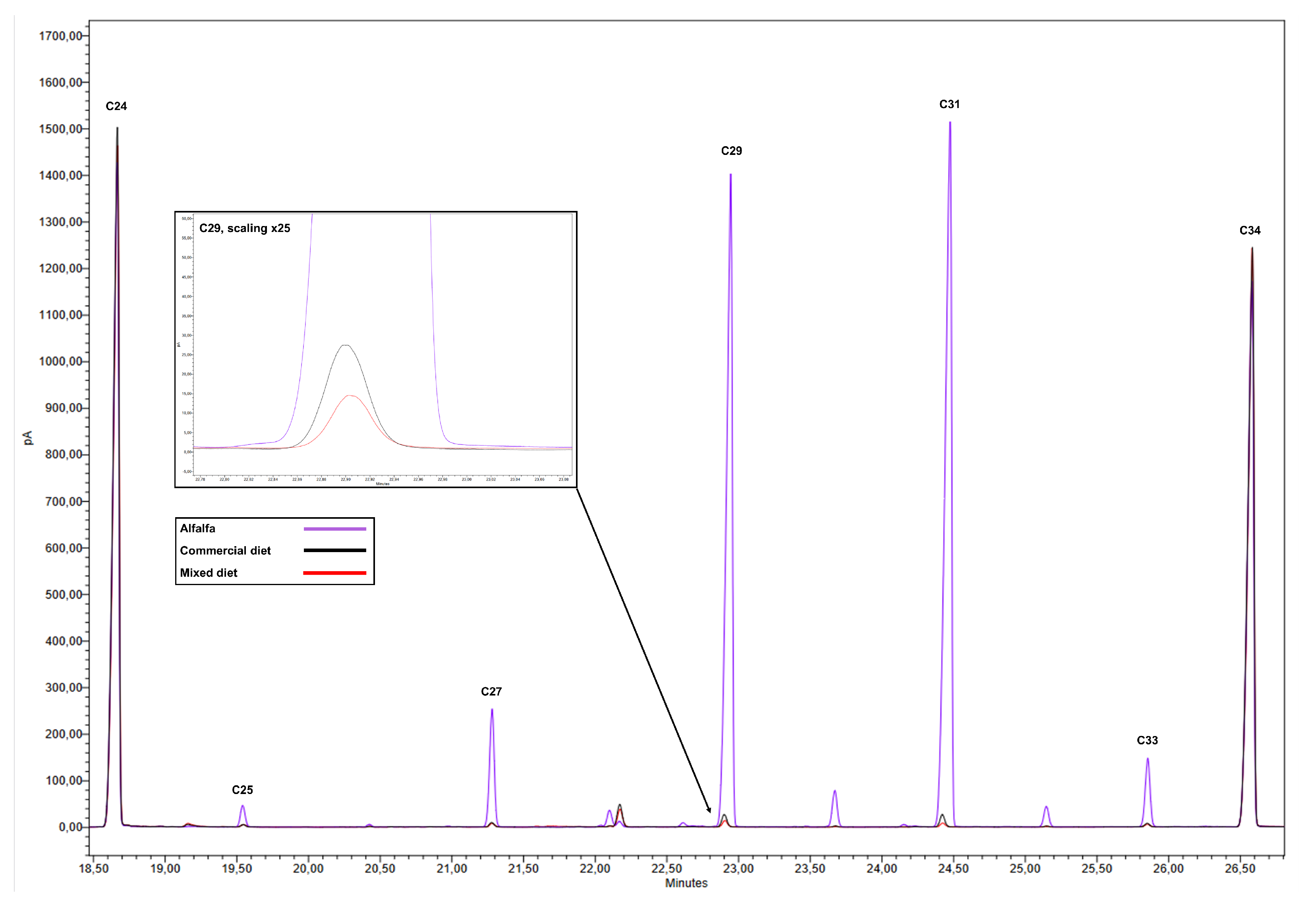
Due to the high concentrations of n-alkanes in the epicuticular wax of the upper part of alfalfa plants, especially C29 and C31, the addition of 1% alfalfa to the commercial diet markedly altered the n-alkane profile of the final mixture (
Figure 1). In particular, an increase of 8%, 38%, 120%, 186%, and 27% was observed for C25, C27, C29, C31, and C33, respectively, compared to the n-alkane profile of the commercial diet (
Table 2).
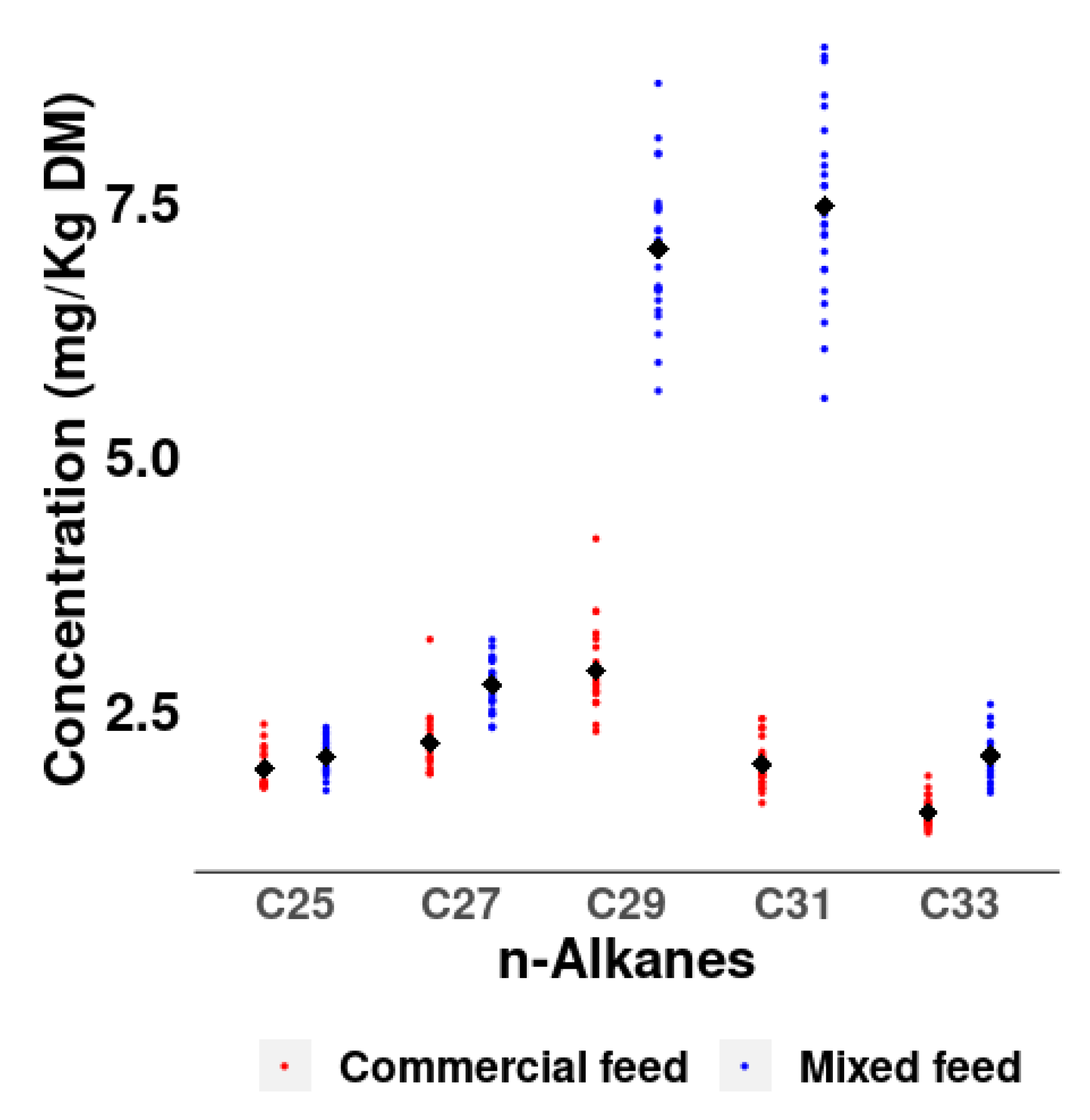
The different n-alkane profiles in the diets are also reflected in the concentration of n-alkanes in the excreta, especially in the n-alkanes C29 (2.87 vs. 7.03 mg/kg DM) and C31 (1.97 vs. 7.45 mg/kg DM) with much higher concentrations in the mixed than in the commercial diet (
Figure 2).
Approximately 36% of the total n-alkanes assayed were indigestible in the commercial diet, whereas this figure increased to 41% with the inclusion of alfalfa in the diet (
Table 3). One animal in the control group was identified as an outlier with a very high recovery rate (66% for the total alkanes) due to relatively low feed intake (42 g/day), and was therefore disregarded in the subsequent analysis.
Table 3 provides the estimated means of the recovery rates of n-alkanes in each diet. The interaction between diet and type of n-alkane was significant (
p value < 0.001), indicating that the mean differences between the n-alkanes depend on the type of diet consumed by the animal. Although the mean recovery rate was always higher in the diet with 1% alfalfa than in the diet without alfalfa, the differences were greater in the n-alkanes with greater carbon length (C29, C31, C33). In the commercial feed diet, the average recovery rate decreased as the carbon chain length increased until the minimum was obtained at the n-alkane C31. However, in the mixed feed diet the minimum was reached in the n-alkanes C27 and C29 and increased for the n-alkanes C31 and C33. These estimated mean values were used for the adjustments made to the concentrations of n-alkanes in excreta necessary for the application of the alfalfa consumption prediction.
The results of qualitative evaluation using the discriminant analysis, as shown in
Table 4, demonstrate the effectiveness of both the comprehensive use of all n-alkanes and individual n-alkanes in classifying animals based on their diets as reflected in their fecal n-alkane profiles. Using all n-alkanes together results in a high degree of accuracy in grouping animals with similar diets. However, when each n-alkane is considered individually, C29 and C31 consistently provide accurate results. In contrast, C25 shows some discrepancies, misclassifying eight animals from the mixed diet as part of the control group and seven animals from the control diet as part of the mixed-diet group. Conversely, C27 and C33 demonstrate lower levels of error, with only one control-diet animal misclassified as part of the mixed-diet group and two mixed-diet animals misclassified as part of the control group. These findings have led to an investigation to identify the most accurate combination of the n-alkanes studied under the proposed equation, using all possible combinations to optimize accuracy.
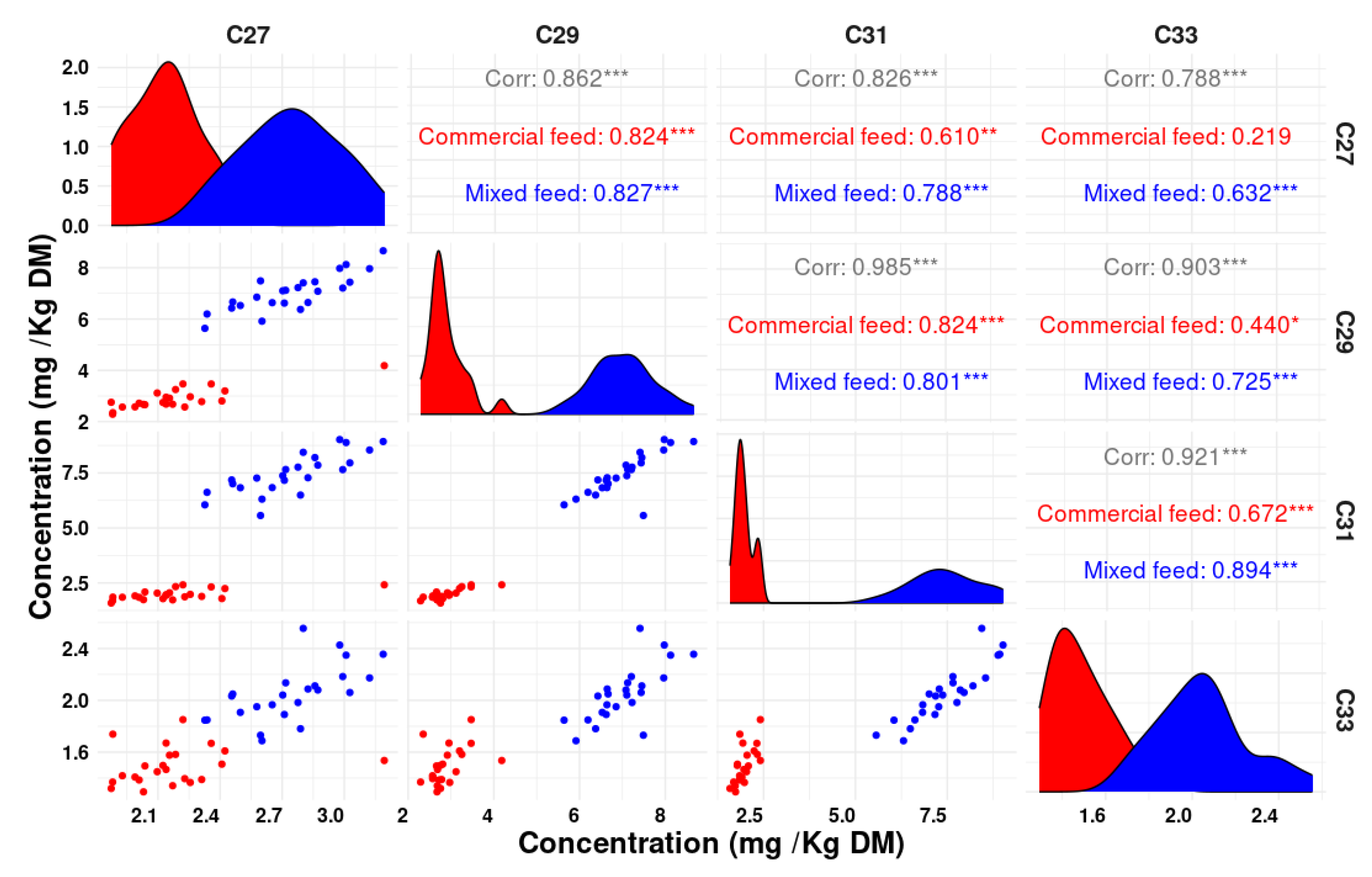
Figure 3 presents notable correlations observed between various n-alkanes in feces, where strong associations were identified. In particular, the correlation between C31 and C29 showed a Pearson correlation coefficient (r) value of 0.98, indicating a strong relationship. Additionally, C31 and C33 presented a high r of 0.92, while the correlation between C29 and C33 provided an r of 0.90, indicating substantial associations between these pairs of n-alkanes. Furthermore, the correlations between C31 and C27, as well as C27 and C29, demonstrated substantial links with r values of 0.82 and 0.86, respectively, further highlighting the strength of these associations.
Table 5 presents key evaluation metrics, including root mean square error (RMSE), variance and bias, used to assess the accuracy of predictions regarding alfalfa consumption in animals fed the two experimental diets. In particular, the coefficient x
a was close to zero (x
a = 0.001) for all animals assigned to the commercial diet, indicating that the method effectively predicted the absence of alfalfa in this particular diet, with an average alfalfa inclusion rate of approximately 0.02%. When considering animals on the mixed diet, the variance and bias were calculated to be 0.012 and −0.196, respectively. The mean prediction of the proportion of alfalfa in the diet stood at 0.804, revealing a moderate underestimation of 0.196. The RMSE, quantified at 0.224, represents an error equivalent to 28% of the mean (0.224/0.804). Importantly, the overall application of the entire range of alkanes examined in solving the equation showed a relatively accurate prediction of the proportion of alfalfa added to the diet on a dry matter basis.
Table 6 provides an overview of the outcomes resulting from a systematic evaluation of various combinations of the n-alkanes studied to solve the equation, showing the best solutions based on the minimum error committed. In the context of a five-component combination, it is noteworthy that an underestimation of 19.6% was observed, with the estimated mean value of alfalfa incorporated in the diet at approximately 0.804%. As for the four-component combination, the results showed that the most accurate solution was achieved using the combination C25–C27–C29–C33, which had a minimum committed error of 3.5% and an estimated mean value of 0.965% for alfalfa inclusion in the diet. However, the highest error was associated with the combination C27–C29–C31–C33, resulting in a significant bias of 24.4% (
Appendix A Table A1). Among the three-component combinations, the optimal accuracy was associated with the combination C25–C29–C33, which resulted in a slight overestimation of 2.80% with an estimated mean of 1.028% for alfalfa inclusion in the diet. On the other hand, the combination C25–C27–C33 showed a maximum bias of about 41.7%. Finally, the two-component combinations demonstrated a minimum committed error with a slight overestimation of 4.40% when using the combination C25–C29, giving an estimated mean of 1.044% for alfalfa in the diet. The largest error in this category, and in general, was found in the combination C27–C33, which estimated the inclusion of alfalfa in the diet at about 14.25%.
4. Discussion
n-Alkane recovery in feces is generally incomplete, and is known to increase, in a predictable way, with carbon-chain length of individual n-alkanes [
24]. The recovery rates of n-alkanes (C27–C33) in this study varied from 0.30 to 0.44 (
Table 3), dependently of the diet and the carbon chain length of these n-alkanes, as seen in the significant interaction (
p-value < 0.05), where recovery rate was higher in alfalfa including diet. These findings are consistent with the published report by [
19]. However, higher recovery values were reported by [
16] and utilized by [
20], where the range was 0.55 to 0.75 for C27 and C33, respectively. This substantial difference can be attributed to the higher concentration of n-alkanes presents in the diets used by [
16], where a grass-fed treatment was administered via tube feeding, with the examined dried grass containing 12.0, 31.5, 131.2, 168.2, 78.5 mg/kg DM of C25, C27, C29, C31, and C33, respectively, in comparison to 1.28, 2.20, 5.69, 5.13, 1.51 mg/kg DM tested in the mixed diet as part of the current study. The high concentration of n-alkanes in plants is primarily associated with C29 and C31, measuring at 289 and 358 (mg/kg DM) in the alfalfa batch used (
Table 2), respectively, exhibiting a notably favorable recovery rate. This observation aligns with the results reported by both [
16,
19], which highlights the potential of these two n-alkanes as reliable markers compared to the rest.
The applied methodology demonstrated favorable outcomes despite a relatively low plant inclusion rate of 1% in the diet, contrasting with the findings of Rivera [
31] in a field-based case study on broilers where plant inclusion was approximately 10% of the daily intake (11 g DM herbage per 100 g DM feed). However, this effect might be significant in laying hens, as they can consume as much as 30–40 g of dry matter (DM) from a grass/clover pasture per 110 g DM of feed daily [
7,
32,
33]. This difference represents a ten-fold higher inclusion level. Furthermore, this increased plant inclusion rate can minimize potential errors due to the low concentration of n-alkanes in the experimental diet used in the current study.
The investigated method moderately underestimated the proportion of alfalfa (bias = −0.196) when using the entire set of the studied n-alkanes. This underestimation was attributed to the higher variability observed, where approximately 10 g of daily leftovers per animal accounted for approximately 13% of the diet offered daily. Given that the planned inclusion of alfalfa was 1%, this indicates a potential for lower alfalfa consumption compared to the leftovers if animals showed selectivity in their feeding behavior. However, despite this observed discrepancy, the methodology demonstrated notably high accuracy and did exhibit a very low error in determining whether the animals consumed only one source of n-alkanes, such as the commercial diet. In a practical context, it is important to consider that other sources of n-alkanes, such as those from soil, as reported by [
19], could be part of the diet studied. Therefore, the additional inclusion of n-alkanes from sources such as soil may lead to an underestimation of plant consumption. In order to address this, the inclusion of soil n-alkane profiling in the study area may be valuable to improve consumption estimates.
To the best of our knowledge, this research marks the first investigation of both qualitative classifications based on fecal n-alkane profiles using discriminant analysis and the role of various n-alkane combinations in quantitatively estimating plant intake in poultry. Regarding the qualitative assessment of the diet, the proposed methodology demonstrated exceptional accuracy in classifying the type of diet based on n-alkane concentrations, while using individual n-alkanes led to some classification errors. The best results emerged when using the combination of three n-alkanes (C25, C29, C33), whereas the least precise predictions were obtained when employing all five studied n-alkanes. However, it is crucial to acknowledge the impact of multicollinearity (
Appendix A), particularly among C31, C29, and C27, when used together, which can contribute to higher prediction errors. Eliminating C31 from the n-alkanes used for solving the equation led to an 82% improvement in the prediction error, significantly reducing the underestimation. A slight improvement in prediction error was observed when C27 was removed, resulting in a 22% reduction in the error compared to using C25, C27, C29, and C33 together. Even when combining only two n-alkanes (C25 and C29), the prediction error increased slightly, but it remained significantly lower than when using all five n-alkanes together. It is noteworthy that our results deviate from the conclusions reached by [
34] and Dove and [
35], where the use of more n-alkanes was associated with better predictive precision. These findings highlight the critical role of n-alkane selection in predictive accuracy.
5. Conclusions
In conclusion, the use of fecal n-alkane profiles for the qualitative grouping of animals based on intake type has proven to be a successful and valuable approach. Similarly, the use of n-alkanes for quantitative estimation of plant intake in laying hens has shown promise, offering the advantage of avoiding the need for artificial dosing with external markers. Furthermore, this method offers the potential to not only estimate diet composition, but also to break down intake into individual n-alkane sources, which holds significant value for future free-range research. In order to achieve the highest predictive accuracy, careful selection of optimal n-alkane combinations is of major importance. However, refinement is needed to accurately estimate the contribution of n-alkanes from other sources, which requires in-depth analysis of the initial dietary n-alkane profile and its impact on the recovery rate. Furthermore, it is imperative that future studies explore additional sources of n-alkanes, particularly in the context of different plant and insect species under outdoor conditions. Such efforts are essential to achieve a more accurate estimation of plant intake in real-world scenarios, thereby improving our understanding of the dietary factors that influence the performance of laying hens in a free-range system.