The Antioxidant and Hepatoprotective Potential of Berberine and Silymarin on Acetaminophen Induced Toxicity in Cyprinus carpio L.
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Fish Maintenance
2.2. Water Quality Parameters
2.3. Growth Performance and Organosomatic Indices
- Weight gain (WG, g) = final body weight (g) − initial body weight (g);
- Specific growth rate (SGR, %/day) = 100 × [Ln (final body weight) − Ln (initial body weight)]/days;
- Feed conversion ratio (FCR) = Consumed feed (g)/WG (g).
- Hepatosomatic index (HSI, %) = [liver weight (g)/body weight (g)] × 100;
- Gonadosomatic index (GSI, %) = [gonads weight (g)/body weight (g)] × 100;
- Spleen somatic index (SSI, %) = [spleen weight (g)/body weight (g)] × 100.
2.4. Biochemical Composition of Carp Muscle
2.5. Hematological Analysis
2.6. Analysis of Blood Biochemistry
2.7. Analysis of Oxidative Stress, Lyzozyme Activity, and Total Antioxidant Capacity
2.8. Data Analysis
3. Results
3.1. Evaluation of Growth Performance
3.2. Evaluation of Organosomatic Indices and Muscle Biochemical Composition of Carp
3.3. Evaluation of Hematological and Biochemical Blood Parameters
3.4. Blood Metabolic Profile
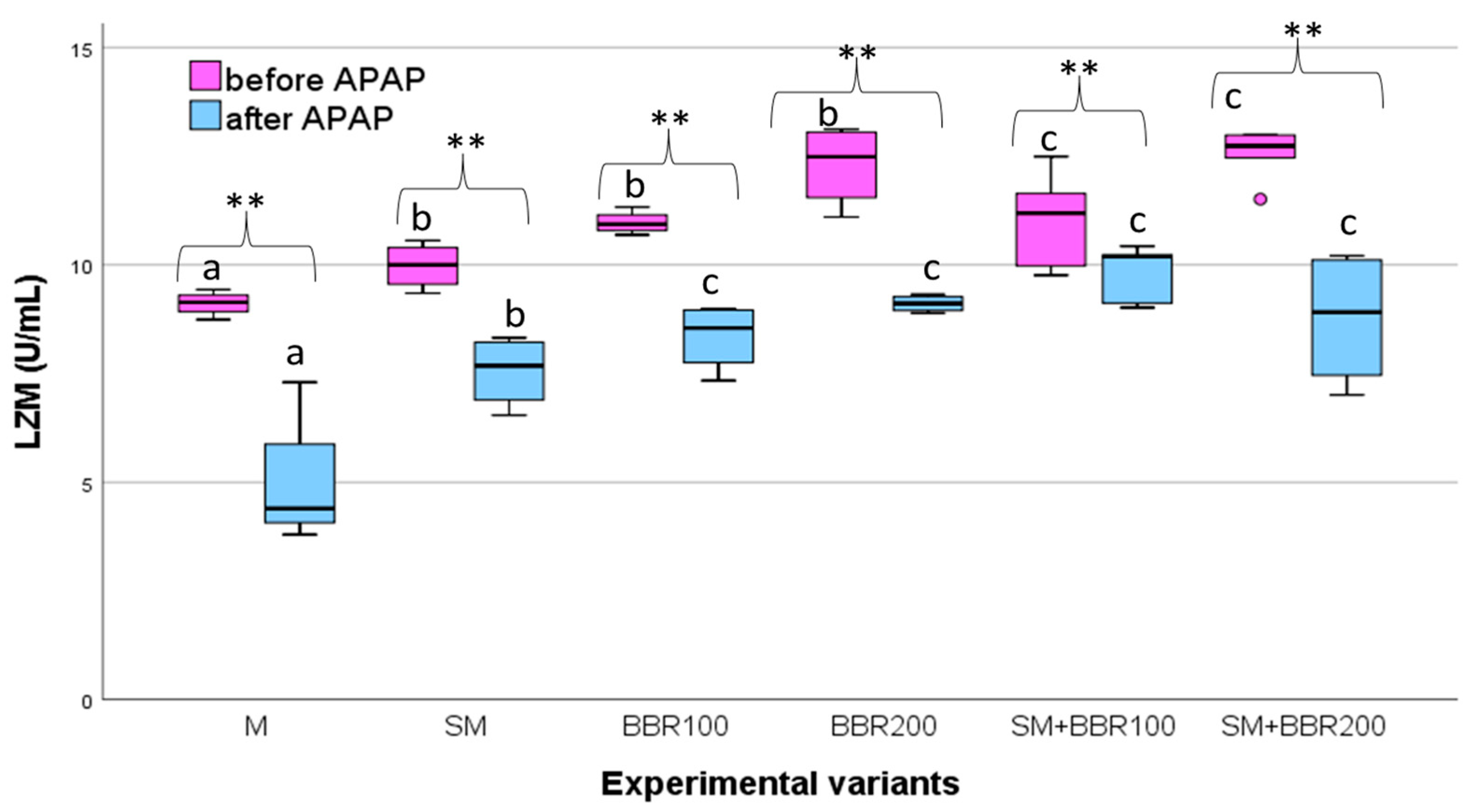
3.5. Oxidative Stress Parameters and Lysozyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022. In Towards Blue Transformation; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Ramos, A.S.; Correia, A.T.; Antunes, S.C.; Gonçalves, F.; Nunes, B. Effect of acetaminophen exposure in Oncorhynchus mykiss gills and liver: Detoxification mechanisms, oxidative defence system and peroxidative damage. Environ. Toxicol. Pharmacol. 2014, 37, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, J.L.; Rachinas-Lopes, P.; Arechavala-Lopez, P. Finding the “golden stocking density”: A balance between fish welfare and farmers’ perspectives. Front. Vet. Sci. 2022, 9, 1099. [Google Scholar] [CrossRef]
- Matthee, C.; Brown, A.R.; Lange, A.; Tyler, C.R. Factors Determining the Susceptibility of Fish to Effects of Human Pharmaceuticals. Environ. Sci. Technol. 2023, 57, 8845–8862. [Google Scholar] [CrossRef] [PubMed]
- Hawash, H.B.; Moneer, A.A.; Galhoum, A.A.; Elgarahy, A.M.; Mohamed, W.A.; Samy, M.; El-Seedi, H.R.; Gaballah, S.M.; Mubarak, M.F.; Attia, N.F. Occurrence and spatial distribution of pharmaceuticals and personal care products (PPCPs) in the aquatic environment, their characteristics, and adopted legislations. J. Water Process Eng. 2023, 52, 103490. [Google Scholar] [CrossRef]
- Priyadarshinee, S.; Umamaheswari, S.; Ramesh, M. Non-steroidal Anti-inflammatory Drug (NSAID) Naproxen-Induced Hepatotoxicity in a Freshwater Fish Labeo rohita. Water Air Soil. Pollut. 2023, 234, 28. [Google Scholar] [CrossRef]
- Bardhan, A.; Abraham, T.J.; Singha, J.; Rajisha, R.; Krishna, E.K.N.; Panda, S.K.; Patil, P.K. Impacts of Oral Florfenicol Medication and Residues on the Kidney and Liver of Nile Tilapia Oreochromis niloticus (L.). Vet. Sci. 2023, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, T.; Li, M.; Mortimer, M.; Guo, L.H. Direct and gut microbiota-mediated toxicities of environmental antibiotics to fish and aquatic invertebrates. Chemosphere 2023, 329, 138692. [Google Scholar] [CrossRef]
- Grant, D.M. Detoxification pathways in the liver. J. Inherit. Metab. Dis. 1991, 14, 421–430. [Google Scholar] [CrossRef]
- Nunes, B.; Verde, M.F.; Soares, A.M.V.M. Biochemical effects of the pharmaceutical drug paracetamol on Anguilla anguilla. Environ. Sci. Pollut. Res. 2015, 22, 11574–11584. [Google Scholar] [CrossRef]
- Schwaiger, J.; Ferling, H.; Mallow, U.; Wintermayr, H.; Negele, R.D. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I: Histopathological alterations and bioaccumulation in rainbow trout. Aquat. Toxicol. 2004, 68, 141–150. [Google Scholar] [CrossRef]
- Ajima, M.N.O.; Kumar, K.; Poojary, N.; Pandey, P.K. Oxidative stress biomarkers, biochemical responses and Na+-K+-ATPase activities in Nile tilapia, Oreochromis niloticus exposed to diclofenac. Comp. Biochem. Physiol. Part-C Toxicol. 2021, 240, 108934. [Google Scholar] [CrossRef] [PubMed]
- Rastiannasab, A.; Afsharmanesh, S.; Rahimi, R.; Sharifian, I. Alternations in the liver enzymatic activity of Common carp, Cyprinus carpio in response to parasites, Dactylogyrus spp. and Gyrodactylus spp. J. Parasit. Dis. 2016, 40, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- Bojarski, B.; Kot, B.; Witeska, M. Antibacterials in aquatic environment and their toxicity to fish. Pharmaceuticals 2020, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Manautou, J.E. Molecular mechanisms underlying chemical liver injury. Expert. Rev. Mol. Med. 2012, 14, e4. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.M.; Zhou, L.; Sun, S.X.; Zhang, M.L.; Du, Z.Y. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics causes systemic adverse effects in Nile tilapia and provoke differential human health risk. Environ. Int. 2018, 115, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.P.; Almeida, C.M.R.; Salgado, M.A.; Carvalho, M.F.; Mucha, A.P. Pharmaceutical Compounds in Aquatic Environments—Occurrence, Fate and Bioremediation Prospective. Toxics 2021, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- Guiloski, I.C.; Ribas, J.L.C.; Piancini, L.D.S.; Dagostim, A.C.; Cirio, S.M.; Fávaro, L.F.; de Assis, H.C.S. Paracetamol causes endocrine disruption and hepatotoxicity in male fish Rhamdia quelen after subchronic exposure. Environ. Toxicol. Pharmacol. 2017, 53, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M. Toxicity of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) acetylsalicylic acid, paracetamol, diclofenac, ibuprofen and naproxen towards freshwater invertebrates: A review. Sci. Total Environ. 2020, 740, 140043. [Google Scholar] [CrossRef]
- Matus, G.N.; Pereira, B.V.R.; Silva-Zacarin, E.C.M.; Costa, M.J.; Cordeiro Alves dos Santos, A.; Nunes, B. Behavior and histopathology as biomarkers for evaluation of the effects of paracetamol and propranolol in the neotropical fish species Phalloceros harpagos. Environ. Sci. Pollut. Res. 2018, 25, 28601–28618. [Google Scholar] [CrossRef]
- Raja, D.S.S.; Bharathi, U.D. Histopathological and biochemical effects of paracetamol-induced toxicity in freshwater fish. Labeo rohita. World J. Pharm. Res. 2018, 7, 1223–1233. [Google Scholar]
- Bethke, K.; Kropidłowska, K.; Stepnowski, P.; Caban, M. Review of warming and acidification effects to the ecotoxicity of pharmaceuticals on aquatic organisms in the era of climate change. Sci. Total Environ. 2023, 877, 162829. [Google Scholar] [CrossRef] [PubMed]
- Kuebutornye, F.K.A.; Roy, K.; Folorunso, E.A.; Mraz, J. Plant-based feed additives in Cyprinus carpio aquaculture. Rev. Aquac. 2024, 16, 309–336. [Google Scholar] [CrossRef]
- Reverter, M.; Tapissier-Bontemps, N.; Sarter, S.; Sasal, S.; Caruso, D. Moving towards more sustainable aquaculture practices: A meta-analysis on the potential of plant-enriched diets to improve fish growth, immunity and disease resistance. Rev. Aquac. 2021, 13, 537–555. [Google Scholar] [CrossRef]
- Surai, P.F. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef] [PubMed]
- Banaee, M.; Sureda, A.; Mirvaghefi, A.R.; Rafei, G.R. Effects of long-term silymarin oral supplementation on the blood biochemical profile of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2011, 37, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Freitag, A.F.; Cardia, G.F.E.; Da Rocha, B.A.; Aguiar, R.P.; Silva-Comar, F.M.D.S.; Spironello, R.A.; Grespan, R.; Caparroz-Assef, S.M.; Bersani-Amado, C.A.; Cuman, R.K.N. Hepatoprotective effect of silymarin (Silybum marianum) on hepatotoxicity induced by acetaminophen in spontaneously hypertensive rats. Evid. Based Complement. Alternat. Med. 2015, 2015, 538317. [Google Scholar] [CrossRef] [PubMed]
- Hermenean, A.; Damache, G.; Albu, P.; Ardelean, A.; Ardelean, G.; Ardelean, D.P.; Horge, M.; Nagy, T.; Braun, M.; Zsuga, M.; et al. Histopatological alterations and oxidative stress in liver and kidney of Leuciscus cephalus following exposure to heavy metals in the Tur River, North Western Romania. Ecotoxicol. Environ. Saf. 2015, 119, 198–205. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wu, S.C. Health Benefits of Silybum marianum: Phytochemistry, Pharmacology, and Applications. J. Agric. Food Chem. 2020, 68, 11644–11664. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Shukry, M.; Noreldin, A.E.; Ahmed, H.A.; El-Bahrawy, A.; Ghetas, H.A.; Khalifa, E. Milk thistle (Silybum marianum) extract improves growth, immunity, serum biochemical indices, antioxidant state, hepatic histoarchitecture, and intestinal histomorphometry of striped catfish, Pangasianodon hypophthalmus. Aquaculture 2023, 562, 738761. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Wang, X.; Mai, K.; He, G. Effects of silymarin on growth performance, antioxidant capacity and immune response in turbot (Scophthalmus maximus L.). J. World Aquac. Soc. 2019, 50, 1168–1181. [Google Scholar] [CrossRef]
- Owatari, M.S.; Alves Jesus, G.F.; Brum, A.; Pereira, S.A.; Lehmann, N.B.; de Pádua Pereira, U.; Martins, M.L.; Pedreira Mouriño, J.L. Sylimarin as hepatic protector and immunomodulator in Nile tilapia during Streptococcus agalactiae infection. Fish Shellfish. Immunol. 2018, 82, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Jo, S.; Yun, K.S.; Lee, K.J. Effects of dietary micelle silymarin on the growth performance, feed utilization and health of olive flounder (Paralichthys olivaceus). Aquacult. Int. 2023, 31, 3419–3436. [Google Scholar] [CrossRef]
- Shahin, S.A.; Mansour, A.T.; Abdel-Rahim, M.M.; El-Dahhar, A.A.; El Basuini, M.F.; Elhetawy, A.I.G. Silymarin, Silybum marianum, Supplemented Weaning Diet Boosted Survival, Growth, Antioxidant Status, and Fatty Acids Profile of Seabass, Dicentrarchus labrax. Ann. Anim. Sci. 2023, 23, 253–264. [Google Scholar] [CrossRef]
- Nahavandi, R.; Ahmadi, M.; Jafari, H.; Sadeghi, A.; Jahanbakhshi, A.; Tamadoni Jahromi, S.; Pourmozaffar, S. Evaluation of the Effects of Silybum marianum Methanolic Extract on Liver Function and Growth Parameters in Common Carp (Cyprinus carpio). J. Mar. Med. 2021, 3, 162–168. [Google Scholar] [CrossRef]
- Zhou, M.; Deng, Y.; Liu, M.; Liao, L.; Dai, X.; Guo, C.; Zhao, X.; He, L.; Peng, C.; Li, Y. The pharmacological activity of berberine, a review for liver protection. Eur. J. Pharmacol. 2021, 890, 173655. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, F.; Shekarabi, S.P.H.; Mehrgan, M.S.; Foroudi, F.; Islami, H.R. Supplementation of Siberian sturgeon (Acipenser baerii) diet with barberry (Berberis vulgaris) fruit extract: Growth performance, hemato-biochemical parameters, digestive enzyme activity, and growth-related gene expression. Aquacuaculture 2021, 540, 736750. [Google Scholar] [CrossRef]
- Doan, H.; Van Hoseinifar, S.H.; Jaturasitha, S.; Dawood, M.A.O.; Harikrishnan, R. The effects of berberine powder supplementation on growth performance, skin mucus immune response, serum immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 2020, 520, 734927. [Google Scholar] [CrossRef]
- Shekarabi, S.P.H.; Mehrgan, M.S.; Ramezani, F.; Dawood, M.A.O.; Van Doan, H.; Moonmanee, T.; Hamid, N.K.A.; Kari, Z.A. Effect of dietary barberry fruit (Berberis vulgaris) extract on immune function, antioxidant capacity, antibacterial activity, and stress related gene expression of Siberian sturgeon (Acipenser baerii). Aquac. Rep. 2022, 23, 101041. [Google Scholar] [CrossRef]
- Wang, L.; Sagada, G.; Wang, C.; Gao, C.; Wang, B.; Shao, Q.; Yan, Y. Berberine in fish nutrition: Impact on hepatoenteric health, antioxidative and immune status. Front. Mar. Sci. 2022, 9, 967748. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Fatemi, I.; Esmaeilizadeh, M.; Ghaznavi, H.; Kalantar, H.; Goudarzi, M. Hepatoprotective effect of berberine against methotrexate induced liver toxicity in rats. Biomed. Pharmacother. 2018, 97, 233–239. [Google Scholar] [CrossRef]
- Tan, P.; Wang, L.; Chen, R.; Xu, D. Berberine Chloride Supplementation Ameliorates Excessive Hepatic Lipid Deposition and Proinflammatory Gene Upregulation in the Soybean-Oil-Based Diet of Juvenile Yellow Drum (Nibea albiflora). Aquac. Nutr. 2022, 2022, 8690138. [Google Scholar] [CrossRef]
- Jindal, R.; Sinha, R.; Brar, P. Evaluating the protective efficacy of Silybum marianum against deltamethrin induced hepatotoxicity in piscine model. Environ. Toxicol. Pharmacol. 2019, 66, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Al-Shawi, S.G.; Yousif, A.Y.; Al-Younis, Z.K.; Shichiyakh, R.A.; Zekiy, A.O.; Naserabad, S.S. Dietary silymarin, Silybum marianum extract ameliorates cadmium chloride toxicity in common carp, Cyprinus carpio. Ann. Anim. Sci. 2021, 22, 741–750. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Abd El-Hakim, Y.M.; Metwally, M.M.; Ghfar, S.S.A.; Khalil, A.A. The single or combined Silybum marianum and co-enzyme Q10 role in alleviating fluorideinduced impaired growth, immune suppression, oxidative stress, histological alterations, and reduced resistance to Aeromonas sobria in African catfish (Clarias gariepinus). Aquaculture 2022, 548, 737693. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Yang, L.; Gao, C.; Wang, B.; Shu, Y.; Wang, H.; Yan, Y. Protective effects of berberine in chronic copper-induced liver and gill injury in freshwater grouper (Acrossocheilus fasciatus). Ecotoxicol. Environ. Saf. 2023, 267, 115672. [Google Scholar] [CrossRef] [PubMed]
- Sabiu, S.; Sunmonu, T.O.; Ajani, E.O.; Ajiboye, T.O. Combined administration of silymarin and vitamin C stalls acetaminophen-mediated hepatic oxidative insults in Wistar rats. Rev. Bras. Farmacogn. 2015, 25, 29–34. [Google Scholar] [CrossRef]
- Papackova, Z.; Heczkova, M.; Dankova, H.; Sticova, E.; Lodererova, A.; Bartonova, L.; Poruba, M.; Cahova, M. Silymarin prevents acetaminophen-induced hepatotoxicity in mice. PLoS ONE 2018, 13, e0191353. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, X.; Yang, M.; Xu, J.; Chen, Z.; Yu, Z.; Liu, J. Ameliorative effect of berberine coated bio-active nanoparticles in acetaminophen induced hepato-renal damage in diabetic rats. J. Photochem. Photobiol. B Biol. 2018, 189, 250–257. [Google Scholar] [CrossRef]
- Zhao, Z.; Wei, Q.; Hua, W.; Liu, Y.; Liu, X.; Zhu, Y. Hepatoprotective effects of berberine on acetaminophen-induced hepatotoxicity in mice. Biomed. Pharmacother. 2018, 103, 1319–1326. [Google Scholar] [CrossRef]
- Dediu, L.; Docan, A.; Crețu, M.; Grecu, I.; Mogodan, A.; Maereanu, M.; Oprea, L. Effects of stocking density on growth performance and stress responses of bester and bester♀× beluga♂ juveniles in recirculating aquaculture systems. Animals 2021, 11, 2292. [Google Scholar] [CrossRef]
- EC Directive 86/609/EEC. Available online: https://op.europa.eu/en/publication-detail/-/publication/cc3a8ccb-5a30-4b6e-8da8-b13348caeb0c/language-en, (accessed on 30 November 2023).
- Goran, S.M.A.; Omar, S.S.; Anwer, A.A. Water Quality and Physiological Parameters of Common Carp Fingerling Fed on Jerusalem artichoke Tubers. Polytechnic 2016, 3, 502–516. [Google Scholar]
- AOAC International. 2016 Official Methods of Analysis of AOAC International 2016, 20th ed.; AOAC International: Rockville, MD, USA, 2016; p. 3172. ISBN 0935584870. [Google Scholar]
- Velisek, J.; Svobodová, Z. Anaesthesia of Common Carp (Cyprinus carpio L.) with 2-phenoxyethanol: Acute Toxicity and Effects on Biochemical Blood Profile. Acta Vet. Brno 2004, 73, 247–252. [Google Scholar] [CrossRef]
- Svobodova, Z.; Pravda, D.; Modra, H. Metody hematologickeho vysetrovani ryb. In Unified Methods of Fish Haematological Investigations, Edice Metodik; Research Institute of Fish Culture and Hydrobiology: Vodňany, Czech Republic, 2012; 29p. [Google Scholar]
- Hesser, E.F. Methods for routine fish hematology. Progress. Fish Cult. 1960, 22, 164–171. [Google Scholar] [CrossRef]
- Ghergariu, S.; Pop, A.; Kadar, L. Veterinary Clinical Laboratory Guide; Ceres Publishing House: Bucharest, Romania, 1985; pp. 82–90. (In Romanian) [Google Scholar]
- Svobodova, Z. Stress in fishes (a review). Bull. Vurh Vodnany 2001, 4, 169–191. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Roberta, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sudová, E.; Piačková, V.; Kroupová, H.; Pijáček, M.; Svobodová, Z. The effect of praziquantel applied per os on selected haematological and biochemical indices in common carp (Cyprinus carpio L.). Fish Physiol. Biochem. 2009, 35, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.K.; Latimer, K.S.; Burnley, V.V. Hematologic reference intervals for koi (Cyprinus carpio), including blood cell morphology, cytochemistry, and ultrastructure. Vet. Clin. Pathol. 2004, 33, 74–83. [Google Scholar] [CrossRef]
- Mikula, P.; Modra, H.; Nemethova, D.; Groch, L.; Svoboda, Z. Effects of subchronic exposure to LASSO MTX (Alachlor 42% w/v) on hematological indices and histology of the common carp, Cyprinus carpio L. Bull. Environ. Contam. Toxicol. 2008, 81, 475–479. [Google Scholar] [CrossRef]
- Velisek, J.; Sudova, E.; Machova, J.; Svobodova, Z. Effects of sub-chronic exposure to terbutryn in common carp (Cyprinus carpio L.). Ecotoxicol. Environ. Saf. 2010, 73, 384–390. [Google Scholar] [CrossRef]
- Velisek, J.; Stara, A.; Machova, J.; Svobodova, Z. Effects of long-term exposure to simazine in real concentrations on common carp (Cyprinus carpio L.). Ecotoxicol. Environ. Saf. 2012, 76, 79–86. [Google Scholar] [CrossRef]
- Gholami-Seyedkolaei, S.J.; Mirvaghefi, A.; Farahmand, H.; Kosari, A.A. Effect of ofaglyphosate -basedherbicide in Cyprinus carpio: Assessment of acetylcholinesterase activity, hematologial response and serum biochemical parameters. Ecotoxicol. Environ. Saf. 2013, 98, 35–141. [Google Scholar] [CrossRef]
- Yonar, S.M. Toxic effects of malathion in carp, Cyprnus carpio: Protective role of lycopene. Ecotoxicol. Environ. Saf. 2013, 97, 223–229. [Google Scholar] [CrossRef]
- Kuhlwein, H.; Merrifield, D.L.; Rawling, M.D.; Foey, A.D. Efect of dietary b-(1,3), (1,6)-D-glucan supplementation on growth performance, intestinal morphology and hematoimmunological profile of mirror carp (Cyprnius carpio L). J. Anim. Physiol. Anim. Nutr. 2014, 98, 279–289. [Google Scholar] [CrossRef]
- Nicula, M.; Bura, M.; Simiz, E.; Banatean-Dunea, I.; Patruica, S.; Marcu, A.; Lunca, M.; Szelei, Z. Researches Concerning Reference Values Assessment of Serum Biochemical Parameters in some Fish Species from Acipenseridae, Cyprinidae, Esocidae and Salmonidae Family. Sci. Pap. J. Anim. Sci. Biotechnol. 2010, 43, 498–505. Available online: https://www.usab-tm.ro/fileadmin/fzb/Simp%202010/vol1/FUNDAMENTAL_SCIENCES_IN_ANIMAL_HUSBANDRY/MORPHOLOGY_AND_PHYSIOLOGY/Nicula.pdf (accessed on 17 December 2023).
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Karmakar, S.; Jana, P.; Roy, U.; Paul, M.; Singh, A.K.; Bera, K.K. Phytobiotics in aquaculture health management: A review. J. Entomol. Zool. Stud. 2018, 6, 1422–1429. Available online: https://www.researchgate.net/publication/335455760 (accessed on 13 December 2023).
- Ahmadifar, E.; Fallah, H.P.; Yousefi, M.; Dawood, M.A.O.; Hoseinifar, S.H.; Adineh, H.; Yilmaz, S.; Paolucci, M.; Van Doan, H. The gene regulatory roles of herbal extracts on the growth, immune system, and reproduction of fish. Animals 2021, 11, 2167. [Google Scholar] [CrossRef]
- Tadese, D.A.; Song, C.; Sun, C.; Liu, B.; Liu, B.; Zhou, Q.; Xu, P.; Ge, X.; Liu, M.; Xu, X.; et al. The role of currently used medicinal plants in aquaculture and their action mechanisms: A review. Rev. Aquac. 2021, 14, 816–847. [Google Scholar] [CrossRef]
- Zhou, W.; Rahimnejad, S.; Lu, K.; Wang, L.; Liu, W. Effects of berberine on growth, liver histology, and expression of lipid-related genes in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Fish Physiol. Biochem. 2019, 45, 83–91. [Google Scholar] [CrossRef]
- Ji, C.; Zhang, D.F.; Li, A.H.; Gong, X.N. Effect of berberine hydrochloride on grass carp Ctenopharyngodon idella serum bactericidal activity against Edwardsiella ictalurid. Fish Shellfish Immunol. 2012, 33, 143–145. [Google Scholar] [CrossRef]
- Xu, W.N.; Chen, D.H.; Chen, Q.Q.; Liu, W.B. Growth performance, innate immune responses and disease resistance of fingerling blunt snout bream, Megalobrama amblycephala adapted to different berberine-dietary feeding modes. Fish. Shellfish. Immunol. 2017, 68, 458–465. [Google Scholar] [CrossRef]
- Wei, L.; Wu, P.; Zhou, X.Q.; Jiang, W.D.; Liu, Y.; Kuang, S.Y.; Tang, L.; Feng, L. Dietary silymarin supplementation enhanced growth performance and improved intestinal apical junctional complex on juvenile grass carp (Ctenopharyngodon idella). Aquaculture 2020, 525, 735311. [Google Scholar] [CrossRef]
- Xiao, P.; Ji, H.; Ye, Y.; Zhang, B.; Chen, Y.; Tian, J.; Du, Z. Dietary silymarin supplementation promotes growth performance and improves lipid metabolism and health status in grass carp (Ctenopharyngodon idellus) fed diets with elevated lipid levels. Fish. Physiol. Biochem. 2017, 43, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.H.; Wang, T.; Wang, T.H.; Ye, J.Y.; Zhang, Y.X.; Yang, X.; Shao, X.P.; Ding, Z.Y. Effects of dietary berberine on growth performance, lipid metabolism, antioxidant capacity and lipometabolism-related genes expression of AMPK signaling pathway in juvenile black carp (Mylopharyngodon piceus) fed high-fat diets. Fish. Physiol. Biochem. 2022, 49, 769–786. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, H.C.; Zhang, K.; Tian, J.J.; Li, Z.F.; Yu, E.M.; Li, H.Y.; Gong, W.B.; Xie, W.P.; Wang, G.J.; et al. Berberine regulates glucose metabolism in largemouth bass by modulating intestinal microbiota. Front. Physiol. 2023, 14, 1147001. [Google Scholar] [CrossRef]
- Shan, Y.Q.; Zhu, Y.P.; Pang, J.; Wang, Y.X.; Song, D.Q.; Kong, W.J. Tetrandrine potentiates the hypoglycemic efficacy of berberine by inhibiting p-glycoprotein function. Biol. Pharm. Bull. 2013, 36, 1562–1569. [Google Scholar] [CrossRef]
- Dai, W.; Wang, K.; Zheng, X.; Chen, X.; Zhang, W.; Zhang, Y.; Hou, J.Y.; Liu, L. High fat plus high cholesterol diet lead to hepatic steatosis in zebrafish larvae: A novel model for screening anti-hepatic steatosis drugs. Nutr. Metab. 2015, 12, 42. [Google Scholar] [CrossRef]
- Maran, B.A.V.; Shaleh, S.R.M. Fish Diet, Health and Control in Aquaculture; University Malaysia Sabah Press: Kota Kinabalu, Malaysia, 2023. [Google Scholar]
- Rønsholdt, B. Effect of size/age and feed composition on body composition and phosphorus content of rainbow trout, Oncorhynchus mykiss. Water Sci. Technol. 1995, 31, 175–183. [Google Scholar] [CrossRef]
- Ahmadi, K.; Banaee, M.; Vosoghei, A.R.; Mirvaghefei, A.R.; Ataeimehr, B. Evaluation of the immunomodulatory effects of silymarin extract (Silybum Marianum) on some immune parameters of rainbow trout, Oncorhynchus mykiss (Actinopterygii: Salmoniformes: Salmonidae). Acta Ichthyol. Piscat. 2012, 42, 113–120. [Google Scholar] [CrossRef]
- Ramezanzadeh, S.; Abedian Kenari, A.; Esmaeili, M. Immunohematological parameters of rainbow trout (Oncorhynchus mykiss) fed supplemented diet with different forms of barberry root (Berberis vulgaris). Comp. Clin. Path. 2020, 29, 177–187. [Google Scholar] [CrossRef]
- Wang, L.; Gao, C.; Yang, L.; Wang, C.; Wang, B.; Wang, H.; Shu, Y.; Yan, Y. The growth-promoting and lipid-lowering effects of berberine are associated with the regulation of intestinal bacteria and bile acid profiles in yellow catfish (Pelteobagrus fulvidraco). Aquac. Rep. 2023, 33, 101848. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Li, W.; Li, X. Hepatotoxicity of paraquat on common carp (Cyprinus carpio L.). Sci. Total Environ. 2018, 616–617, 889–898. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Khater, M.R. Evaluation of the protective potential of Ambrosia maritima extract on acetaminophen-induced liver damage. J. Ethnopharmacol. 2001, 75, 169–174. [Google Scholar] [CrossRef]
- Kumar, G.; Banu, G.S.; Pappa, P.V.; Sundararajan, M.; Pandian, M.R. Hepatoprotective activity of Trianthema portulacastrum L. against paracetamol and thioacetamide intoxication in albino rats. J. Ethnopharmacol. 2004, 92, 37–40. [Google Scholar] [CrossRef]
- Kavitha, P.; Ramesh, R.; Bupesh, G.; Stalin, A.; Subramanian, P. Hepatoprotective activity of Tribulus terrestris extract against acetaminophen-induced toxicity in a freshwater fish (Oreochromis mossambicus). In Vitro Cell. Dev. Biol. Anim. 2011, 47, 698–706. [Google Scholar] [CrossRef]
- Drotman, R.; Lawhan, G. Serum enzymes are indications of chemical induced liver damage. Drug Chem. Toxicol. 1978, 1, 163–171. [Google Scholar] [CrossRef]
- Abdulrazzaq, A.M.; Badr, M.; Gammoh, O.; Abu Khalil, A.A.; Ghanim, B.Y.; Alhussainy, T.M.; Qinna, N.A. Hepatoprotective actions of ascorbic acid, alpha lipoic acid and silymarin or their combination against acetaminophen-induced hepatotoxicity in rats. Medicina 2019, 55, 181. [Google Scholar] [CrossRef]
- Shivashri, C.; Rajarajeshwari, T.; Rajasekar, P. Hepatoprotective action of celery (Apium graveolens) leaves in acetaminophen-fed freshwater fish (Pangasius sutchi). Fish Physiol. Biochem. 2013, 39, 1057–1069. [Google Scholar] [CrossRef]
- Almani, S.A.; Memon, I.A.; Shaikh, T.Z.; Khoharo, H.K.; Ujjan, I. Berberine protects against metformin-associated lactic acidosis in induced diabetes mellitus. Iran. J. Basic Med. Sci. 2017, 20, 511–515. [Google Scholar] [CrossRef]
- Al-Khamas, A.J.H.; Kadhim, Z.H.; Al-Charak, A.G.H.; Faris, J.K. Biochemical and histological study of rat liver toxicity induced by gentamicin and protective action of berberine. Plant Arch. 2020, 20, 3073–3078. [Google Scholar]
- Tang, J.; Feng, Y.; Tsao, S.; Wang, N.; Curtain, R.; Wang, Y. Berberine and Coptidis Rhizoma as novel antineoplastic agents: A review of traditional use and biomedical investigations. J. Ethnopharmacol. 2009, 126, 5–17. [Google Scholar] [CrossRef]
- Ardestani, S.B.; Sahari, M.A.; Barzegar, M.; Abbasi, S. Some Physicochemical Properties of Iranian Native Barberry Fruits (abi and poloei): Berberis integerrima and Berberis vulgaris. J. Food. Pharm. Sci. 2013, 1, 60–67. Available online: https://jurnal.ugm.ac.id/jfps/article/download/1846/1653 (accessed on 20 January 2024).
- Zarei, A.; Changizi-Ashtiyani, S.; Taheri, S.; Ramezani, M. A quick overview on some aspects of endocrinological and therapeutic effects of Berberis vulgaris L. Avicenna J. Phytomed. 2015, 5, 485–497. [Google Scholar]
- Sheridan, M.A. Regulation of lipid metabolism in poikilothermic vertebrates. Comp. Biochem. Physiol. 1994, 107B, 495–508. [Google Scholar] [CrossRef]
- Brusq, J.M.; Ancellin, N.; Grondin, P.; Guillard, R.; Martin, S.; Saintillan, Y.; Issandou, M. Inhibition of lipid synthesis through activation of AMP kinase: An additional mechanism for the hypolipidemic effects of berberine. J. Lipid Res. 2006, 47, 1281–1288. [Google Scholar] [CrossRef]
- Wang, L.; Xu, B.; Sagada, G.; Ng, W.K.; Chen, K.; Zhang, J.; Shao, Q. Dietary berberine regulates lipid metabolism in muscle and liver of black sea bream (Acanthopagrus schlegelii) fed normal or high-lipid diets. Br. J. Nutr. 2021, 125, 481–493. [Google Scholar] [CrossRef]
- Wolf, J.C.; Wolfe, M.J. A brief overview of nonneoplastic hepatic toxicity in fish. Toxicol. Pathol. 2005, 33, 75–85. [Google Scholar] [CrossRef]
- Cui, Y.; Paules, R.S. Use of transcriptomics in understanding mechanisms of drug-induced toxicity. Pharmacogenomics 2010, 11, 573–585. [Google Scholar] [CrossRef]
- Clifton, J.D.; Lucumi, E.; Myers, M.C.; Napper, A.; Hama, K.; Farber, S.A.; Smith, A.B.; Huryn, D.M.; Diamond, S.L.; Pack, M. Identification of novel inhibitors of dietary lipid absorption using zebrafish. PLoS ONE 2010, 5, e12386. [Google Scholar] [CrossRef]
- Tomaro, M.L.; Batlle, A.M.D.C. Bilirubin: Its role in cytoprotection against oxidative stress. Int. J. Biochem. Cell Biol. 2002, 34, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Mirvaghefi, A.; Amoozegar, M.A.; Merrifield, D.; Ringø, E. In vitro selection of a synbiotic and in vivo evaluation on intestinal microbiota, performance and physiological response of rainbow trout (Oncorhynchus mykiss) fingerlings. Aquacult Nutr. 2015, 23, 111–118. [Google Scholar] [CrossRef]
- Lu, K.L.; Wang, L.N.; Zhang, D.D.; Liu, W.B.; Xu, W.N. Berberine attenuates oxidative stress and hepatocytes apoptosis via protecting mitochondria in blunt snout bream Megalobrama amblycephala fed high-fat diets. Fish Physiol. Biochem. 2017, 43, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Sun, Y.; Xu, B.; Sagada, G.; Chen, K.; Xiao, J.; Zhang, J.; Shao, Q. Effects of berberine supplementation in high starch diet on growth performance, antioxidative status, immune parameters and ammonia stress response of fingerling black sea bream (Acanthopagrus schlegelii). Aquaculture 2020, 527, 735473. [Google Scholar] [CrossRef]
- Jia, R.; Cao, L.; Du, J.; Xu, P.; Jeney, G.; Yin, G. The protective effect of silymarin on the carbon tetrachloride (CCl4)- induced liver injury in common carp (Cyprinus carpio). In Vitro Cell Dev. Biol. Anim. 2013, 49, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Lupoae, M.; Cristea, V.; Coprean, D.; Mocanu, M.; Patriche, T.; Bocioc, E. Biochemical determination and oxidative stres evaluation on O. mykiss grown in recirculation system. Sci. Pap. Anim. Husb. 2011, 55, 306–310. [Google Scholar]
- Lee, D.; Bae, J.; Kim, Y.K.; Gil, M.; Lee, J.Y.; Park, C.S.; Lee, K.J. Inhibitory effects of berberine on lipopolysaccharide-induced inducible nitric oxide synthase and the high-mobility group box 1 release in macrophages. Biochem. Biophys. Res. Commun. 2013, 431, 506–511. [Google Scholar] [CrossRef]
- Chu, M.; Ding, R.; Chu, Z.Y.; Zhang, M.B.; Liu, X.Y.; Xie, S.H.; Zhai, Y.-J.; Wang, Y.D. Role of berberine in anti-bacterial as a high-affinity LPS antagonist binding to TLR4/MD-2 receptor. BMC Complement. Altern. Med. 2014, 14, 89. [Google Scholar] [CrossRef]
- Chen, C.; Yu, Z.; Li, Y.; Fichna, J.; Storr, M. Effects of berberine in the gastrointestinal tract—A review of actions and therapeutic implications. Am. J. Chin. Med. 2014, 42, 1053–1070. [Google Scholar] [CrossRef]
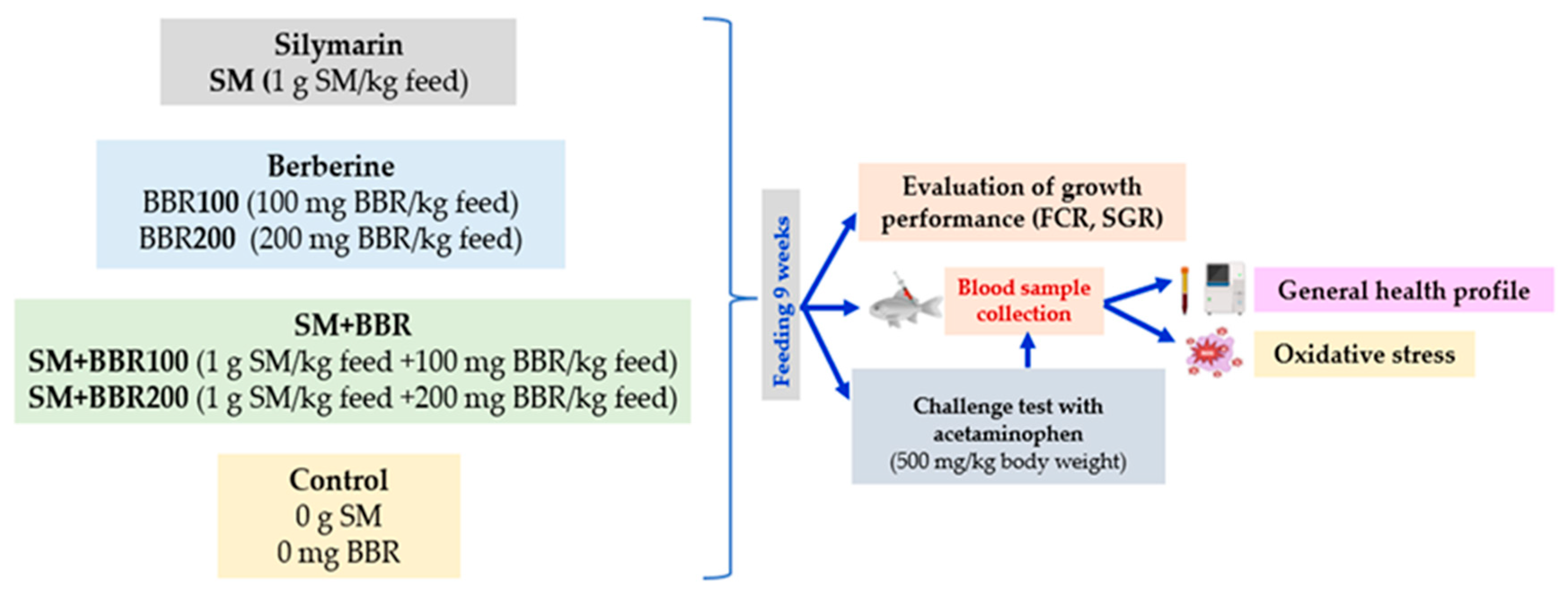
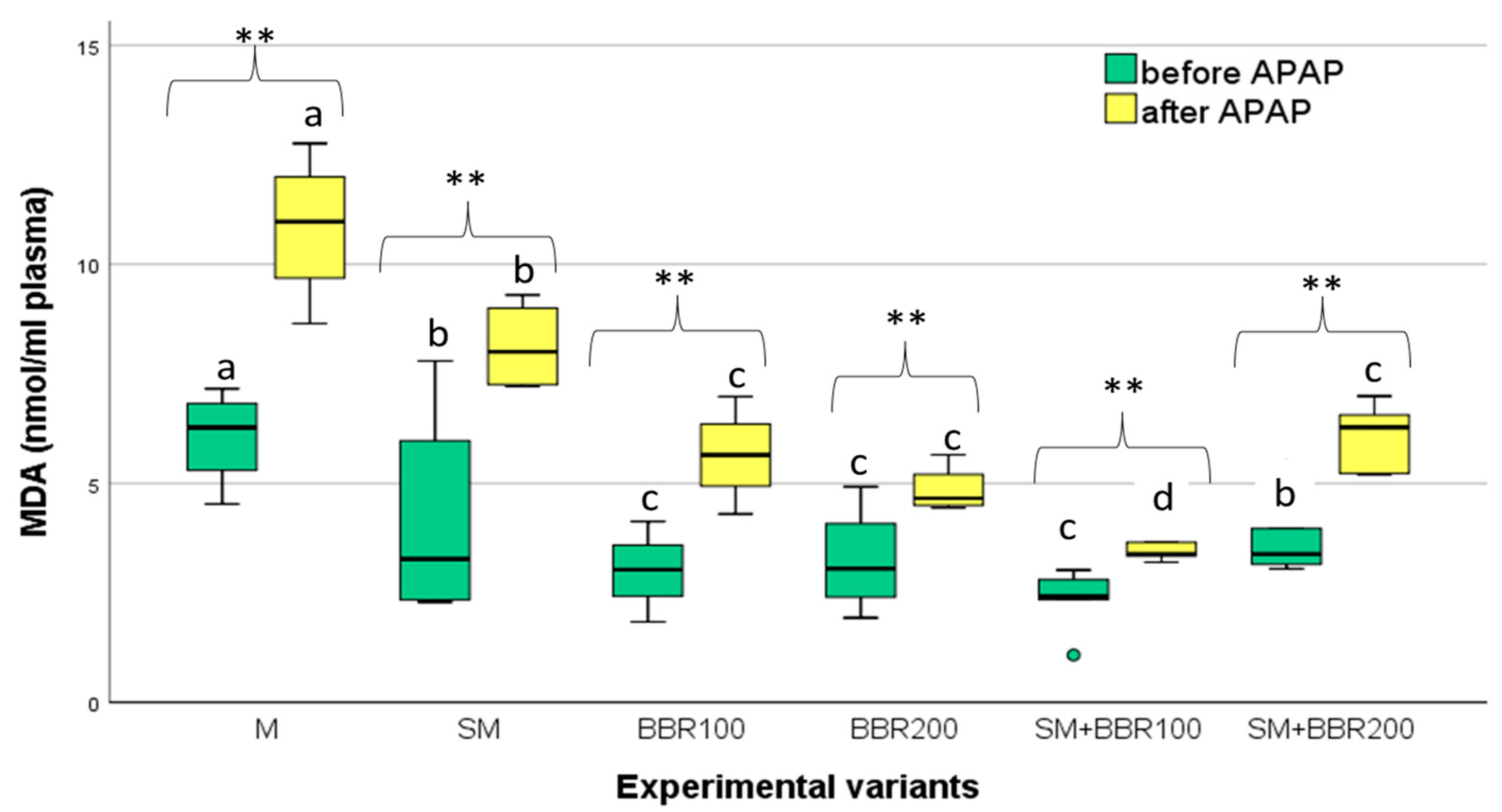
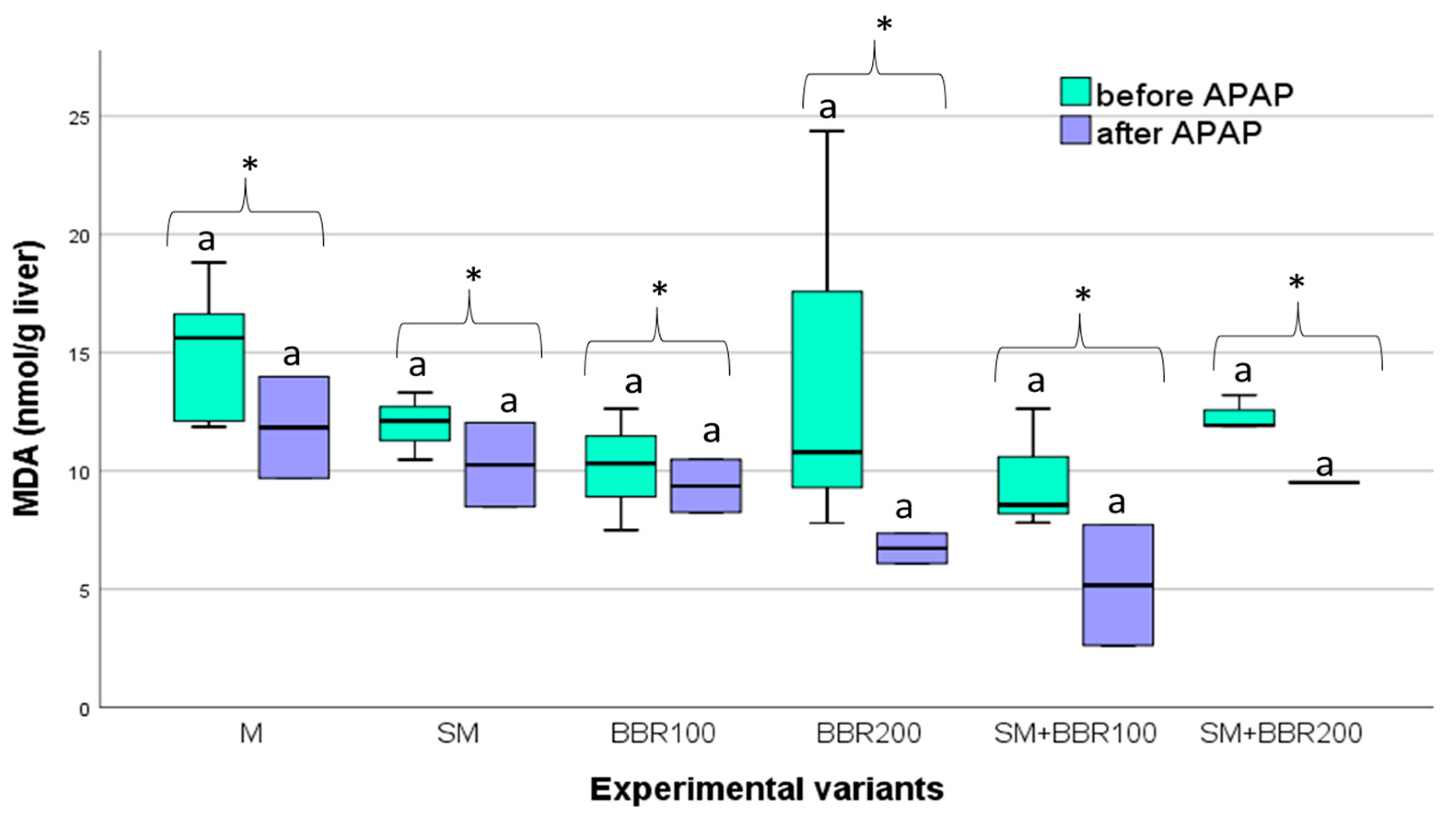
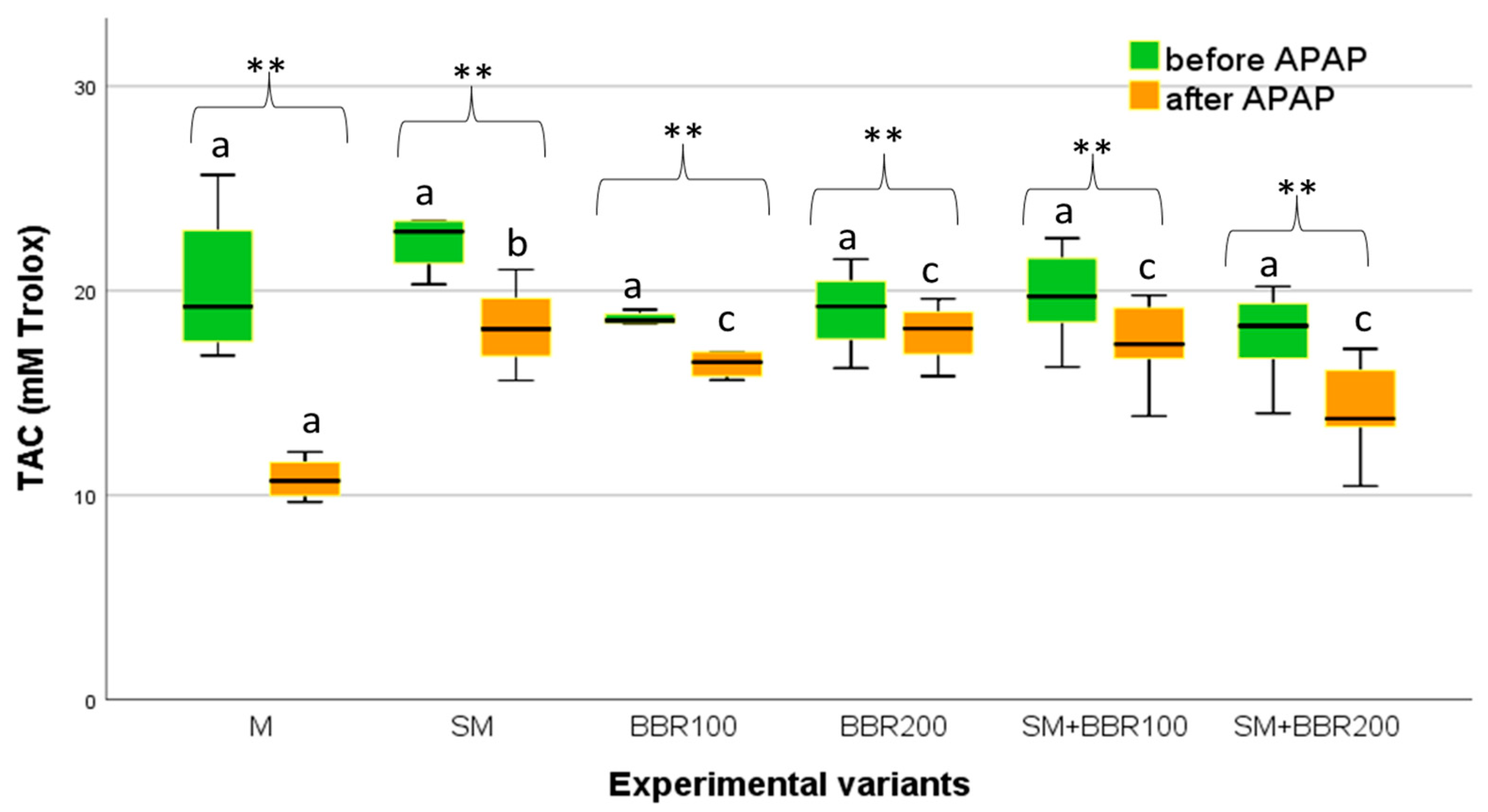
| Growth Parameters | M | SM | BBR100 | BBR200 | SM + BBR100 | SM + BBR200 |
|---|---|---|---|---|---|---|
| IW (g) | 122.70 ± 10.25 a | 121.60 ± 9.12 a | 113.10 ± 7.16 a | 124.50 ± 5.2 a | 115.10± 6,2 a | 117.40 ± 4.65 a |
| FW(g) | 179.15 ± 18.61 a | 195.60 ± 10.12 b | 180.40 ± 19.65 a | 198.70 ± 18.16 b | 179.03± 17.46 a | 180.61 ± 10.12 a |
| SGR (%/day) | 0.69 ± 0.02 a | 0.74 ± 0.01 b | 0.73 ± 0.04 b | 0.73 ± 0.02 b | 0.69± 0.03 a | 0.68 ± 0.03 a |
| FCR (g/g) | 2,21 ± 0.04 b | 1.47 ± 0.08 a | 1.61 ± 0.05 b | 1.46 ± 0.03 a | 1.68± 0.02 b | 2.10 ± 0.05 c |
| Body Indices | M | SM | BBR100 | BBR200 | SM + BBR100 | SM + BBR200 |
|---|---|---|---|---|---|---|
| HSI (%) | 2.29 ± 0.18 b | 1.91 ± 0.24 a | 1.92 ± 0.38 a | 1.69 ± 0.68 a | 1.65 ± 0.21 a | 2.38 ± 0.74 b |
| GSI (%) | 5.07 ± 2.36 b | 3.03 ± 2.35 a | 4.32 ± 1.43 b | 2.96 ± 0.09 a | 5.40 ± 1.80 b | 7.28 ± 5.68 c |
| SSI (%) | 0.27 ± 0.07 a | 0.28 ± 0.06 a | 0.28 ± 0.01 a | 0.33 ± 0.19 a | 0.24 ± 0.06 a | 0.23 ± 0.08 a |
| Composition | M | SM | BBR100 | BBR20 | SM + BBR100 | SM + BBR200 |
|---|---|---|---|---|---|---|
| Water (%) | 78.44 ± 1.26 a | 78.52 ± 0.64 a | 79.30 ± 0.22 a | 79.04 ± 1.32 a | 79.09 ± 1.32 a | 79.54 ± 0.26 a |
| Protein (%) | 17.06 ± 0.73 a | 18.63 ± 0.17 a | 17.54 ± 0.47 a | 17.94 ± 0.53 a | 17.69 ± 0.61 a | 17.42 ± 0.56 a |
| Lipids (%) | 2.44 ± 0.88 b | 1.44 ± 0.46 a | 1.39 ± 0.08 a | 1.32 ± 0.22 a | 1.68 ± 0.28 a | 1.31 ± 0.13 a |
| Ash (%) | 1.12 ± 0.07 a | 1.29 ± 0,09 a | 1.26 ± 0.05 a | 1.07 ± 0.05 a | 1.72 ± 0.65 a | 1.20 ± 0.24 a |
| Time | Exp. Variants | Ht (%) | Hb (g/dL) | RBC (×106/mm3) | MCV (μm3) | MCH (pg) | MCHC (g/dL) |
|---|---|---|---|---|---|---|---|
| Before challenge | M | 43.15 ± 2.94 a* | 10.09 ± 3.24 a* | 1.28 ± 0.22 b* | 340.84 ± 35.37 a* | 82.90 ± 35.96 a* | 24.22 ± 4.22 a* |
| SM | 36.62 ± 0.31 a* | 8.79 ± 0.49 a* | 1.23 ± 0.14 a* | 299.70 ± 32.24 a* | 72.10 ± 10.92 a* | 24.00 ± 1.42 a* | |
| BBR100 | 42.14 ± 3.80 a* | 10.36 ± 0.38 a* | 1.34 ± 0.10 b* | 318.73 ± 53.26 a* | 78.13 ± 8.95 a* | 24.66 ± 1.38 a* | |
| BBR200 | 44.36 ± 1.11 a* | 11.88 ± 1.18 a* | 1.42 ± 0.47 b* | 333.38 ± 98.70 a* | 88.13 ± 22.24 a* | 26.83 ± 7.42 a* | |
| SM + BBR100 | 43.98 ± 3.78 a* | 12.70 ± 3.25 a* | 1.40 ± 0.08 b* | 315.58 ± 39.75 a* | 97.41 ±20.10 a* | 31.55 ± 8.61 a* | |
| SM + BBR200 | 44.11 ± 3.63 a* | 12.44 ± 2.12 a* | 1.91± 0.39 c* | 240.85 ± 58.82 a* | 67.04 ± 14.48 a* | 28.17 ± 3.81 a* | |
| After challenge | M | 31.86 ± 4.75 a** | 12.67 ± 2.33 a* | 1.52 ± 0.14 a** | 206.95 ± 37.10 a** | 80.95 ± 10.40 a* | 40.55 ± 9.76 a* |
| SM | 28.57 ± 2.76 a** | 13.77 ± 4.70 a* | 1.31 ± 0.26 a* | 235.44 ± 49.96 a* | 105.66 ± 25.77 a* | 49.43 ± 7.16 a** | |
| BBR100 | 24.41 ± 3.63 a** | 10.33 ± 5.03 a* | 1.44 ± 0.28 a* | 180.50 ± 31.01 a** | 84.21 ± 22.22 a* | 49.38 ± 5.74 a** | |
| BBR200 | 28.13 ± 6.05 a** | 12.52 ± 0.70 a* | 1.30 ± 0.27 a* | 225.99 ± 74.15 a* | 91.63 ± 18.34 a* | 42.11 ± 8.41 a* | |
| SM + BBR100 | 23.99 ± 5.88 a** | 12.99 ± 1.57 a* | 1.32 ± 0.48 a* | 215.07 ± 79.54 a** | 91.94 ± 28.43 a* | 48.21 ± 6.16 a** | |
| SM + BBR200 | 26.75 ± 8.19 a** | 13.40 ± 3.37 a* | 1.19 ± 0.23 a** | 247.57 ± 94.17 a* | 109.65 ± 13.83 a* | 52.35 ± 4.66 a* |
| Carp Body Weight (g) | Ht (%) | Hb (g/dL) | RBC (×106/mm3) | MCV (μm3) | MCH (pg) | MCHC (g/dL) | References |
|---|---|---|---|---|---|---|---|
| 138.3 ± 28.7 | 29 ± 3 | 8.63 ± 0.76 | 1.64 ± 0.14 | 180.30 ±15.3 | 52.9 ± 4.7 | 29 ± 2.0 | [62] |
| 200 | 31.8 ± 5.5 | 6.94 ± 1.6 | 1.81 ± 0.2 | 178.2 ± 31.7 | 40.2 ± 6.5 | 21.6 ± 3.3 | [63] |
| 61.2 ± 7.3 | 22–39 | 3.76–8.76 | 0.90–2.02 | 133.7–248.4 | 36.9–57.8 | 15–32 | [64] |
| 138 ± 28.7 | 26 ± 3 | 6.84 ± 0.9 | 1.05 ± 0.03 | 248.1 ± 36 | 65.2 ± 10 | 26.3 ± 0.13 | [65] |
| 297.4 ± 55.6 | 29.2 ± 2.7 | 7.43 ± 0.7 | 1.63 ± 0.13 | 179.5 ± 13.5 | 45.8 ± 4.2 | 25.5 ± 0.78 | [66] |
| 41 ± 0.20 | 42.2 ± 0.5 | 11.24 ± 0.41 | 1.80 ± 0.02 | 234.5 ± 2.0 | 62.5 ± 1.6 | 26.67 ± 0.7 | [67] |
| 67.5 ± 9.1 | 30.9 ± 3.5 | 7.18 ± 0.13 | 1.40 ± 0.09 | 217.2 ± 24.2 | 51.9 ± 8.2 | 24.1 ± 0.31 | [68] |
| 61.9 ± 2.4 | 42.8 ± 4 | 9.4 ± 0.8 | 1.37 ± 0.19 | 317.9 ± 60.9 | - | 22.2 ± 3.3 | [69] |
| Parameter | After the Feeding Experiment/Before APAP Challenge Test | |||||
|---|---|---|---|---|---|---|
| M | SM | BBR100 | BBR200 | SM + BBR100 | SM + BBR200 | |
| TG (mg/dL) | 458 ± 81.93 c | 370.33 ± 76.44 b | 367.00 ± 67.39 b | 347.29 ± 45.74 b | 343.5 ± 53.78 a | 342.6+ ± 48.71 a |
| CHOL (mg/dL) | 268.33 ± 33.07 c | 234.00 ± 16.7 b | 206.75 ± 9.81 a | 225.00 ± 22.99 b | 225.66 ± 12.5 b | 214.5 ± 20.69 a |
| LDL (mg/dL) | 115.10 + 4.56 d | 98.22 ± 11.22 c | 53.67 ± 7.55 b | 46.67 ± 9.34 a | 64.34 ± 8.56 b | 54.76 ± 6.55 b |
| HDL (mg/dL) | 44.71 ± 6.32 a | 42.73 ± 8.12 a | 77.50 ± 9.10 b | 89.88 ± 8.45 c | 75.43 ± 6.77 b | 82.12 ± 7.45 b |
| ALT (U/L) | 10.25 ± 1.25 b | 10.00 ± 3.00 b | 8.00 ± 1.82 a | 8.12 ± 0.63 a | 9.33 ± 4.03 a | 8.33 ± 1.96 a |
| AST (U/L) | 174 ± 19.71 a | 142.67 ± 49.03 a | 134.79 ± 8.86 a | 188.67 ± 37.11 a | 199.5 ± 55.47 a | 147.8 ± 22.81 a |
| ALP (U/L) | 186 ± 48.46 a | 158.33 ± 27.28 a | 144.67 ± 20.71 a | 147 ± 54.72 a | 185.66 ± 68.19 a | 155.25 ± 77.10 a |
| GGT (U/L) | 1.33 ± 0.47 a | 1.23 ± 0.75 a | 0.92 ± 0.09 a | 1.00 ± 0.44 a | 1.38 ± 0.33 a | 1.45 ± 0.32 a |
| BILD (mg/dL) | 0.18 ± 0.05 a | 0.13 ± 0.03 a | 0.15 ± 0.01 a | 0.18 ± 0.04 a | 0.12 ± 0.03 a | 0.15 ± 0.02 a |
| BILT (mg/dL) | 0.31 ± 0.06 b | 0.20 ± 0.02 a | 0.21 ± 0.06 a | 0.23 ± 0.09 a | 0.19 ± 0.02 a | 0.21 ± 0.01 a |
| Parameter | After APAP Challenge Test | |||||
| M | SM | BBR100 | BBR200 | SM + BBR100 | SM + BBR200 | |
| TG (mg/dL) | 141.00 ± 29.81 a** | 189.25 ±38.59 b* | 186.75 ± 41.55 b* | 181.75 ± 38.81 b* | 182.25 ± 28.50 b* | 163.25 ± 27.73 b* |
| CHOL (mg/dL) | 187.25 ± 32.01 a** | 173.25 ± 14.10 a** | 196.50 ± 27.19 a* | 188.25 ± 24.40 a* | 156.00 ± 25.17 a** | 170.50 ± 18.77 a* |
| LDL (mg/dL) | 95.10 + 4.56 d** | 78.22 ± 11.22 c** | 44.67 ± 7.55 b** | 39.61 ± 7.24 a* | 44.98 ± 7.65 b** | 39.16 ± 8.34 b** |
| HDL (mg/dL) | 34.71 ± 6.32 a** | 35.73 ± 8.12 a** | 68.20 ± 9.10 b* | 82.33 ± 4.95 c* | 70.43 ± 7.37 b* | 77.12 ± 7.45 b* |
| ALT (U/L) | 42.50 ± 8.58 b** | 25.50 ± 8.54 a* | 26.00 ± 2.58 a* | 29.75 ± 12.39 a* | 25.30 ± 0.57 a* | 29.75 ± 4.71 a* |
| AST(U/L) | 364.25 ± 78.88 b** | 218.00 ± 44.21 a* | 295.25 ± 88.68 a** | 304.50 ± 32.27 a** | 242.00 ± 24.12 a* | 273.20 ± 79.95 a* |
| ALP (U/L) | 294.50 ± 15.02 c** | 183.67 ± 64.01 b* | 173.33 ± 49.80 a* | 154.00 ± 77.6 a* | 199.5 ± 23.01 b* | 179.25 ± 78.54 b* |
| GGT (U/L) | 2.90 ± 0.85 c** | 1.55 ± 0.42 b* | 1.27 ± 0.25 a* | 1.15 ± 0.20 a* | 1.50 ± 0.57 b* | 2.00 ± 1.55 b* |
| BILD (mg/dL) | 0.26 ± 0.04 c** | 0.16 ± 0.03 a* | 0.22 ± 0.01 b* | 0.24 ± 0.05 b* | 0.15 ± 0.04 a* | 0.20 ± 0.01 b* |
| BILT (mg/dL) | 0.46 ± 0.06 c** | 0.27 ± 0.04 a* | 0.31 ± 0.04 a* | 0.35 ± 0.10 b* | 0.25 ± 0.10 a* | 0.34 ± 0.09 b* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grădinariu, L.; Dediu, L.; Crețu, M.; Grecu, I.R.; Docan, A.; Istrati, D.I.; Dima, F.M.; Stroe, M.D.; Vizireanu, C. The Antioxidant and Hepatoprotective Potential of Berberine and Silymarin on Acetaminophen Induced Toxicity in Cyprinus carpio L. Animals 2024, 14, 373. https://doi.org/10.3390/ani14030373
Grădinariu L, Dediu L, Crețu M, Grecu IR, Docan A, Istrati DI, Dima FM, Stroe MD, Vizireanu C. The Antioxidant and Hepatoprotective Potential of Berberine and Silymarin on Acetaminophen Induced Toxicity in Cyprinus carpio L. Animals. 2024; 14(3):373. https://doi.org/10.3390/ani14030373
Chicago/Turabian StyleGrădinariu, Lăcrămioara, Lorena Dediu, Mirela Crețu, Iulia Rodica Grecu, Angelica Docan, Daniela Ionela Istrati, Floricel Maricel Dima, Maria Desimira Stroe, and Camelia Vizireanu. 2024. "The Antioxidant and Hepatoprotective Potential of Berberine and Silymarin on Acetaminophen Induced Toxicity in Cyprinus carpio L." Animals 14, no. 3: 373. https://doi.org/10.3390/ani14030373
APA StyleGrădinariu, L., Dediu, L., Crețu, M., Grecu, I. R., Docan, A., Istrati, D. I., Dima, F. M., Stroe, M. D., & Vizireanu, C. (2024). The Antioxidant and Hepatoprotective Potential of Berberine and Silymarin on Acetaminophen Induced Toxicity in Cyprinus carpio L. Animals, 14(3), 373. https://doi.org/10.3390/ani14030373








