Dietary Paper Mulberry Silage Supplementation Improves the Growth Performance, Carcass Characteristics, and Meat Quality of Yangzhou Goose
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Experiment Design
2.3. Growth Performance and Sampling
2.4. Meat Quality Determination
2.5. Analysis of Metabolomics
2.5.1. Extraction of Metabolites
2.5.2. LC-MS Analysis
2.5.3. Multivariate Statistical Analysis
2.6. Statistic Analysis
3. Results
3.1. Growth Performance and Carcass Characteristics
3.2. Meat Quality Parameters
3.3. Untargeted Metabolomic
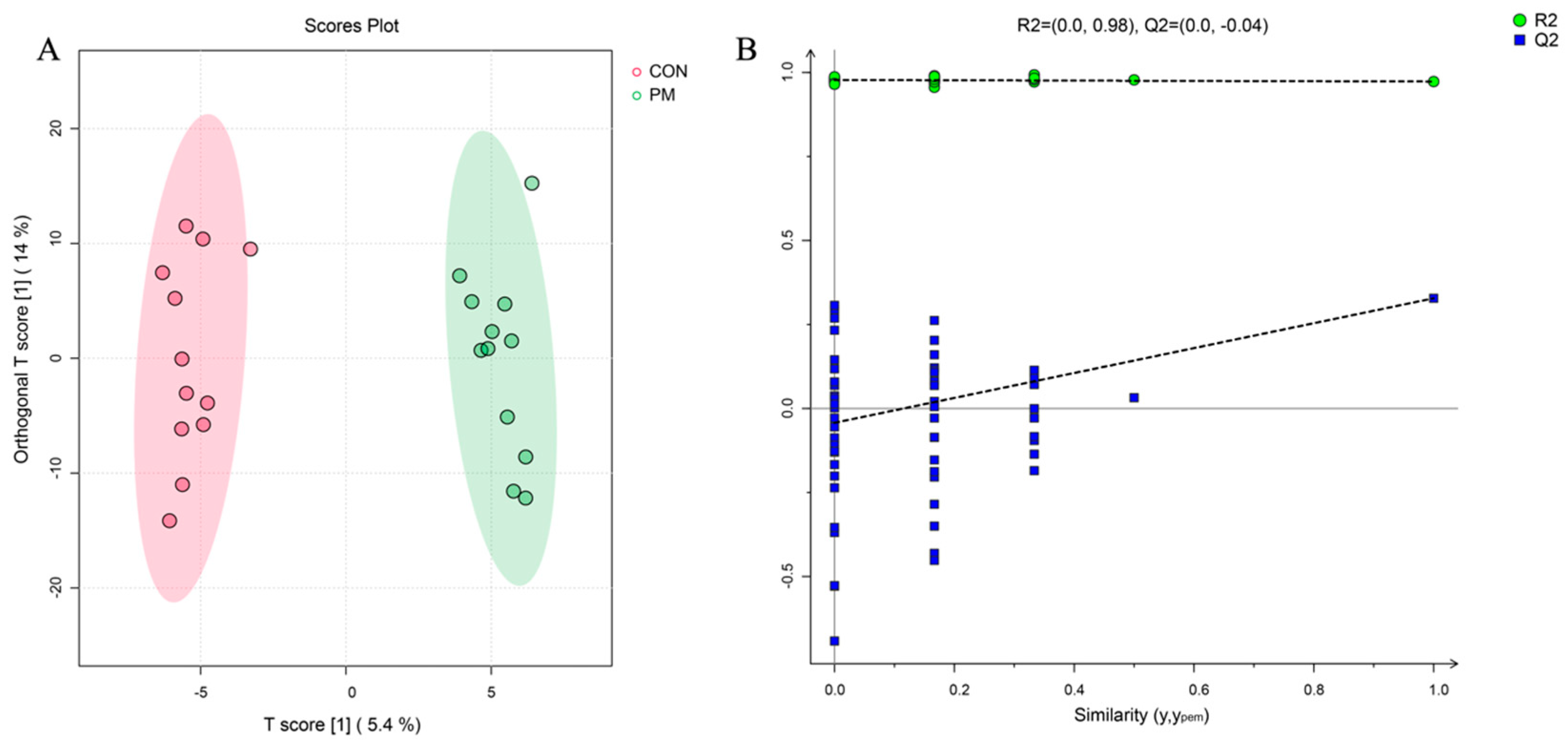
3.3.1. General Information for Metabolomics Data
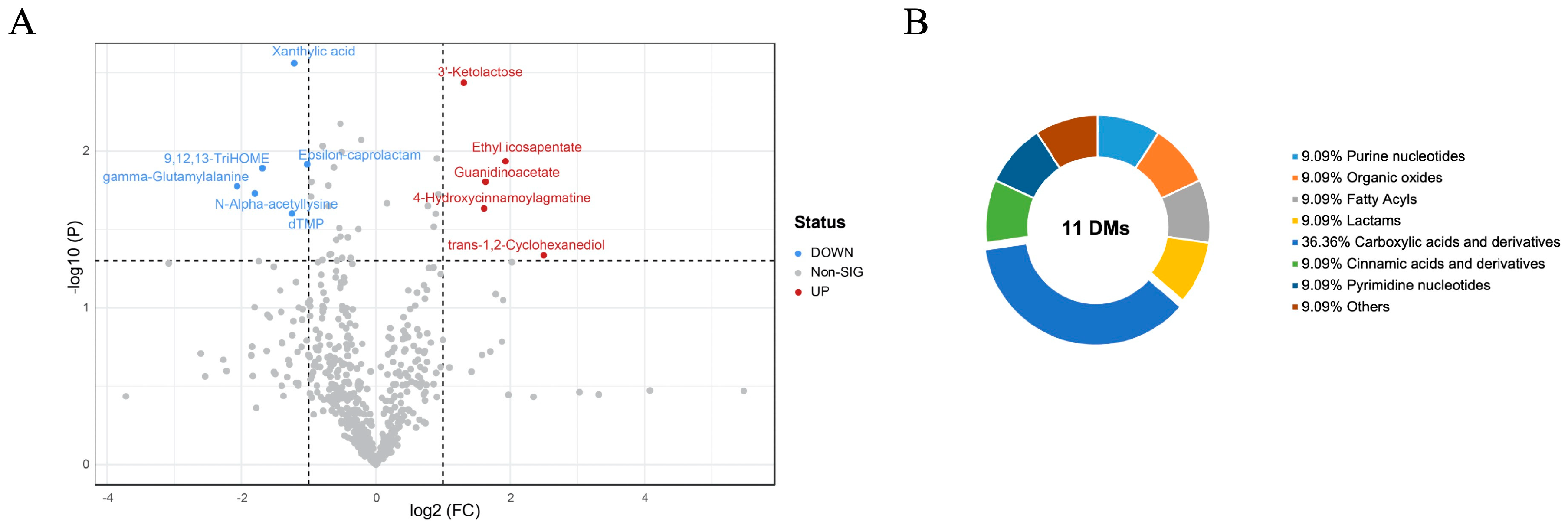
3.3.2. Different Metabolites of Breast Muscle between PM and CON Groups
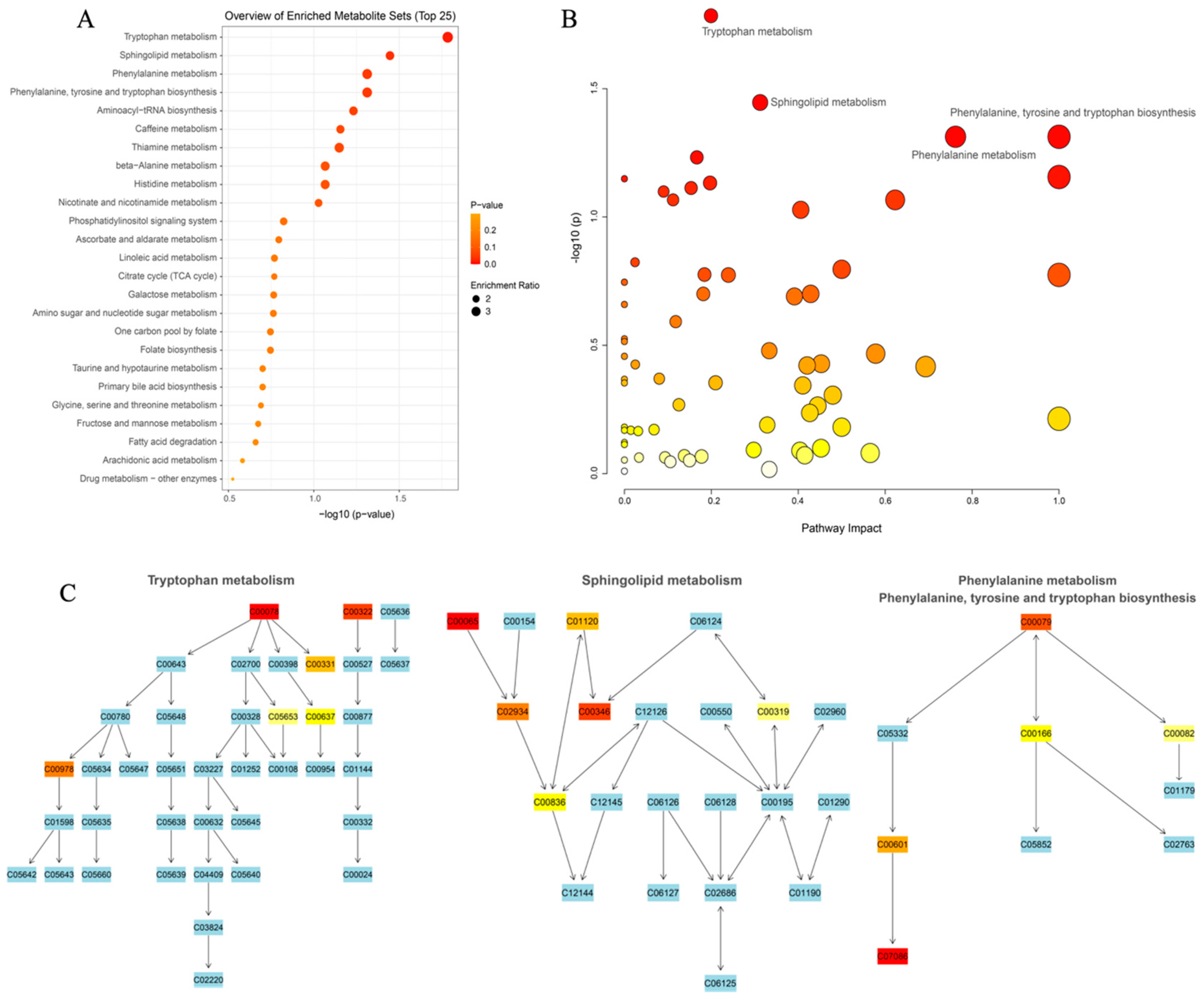
3.3.3. Elucidating the Metabolites Difference and Pathway
4. Discussion
4.1. Growth Performance, Carcass Characteristics, and Meat Quality of Yangzhou Geese
4.2. Untargeted Metabolomic (LC-MS/MS) Analysis of Breast Muscle in the CON and PM Groups
4.3. Flavor Compounds Analysis of Breast Muscle in the CON and PM Groups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, X.N.; Xu, Y.G.; Zhen, Z.Y.; Li, J.J.; Zheng, H.B.; Li, S.H.; Hu, Q.N.; Ye, P.F. Slaughter performance of the main goose breeds raised commercially in China and nutritional value of the meats of the goose breeds: A systematic review. J. Sci. Food Agric. 2023, 103, 3748–3760. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiao, Y.; Gui, G.; Li, J.; Wang, J.; Li, D. Microbial community and short-chain fatty acid profile in gastrointestinal tract of goose. Poult. Sci. 2018, 97, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Wood, K.M.; Salim, H.; McEwen, P.L.; Mandell, I.B.; Miller, S.P.; Swanson, K.C. The effect of corn or sorghum dried distillers grains plus solubles on growth performance and carcass characteristics of cross-bred beef steers. Anim. Feed Sci. Technol. 2011, 165, 23–30. [Google Scholar] [CrossRef]
- Liu, H.W.; Zhou, D.W. Influence of pasture intake on meat quality, lipid oxidation, and fatty acid composition of geese. J. Anim. Sci. 2013, 91, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Wang, Z.Y.; Yang, H.M.; Xu, L.; Xie, Y.J.; Jin, S.L.; Sheng, D.F. Effects of dietary fiber on growth performance, slaughter performance, serum biochemical parameters, and nutrient utilization in geese. Poult. Sci. 2017, 96, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Shi, T.; Wang, B.; Zhang, Y.; Zhao, X.; Wang, Y.; Dai, G.; Xie, K. Comparison on the Contents of Amino Acids and Inosine Monphosphate in Muscle of Yangzhou Geese and Their Crossbred Combinations. China Anim. Husb. Vet. Med. 2018, 45, 1184–1195. [Google Scholar] [CrossRef]
- Guo, B.D.; Li, D.H.; Zhou, B.B.; Jiang, Y.; Bai, H.; Zhang, Y.; Xu, Q.; Yongzhang; Chen, G.H. Research Note: Effect of diet with different proportions of ryegrass on breast meat quality of broiler geese. Poult. Sci. 2020, 99, 2500–2507. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zi, X.J.; Tang, J.; Xu, T.S.; Gu, L.H.; Zhou, H.L. Effects of cassava foliage on feed digestion, meat quality, and antioxidative status of geese. Poult. Sci. 2020, 99, 423–429. [Google Scholar] [CrossRef]
- Xiong, Y.; Guo, C.; Wang, L.; Chen, F.; Dong, X.; Li, X.; Ni, K.; Yang, F. Effects of Paper Mulberry Silage on the Growth Performance, Rumen Microbiota and Muscle Fatty Acid Composition in Hu Lambs. Fermentation 2021, 7, 286. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, X.; Li, X.M.; Guo, L.N.; Yang, F.Y.; Ni, K.K. Exploring the rumen microbiota of Hu lambs in response to diet with paper mulberry. Appl. Microbiol. Biotechnol. 2023, 107, 4961–4971. [Google Scholar] [CrossRef]
- Wang, L.; Gao, H.; Sun, C.; Huang, L. Protective Application of Morus and Its Extracts in Animal Production. Animals 2022, 12, 3541. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.W.; Xiong, Y.; Wang, X.K.; Guo, L.N.; Lin, Y.L.; Ni, K.K.; Yang, F.Y. Effects of Lacto bacillus plantarum on Fermentation Quality and Anti-Nutritional Factors of Paper Mulberry Silage. Fermentation 2022, 8, 144. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Q.; Li, Q.; Liu, R.; Tang, L.; Song, Y.; Luo, J.; Liu, S.; Wang, P. Effects of hybrid Broussonetia papyrifera silage on growth performance, visceral organs, blood biochemical indices, antioxidant indices, and carcass traits in dairy goats. Anim. Feed Sci. Technol. 2022, 292, 115435. [Google Scholar] [CrossRef]
- Sun, H.; Luo, Y.; Zhao, F.F.; Fan, Y.T.; Ma, J.N.; Jin, Y.Q.; Hou, Q.R.; Ahmed, G.; Wang, H.R. The Effect of Replacing Wildrye Hay with Mulberry Leaves on the Growth Performance, Blood Metabolites, and Carcass Characteristics of Sheep. Animals 2020, 10, 2018. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.L.; Hou, Q.R.; Wang, M.Z.; Zhao, W.G.; Feng, D.; Pi, Y.; Sun, X.Z. Effects of dietary mulberry leaf powder on growth performance, blood metabolites, meat quality, and antioxidant enzyme-related gene expression of fattening Hu lambs. Can. J. Anim. Sci. 2020, 100, 510–521. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Dietary supplementation with Moringa oleifera and mulberry leaf affects pork quality from finishing pigs. J. Anim. Physiol. Anim. Nutr. 2021, 105, 72–79. [Google Scholar] [CrossRef]
- Liu, Z.N.; Chen, Q.; Zhong, Y.P.; Wu, Y.J.; Li, J.J.; Kong, Z.W.; Zhang, Q.; Lei, X.W. Effects of earthworm hydrolysate in production performance, serum biochemical parameters, antioxidant capacity and intestinal function of Muscovy ducks. Poult. Sci. 2023, 102, 102409. [Google Scholar] [CrossRef] [PubMed]
- Faustman, C.; Cassens, R.G. The biochemical basis for discoloration in fresh meat: A review. J. Muscle Foods 1990, 1, 217–243. [Google Scholar] [CrossRef]
- Li, Y.C.; Fu, B.; Zhang, J.M.; Wang, G.J.; Gong, W.B.; Tian, J.J.; Li, H.Y.; Zhang, K.; Xia, Y.; Li, Z.F.; et al. Effects of heat stress on the chemical composition, oxidative stability, muscle metabolism, and meat quality of Nile tilapia (Oreochromis niloticus). Food Chem. 2023, 426, 136590. [Google Scholar] [CrossRef]
- Chen, X.; Cao, J.; Geng, A.L.; Zhang, X.Y.; Wang, H.H.; Chu, Q.; Yan, Z.X.; Zhang, Y.; Liu, H.G.; Zhang, J. Integration of GC-MS and LC-MS for metabolite characteristics of thigh meat between fast- and slow-growing broilers at marketable age. Food Chem. 2023, 403, 134362. [Google Scholar] [CrossRef]
- Warren, C.R.; O’Sullivan, J.F.; Friesen, M.; Becker, C.E.; Zhang, X.L.; Liu, P.C.; Wakabayashi, Y.; Morningstar, J.E.; Shi, X.; Choi, J.; et al. Induced Pluripotent Stem Cell Differentiation Enables Functional Validation of GWAS Variants in Metabolic Disease. Cell Stem Cell 2017, 20, 547–557.e7. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Dong, K.; Wang, Q.; Huang, X.; Wang, G.Z.; An, F.P.; Luo, Z.; Luo, P. Changes in volatile flavor of yak meat during oxidation based on multi-omics. Food Chem. 2022, 371, 131103. [Google Scholar] [CrossRef] [PubMed]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Liao, R.Y.; Xia, Q.; Zhou, C.Y.; Geng, F.; Wang, Y.; Sun, Y.Y.; He, J.; Pan, D.D.; Cao, J.X. LC-MS/MS-based metabolomics and sensory evaluation characterize metabolites and texture of normal and spoiled dry-cured hams. Food Chem. 2022, 371, 131156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Yang, H.M.; Xu, L.; Wang, Z.Y.; Zhao, Y.; Chen, X.S. Effects of dietary fiber levels on cecal microbiota composition in geese. Asian-Australas. J. Anim. Sci. 2018, 31, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; He, R.; Mao, Z.; Zhang, B.; Zhang, Y.; Xiang, X. Effect of crude dietary fiber level on production performance, metabolic hormone and blood biochemical index of geese. Chin. J. Vet. Sci. 2007, 27, 914–918. [Google Scholar]
- Bozkurt, M.; Koçer, B.; Ege, G.; Tüzün, A.E.; Biyik, H.H.; Poyrazoglu, E. Influence of the particle size and form of feed on growth performance, digestive tract traits and nutrient digestibility of white egg-laying pullets from 1 to 112 D of age. Poult. Sci. 2019, 98, 4016–4029. [Google Scholar] [CrossRef]
- Aslan, R.; Ozturk, E. Effects of maize silage feeding on growth performance, carcass characteristics, digestive system length, chemical composition, and meat quality of domestic geese. Trop. Anim. Health Prod. 2022, 54, 325. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Zheng, S.; Dai, W.; Shen, X.; Zhang, Y.; Zhao, W.; Chang, G.; Xu, Q.; Chen, G. Effects of forage feeding versus grain feeding on the growth performance and meat quality of Yangzhou geese. Br. Poult. Sci. 2017, 58, 397–401. [Google Scholar] [CrossRef]
- Desbuards, N.; Gourbeyre, P.; Haure-Mirande, V.; Darmaun, D.; Champ, M.; Bodinier, M. Impact of perinatal prebiotic consumption on gestating mice and their offspring: A preliminary report. Br. J. Nutr. 2012, 107, 1245–1248. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.; Luo, J.Q.; Yu, B.; Zheng, P.; Huang, Z.Q.; Mao, X.B.; He, J.; Yu, J.; Chen, J.L.; Chen, D.W. Dietary resveratrol supplementation improves meat quality of finishing pigs through changing muscle fiber characteristics and antioxidative status. Meat Sci. 2015, 102, 15–21. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Ge, W.; Zhang, M.; Yue, B.; Zheng, H.; Ren, M.; Zhang, Z. Linoleic acid on growth performance, slaughter performance, meat quality and nutrient availabilities of meat geese aged from 5 to 16 weeks. Chin. J. Anim. Nutr. 2016, 28, 3473–3482. [Google Scholar]
- Li, H.; Liu, Y.; Wei, L.; Lin, Q.; Zhang, Z.F. Effects of Feeding Fermented Medicago sativa (Plus Soybean and DDGS) on Growth Performance, Blood Profiles, Gut Health, and Carcass Characteristics of Lande (Meat) Geese. Front. Physiol. 2022, 13, 902802. [Google Scholar] [CrossRef]
- Zhao, H.H.; Chong, J.; Tang, R.; Li, L.; Xia, J.G.; Li, D.P. Metabolomics investigation of dietary effects on flesh quality in grass carp (Ctenopharyngodon idellus). Gigascience 2018, 7, giy111. [Google Scholar] [CrossRef]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.Z.; Bourque, G.; Wishart, D.S.; Xia, J.G. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef]
- Plumb, R.S.; Granger, J.H.; Stumpf, C.L.; Johnson, K.A.; Smith, B.W.; Gaulitz, S.; Wilson, I.D.; Castro-Perez, J. A rapid screening approach to metabonomics using UPLC and oa-TOF mass spectrometry: Application to age, gender and diurnal variation in normal/Zucker obese rats and black, white and nude mice. Analyst 2005, 130, 844–849. [Google Scholar] [CrossRef]
- Ostojic, S.M. Creatine as a food supplement for the general population. J. Funct. Foods 2021, 83, 104568. [Google Scholar] [CrossRef]
- Wu, G.Y. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef]
- Bampidis, V.; Azimonti, G.; Bastos, M.D.; Christensen, H.; Dusemund, B.; Durjava, M.F.; Kouba, M.; López-Alonso, M.; Puente, S.L.; Marcon, F.; et al. Safety and efficacy of a feed additive consisting of guanidinoacetic acid for all animal species (Alzchem Trostberg GmbH). Efsa J. 2022, 20, e07269. [Google Scholar] [CrossRef] [PubMed]
- Reicher, N.; Epstein, T.; Gravitz, D.; Cahaner, A.; Rademacher, M.; Braun, U.; Uni, Z. From broiler breeder hen feed to the egg and embryo: The molecular effects of guanidinoacetate supplementation on creatine transport and synthesis. Poult. Sci. 2020, 99, 3574–3582. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D.; Powell, W.S. Metabolism of linoleic-acid by prostaglandin endoperoxide synthase from adult and fetal blood-vessels. Biochim. Biophys. Acta 1983, 754, 57–71. [Google Scholar] [CrossRef]
- Funk, C.D.; Powell, W.S. Release of prostaglandins and monohydroxy and trihydroxy metabolites of linoleic and arachidonic acids by adult and fetal aortae and ductus-arteriosus. J. Biol. Chem. 1985, 260, 7481–7488. [Google Scholar] [CrossRef]
- Lund, M.N.; Heinonen, M.; Baron, C.P.; Estévez, M. Protein oxidation in muscle foods: A review. Mol. Nutr. Food Res. 2011, 55, 83–95. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Szendro, Z.; Cullere, M.; Gerencser, Z.; Matics, Z.; Castellini, C.; Dalle Zotte, A. The effect of dietary Digestarom herbal supplementation on rabbit meat fatty acid profile, lipid oxidation and antioxidant content. Meat Sci. 2016, 121, 238–242. [Google Scholar] [CrossRef]
- Orlowski, S.; Flees, J.; Greene, E.S.; Ashley, D.; Lee, S.O.; Yang, F.L.; Owens, C.M.; Kidd, M.; Anthony, N.; Dridi, S. Effects of phytogenic additives on meat quality traits in broiler chickens. J. Anim. Sci. 2018, 96, 3757–3767. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhou, X.; Liu, M.R.; Zang, H.R.; Zhang, R.S.; Yang, H.; Jin, S.J.; Qi, X.Y.; Shan, A.S.; Feng, X.J. Quality of chicken breast meat improved by dietary pterostilbene referring to up-regulated antioxidant capacity and enhanced protein structure. Food Chem. 2023, 405, 134848. [Google Scholar] [CrossRef]
- Danneskiold-Samsoe, N.B.; Barros, H.; Santos, R.; Bicas, J.L.; Cazarin, C.B.B.; Madsen, L.; Kristiansen, K.; Pastore, G.M.; Brix, S.; Marostica, M.R. Interplay between food and gut microbiota in health and disease. Food Res. Int. 2019, 115, 23–31. [Google Scholar] [CrossRef]
- Aguilera-Romero, A.; Gehin, C.; Riezman, H. Sphingolipid homeostasis in the web of metabolic routes. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2014, 1841, 647–656. [Google Scholar] [CrossRef]
- Li, J.; Vavricka, C.J.; Yang, C.; Han, Q.; Cooper, A.J.L. Amino Acids|Aromatic Amino Acid Metabolism Across Species. In Encyclopedia of Biological Chemistry III, 3rd ed.; Jez, J., Ed.; Elsevier: Oxford, UK, 2021; pp. 22–42. [Google Scholar]
- Ma, X.Y.; Yu, M.; Liu, Z.C.; Deng, D.; Cui, Y.Y.; Tian, Z.M.; Wang, G. Effect of amino acids and their derivatives on meat quality of finishing pigs. J. Food Sci. Technol.-Mysore 2020, 57, 404–412. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, X.G.; Zhang, B.Y.; Cui, Y.M.; Nueraihemaiti, M.; Kou, Q.F.; Luo, H.L. Assessment of components related to flavor and taste in Tan-lamb meat under different silage-feeding regimens using integrative metabolomics. Food Chem.-X 2022, 14, 100269. [Google Scholar] [CrossRef]
- Frank, D.; Kaczmarska, K.; Paterson, J.; Piyasiri, U.; Warner, R. Effect of marbling on volatile generation, oral breakdown and in mouth flavor release of grilled beef. Meat Sci. 2017, 133, 61–68. [Google Scholar] [CrossRef]
- Zhao, X.; Zuo, S.; Guo, Y.; Zhang, C.; Wang, Y.; Peng, S.; Liu, M.; Wang, B.; Zhang, H.; Luo, H. Carcass meat quality, volatile compound profile, and gene expression in Tan sheep under different feeding regimes. Food Biosci. 2023, 56, 103213. [Google Scholar] [CrossRef]
- Tian, H.X.; Yang, R.; Sun, X.F.; Yu, H.Y.; Huang, J.; Yuan, H.B.; Lou, X.M.; Yuan, Z.H.; Chen, C. Screening of goaty flavor-inhibiting lactic acid bacteria and their effects on the flavor profiles of goat milk cakes. Food Biosci. 2023, 53, 102504. [Google Scholar] [CrossRef]
- Szudera-Konczal, K.; Myszka, K.; Kubiak, P.; Drabinska, N.; Majcher, M.A. The combined effect of lactic acid bacteria and Galactomyces geotrichum fermentation on the aroma composition of sour whey. Molecules 2023, 28, 4308. [Google Scholar] [CrossRef]
- Crabo, A.G.; Singh, B.; Nguyen, T.; Emami, S.; Gassner, G.T.; Sazinsky, M.H. Structure and biochemistry of phenylacetaldehyde dehydrogenase from the Pseudomonas putida S12 styrene catabolic pathway. Arch. Biochem. Biophys. 2017, 616, 47–58. [Google Scholar] [CrossRef]
- Li, N.; Fu, J.J.; Zhang, G.R.; Liu, J.; Li, Z.X.; Luo, R.; Li, L. Investigating the mechanism of the flavor formation in Sichuan sun vinegar based on flavor-orientation and metagenomics. Curr. Res. Food Sci. 2023, 6, 100460. [Google Scholar] [CrossRef]
- Benzaldehyde. Food Cosmet. Toxicol. 1976, 14, 693–698. [CrossRef]
- Ha, J.K.; Lindsay, R.C. Volatile alkylphenols and thiophenol in species-related characterizing flavors of red meats. J. Food Sci. 1991, 56, 1197–1202. [Google Scholar] [CrossRef]
- Maga, J.A.; Katz, I. Simple phenol and phenolic compounds in food flavor. CRC Crit. Rev. Food Sci. Nutr. 1978, 10, 323–372. [Google Scholar] [CrossRef]
- Machiels, D.; Istasse, L.; van Ruth, S.M. Gas chromatography-olfactometry analysis of beef meat originating from differently fed Belgian Blue, Limousin and Aberdeen Angus bulls. Food Chem. 2004, 86, 377–383. [Google Scholar] [CrossRef]
- Ramaswamy, H.S.; Richards, J.F. Flavor of poultry meat—A review. Can. Inst. Food Sci. Technol. J. 1982, 15, 7–18. [Google Scholar] [CrossRef]
| Item | Groups | |
|---|---|---|
| CON | PM | |
| Ingredient | ||
| Corn | 60.00 | 57.30 |
| Wheat bran | 15.50 | 12.50 |
| Soybean meal | 20.50 | 18.25 |
| Paper mulberry silage | 0.00 | 8.00 |
| CaHPO4 | 0.20 | 0.20 |
| Shell | 0.15 | 0.05 |
| NaCl | 0.30 | 0.30 |
| Met | 0.15 | 0.20 |
| Premix | 3.20 | 3.20 |
| Total | 100.00 | 100.00 |
| Nutrient level | ||
| ME/(MJ·kg−1) | 12.21 | 12.15 |
| CP | 16.50 | 16.50 |
| CF | 3.82 | 5.20 |
| Ca | 0.76 | 0.77 |
| P | 0.57 | 0.54 |
| Lys | 0.78 | 0.70 |
| Met | 0.77 | 0.76 |
| Item | Groups | SEM | p-Value | |
|---|---|---|---|---|
| CON | PM | |||
| Live weight, g | ||||
| D1 | 632.00 | 612.89 | 8.65 | 0.421 |
| D42 | 3168.5 | 3277.56 | 34.17 | 0.095 |
| ADFI, g | 323.64 | 338.65 | 8.35 | 0.690 |
| ADG, g | 60.39 | 63.44 | 0.79 | 0.056 |
| F/G | 5.58 | 5.60 | 0.08 | 0.841 |
| Dressing percentage, % | 84.15 | 83.12 | 0.51 | 0.343 |
| Eviscerated percentage, % | 68.06 | 68.50 | 0.65 | 1.000 |
| Breast muscle percentage, % | 7.65 | 8.08 | 0.17 | 0.486 |
| Item | Groups | SEM | p-Value | |
|---|---|---|---|---|
| CON | PM | |||
| WHC, % | 28.25 | 27.81 | 0.61 | 0.765 |
| pH24h | 5.85 | 5.87 | 0.02 | 0.629 |
| Meat color | ||||
| L* | 49.59 | 47.77 | 0.62 | 0.157 |
| a* | 22.36 | 19.69 ** | 0.63 | 0.005 |
| b* | 10.44 | 10.63 | 0.10 | 0.407 |
| Dry matter, % | 22.07 | 23.55 | 0.44 | 0.089 |
| Crude protein, % | 79.35 | 80.42 * | 0.28 | 0.041 |
| Intramuscular fat, % | 9.05 | 8.91 | 0.11 | 0.588 |
| Ash, % | 1.53 | 1.55 | 0.03 | 0.728 |
| Name | Adduct | m/z | rt(s) | VIP | FC | Variation | p-Value |
|---|---|---|---|---|---|---|---|
| Xanthylic acid | [M − H]− | 363.0339 | 74.2 | 2.5624 | 0.43003 | ↓ | 0.0027389 |
| 3′-Ketolactose | [M − H]− | 339.0944 | 345.9 | 2.4382 | 2.47560 | ↑ | 0.0036448 |
| Ethyl icosapentate | [M + H]+ | 331.2631 | 846.6 | 1.9362 | 3.81320 | ↑ | 0.0115820 |
| Epsilon-caprolactam | [M + H]+ | 114.0918 | 423.6 | 1.9165 | 0.49254 | ↓ | 0.0121200 |
| 9,12,13-TriHOME | [M − H]− | 329.2334 | 642.8 | 1.8918 | 0.31053 | ↓ | 0.0128290 |
| Guanidinoacetate | [M − H]− | 116.0494 | 366.1 | 1.8054 | 3.10280 | ↑ | 0.0156530 |
| gamma-Glutamylalanine | [M + H]+ | 219.0952 | 774.9 | 1.7771 | 0.23896 | ↓ | 0.0167070 |
| N-Alpha-acetyllysine | [M]+ | 118.0710 | 399.1 | 1.7316 | 0.28660 | ↓ | 0.0185530 |
| 4-Hydroxycinnamoylagmatine | [M]+ | 276.1447 | 237.4 | 1.6351 | 3.05340 | ↑ | 0.0231710 |
| dTMP | [M − H]− | 321.0482 | 123.1 | 1.6030 | 0.42068 | ↓ | 0.0249450 |
| trans-1,2-Cyclohexanediol | [M]− | 115.9194 | 931.1 | 1.3355 | 5.64660 | ↑ | 0.0461810 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Wang, X.; Xiong, Y.; Cao, J.; Nussio, L.G.; Ni, K.; Lin, Y.; Wang, X.; Yang, F. Dietary Paper Mulberry Silage Supplementation Improves the Growth Performance, Carcass Characteristics, and Meat Quality of Yangzhou Goose. Animals 2024, 14, 359. https://doi.org/10.3390/ani14030359
Wang R, Wang X, Xiong Y, Cao J, Nussio LG, Ni K, Lin Y, Wang X, Yang F. Dietary Paper Mulberry Silage Supplementation Improves the Growth Performance, Carcass Characteristics, and Meat Quality of Yangzhou Goose. Animals. 2024; 14(3):359. https://doi.org/10.3390/ani14030359
Chicago/Turabian StyleWang, Ruhui, Xin Wang, Yi Xiong, Jingwen Cao, Luiz Gustavo Nussio, Kuikui Ni, Yanli Lin, Xuekai Wang, and Fuyu Yang. 2024. "Dietary Paper Mulberry Silage Supplementation Improves the Growth Performance, Carcass Characteristics, and Meat Quality of Yangzhou Goose" Animals 14, no. 3: 359. https://doi.org/10.3390/ani14030359
APA StyleWang, R., Wang, X., Xiong, Y., Cao, J., Nussio, L. G., Ni, K., Lin, Y., Wang, X., & Yang, F. (2024). Dietary Paper Mulberry Silage Supplementation Improves the Growth Performance, Carcass Characteristics, and Meat Quality of Yangzhou Goose. Animals, 14(3), 359. https://doi.org/10.3390/ani14030359






