Whole-Transcriptome Analysis Reveals the Regulatory Network of Immune Response in Dapulian Pig
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Quality Control of Raw Data
2.3. Mapping to the Reference Genome
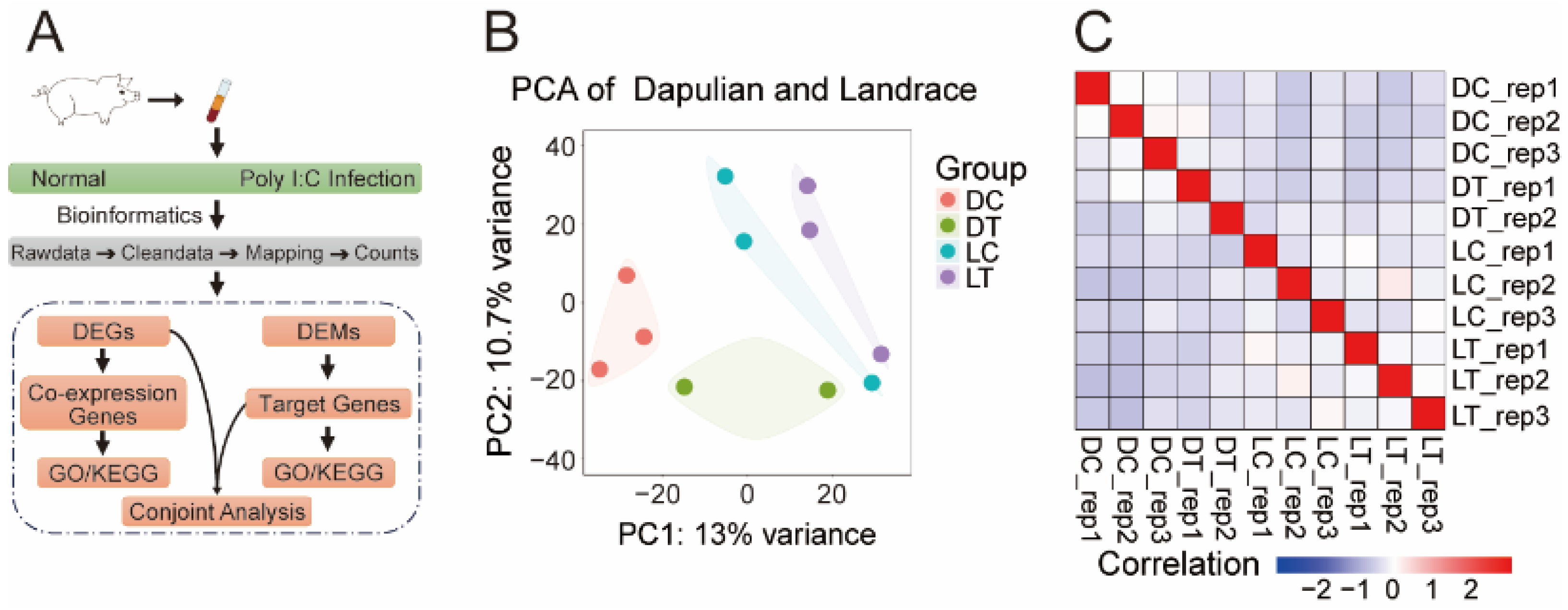
2.4. Principal Components Analysis (PCA) and Sample Correlation Analysis
2.5. Identification of Differentially Expressed Genes
2.6. Gene Functional Enrichment
2.7. Network Analysis of Protein-Protein Interaction (PPI)
2.8. Target Genes of miRNA Prediction
3. Results
3.1. The Different Transcriptome Modes of PBMCs with and Without Poly I:C Treatment in Pig
3.2. The DEGs Identification of PBMCs from Different Groups
3.3. Key Regulatory Genes of Immune Response in Pigs
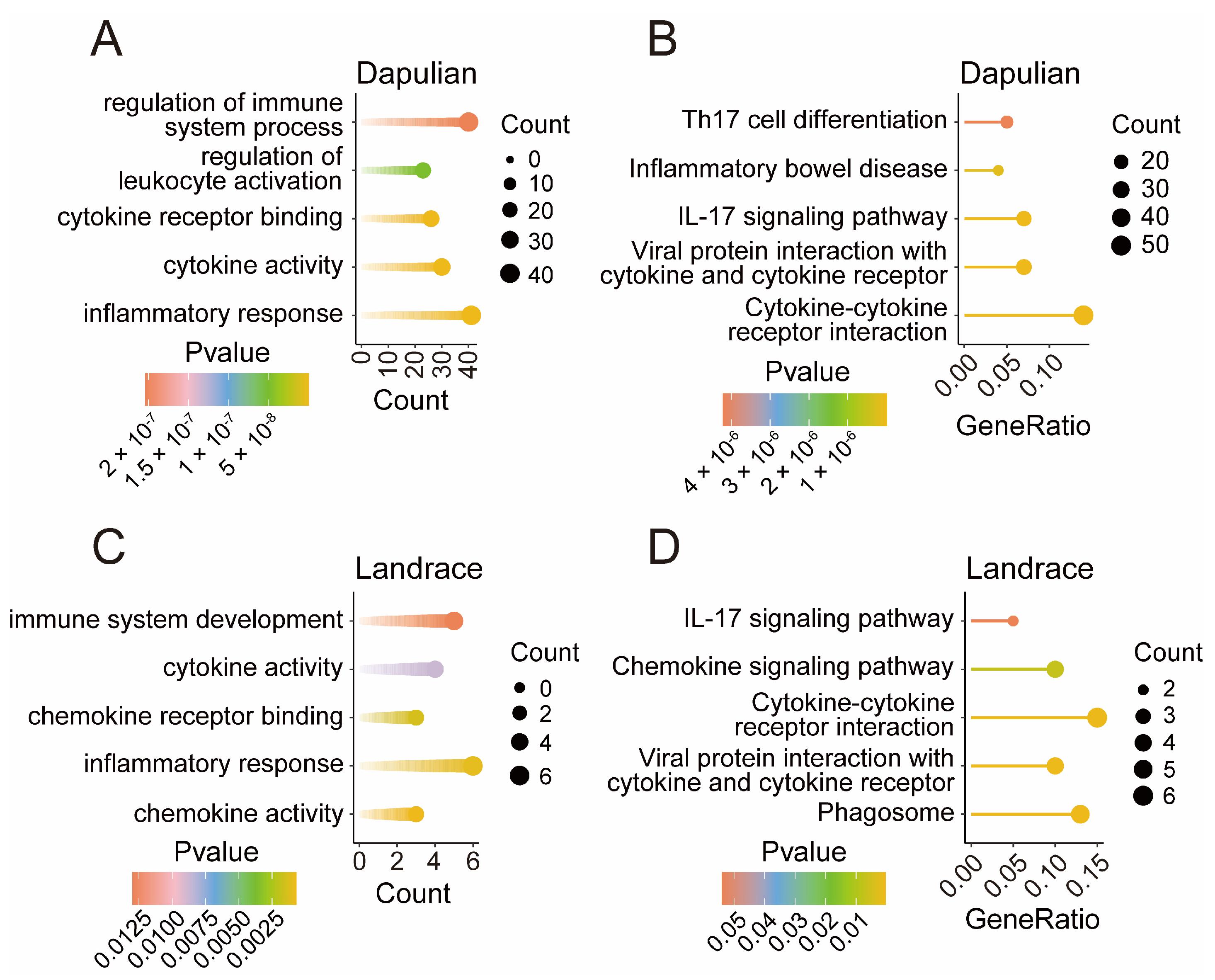
3.4. Functional Enrichment of Genes Specifically Expressed in Dapulian and Landrace
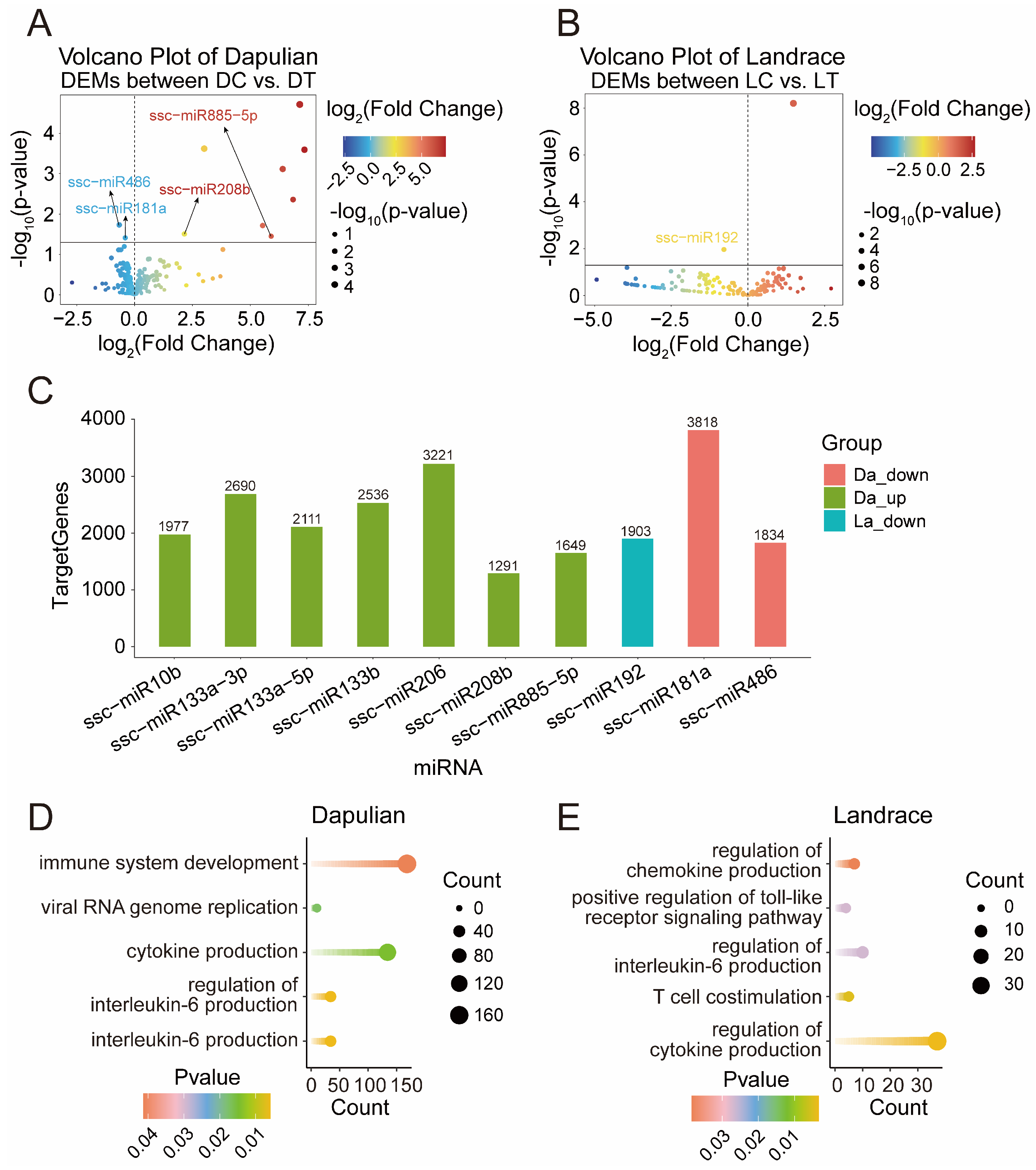
3.5. Differential Expression Analysis of miRNA in Different Groups
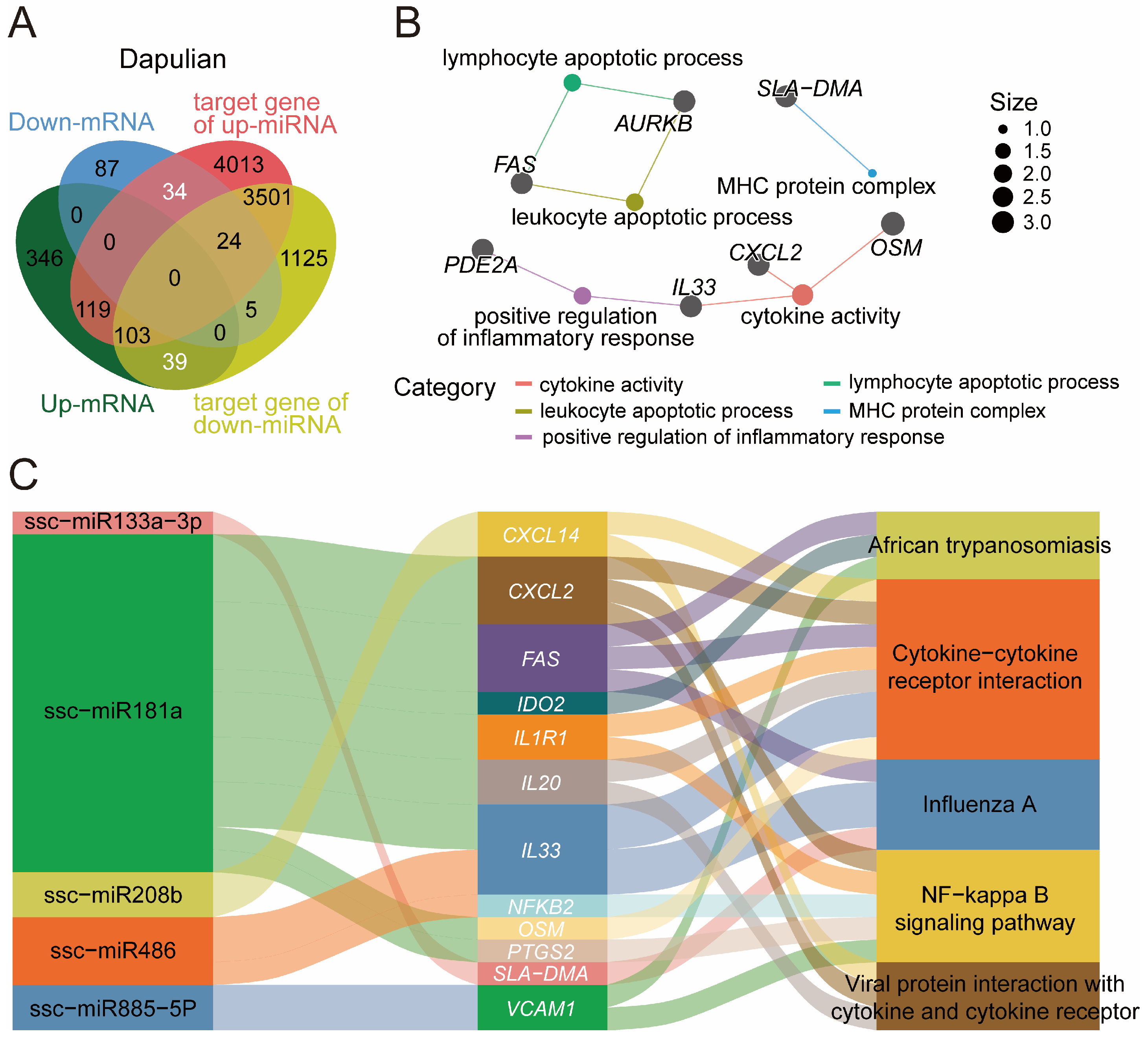
3.6. Construction of Potential Regulatory Network of miRNA-mRNA Regard to Immune Response in Dapulian Pig
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Larson, G.; Dobney, K.; Albarella, U.; Fang, M.; Matisoo-Smith, E.; Robins, J.; Lowden, S.; Finlayson, H.; Brand, T.; Willerslev, E.; et al. Worldwide phylogeography of wild boar reveals multiple centers of pig domestication. Science 2005, 307, 1618–1621. [Google Scholar] [CrossRef] [PubMed]
- Prunier, A.; Heinonen, M.; Quesnel, H. High physiological demands in intensively raised pigs: Impact on health and welfare. Animal 2010, 4, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Cobanovic, N.; Magrin, L. Editorial: Health and welfare problems of farm animals: Prevalence, risk factors, consequences and possible prevention solutions. Front. Vet. Sci. 2023, 10, 1238852. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Siengsanan-Lamont, J.; Halasa, T.; Young, J.R.; Ward, M.P.; Douangngeun, B.; Theppangna, W.; Khounsy, S.; Toribio, J.; Bush, R.D.; et al. The impact of African swine fever virus on smallholder village pig production: An outbreak investigation in Lao PDR. Transbound. Emerg. Dis. 2021, 68, 2897–2908. [Google Scholar] [CrossRef]
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef]
- Qin, M.; Li, C.; Li, Z.; Chen, W.; Zeng, Y. Genetic diversities and differentially selected regions between Shandong indigenous pig breeds and western pig breeds. Front. Genet. 2020, 10, 1351. [Google Scholar] [CrossRef]
- Kofanova, O.; Bellora, C.; Quesada, R.A.; Bulla, A.; Panadero-Fajardo, S.; Keipes, M.; Shea, K.; Stone, M.; Lescuyer, P.; Betsou, F. IL8 and EDEM3 gene expression ratio indicates peripheral blood mononuclear cell (PBMC) quality. J. Immunol. Methods 2019, 465, 13–19. [Google Scholar] [CrossRef]
- Bolen, C.R.; Uduman, M.; Kleinstein, S.H. Cell subset prediction for blood genomic studies. BMC Bioinform. 2011, 12, 258. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, J.; Liu, K.; Gao, L.; Wu, H.; Ma, L.; Zhou, J.; Liu, Z.; Han, J.-D.J. Human PBMC scRNA-seq–based aging clocks reveal ribosome to inflammation balance as a single-cell aging hallmark and super longevity. Sci. Adv. 2023, 9, eabq7599. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Zhong, Y.; Chen, X.; Li, Z.; Li, R.; Qin, P.; Wang, S.; Yin, J.; Liu, S. Pan-cancer scRNA-seq analysis reveals immunological and diagnostic significance of the peripheral blood mononuclear cells. Human Mol. Genet. 2024, 33, 342–354. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Zhang, J.; Zhang, Z.; Guo, H.; Wei, D.; Wu, C.; Hai, T.; Sun, H.-X.; Zhao, Y. Single-cell transcriptomic analysis reveals transcriptional and cell subpopulation differences between human and pig immune cells. Genes Genom. 2024, 46, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Fortier, M.E.; Kent, S.; Ashdown, H.; Poole, S.; Boksa, P.; Luheshi, G.N. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R759–R766. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Seya, T. TLR3: Interferon induction by double-stranded RNA including poly(I:C). Adv. Drug Deliv. Rev. 2008, 60, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Purdy, A.K.; Campbell, K.S. Natural killer cells and cancer. Regulation by the killer cell Ig-like receptors (KIR). Zhongguo Fei Ai Za Zhi 2010, 13, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Uribe, J.; Liu, H.; Byrne, K.A.; Bond, Z.F.; Loving, C.L.; Tuggle, C.K. Changes in H3K27ac at Gene Regulatory Regions in Porcine Alveolar Macrophages Following LPS or PolyIC Exposure. Front. Genet. 2020, 11, 817. [Google Scholar] [CrossRef]
- Wu, J.; Ji, Z.; Qiao, M.; Peng, X.; Wu, H.; Song, Z.; Zhao, H.; Liu, G.; Li, F.; Mei, S. MicroRNA transcriptome analysis of poly I:C-stimulated and PRRSV-infected porcine alveolar macrophages. J. Appl. Genet. 2019, 60, 375–383. [Google Scholar] [CrossRef]
- Zhang, F.-L.; Zhang, S.-E.; Sun, Y.-J.; Wang, J.-J.; Shen, W. Comparative transcriptomics uncover the uniqueness of oocyte development in the donkey. Front. Genet. 2022, 13, 839207. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, H.; Wang, H.; Liu, J.-F.; Wu, Y.; Guo, J. Transcriptomic analysis identifies candidate genes and gene sets controlling the response of porcine peripheral blood mononuclear cells to poly I: C stimulation. G3 Genes Genomes Genet. 2016, 6, 1267–1275. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, H.; Guo, J.; Wang, H.; Wu, Y.; Liu, J. MicroRNA Transcriptome of Poly I:C-Stimulated Peripheral Blood Mononuclear Cells Reveals Evidence for MicroRNAs in Regulating Host Response to RNA Viruses in Pigs. Int. J. Mol. Sci. 2016, 17, 1601. [Google Scholar] [CrossRef]
- Zhang, F.L.; Zhu, W.M.; He, T.R.; Zhao, Y.T.; Ge, W.; Tan, J.H.; Shen, W. Comparative transcriptomic analysis reveals that TPX2 and AURXA are involved in porcine PCV2 infection. Gene 2022, 834, 146649. [Google Scholar] [CrossRef]
- Yu, M.; Feng, Y.; Yan, J.; Zhang, X.; Tian, Z.; Wang, T.; Wang, J.; Shen, W. Transcriptomic regulatory analysis of skeletal muscle development in landrace pigs. Gene 2024, 915, 148407. [Google Scholar] [CrossRef] [PubMed]
- de Sena Brandine, G.; Smith, A.D. Falco: High-speed FastQC emulation for quality control of sequencing data. F1000Research 2019, 8, 1874. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The Subread aligner: Fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013, 41, e108. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Wang, S.; Kim, J.; Jiang, X.; Brunner, S.F.; Ohno-Machado, L. GAMUT: GPU accelerated microRNA analysis to uncover target genes through CUDA-miRanda. BMC Med. Genom. 2014, 7 (Suppl. S1), S9. [Google Scholar] [CrossRef]
- Xing, J.; Xing, F.; Zhang, C.; Zhang, Y.; Wang, N.; Li, Y.; Yang, L.; Jiang, C.; Zhang, C.; Wen, C.; et al. Genome-wide gene expression profiles in lung tissues of pig breeds differing in resistance to porcine reproductive and respiratory syndrome virus. PLoS ONE 2014, 9, e86101. [Google Scholar] [CrossRef]
- Song, G.; Zhang, Y.; Gao, H.; Fu, Y.; Chen, Y.; Yin, Y.; Xu, K. Differences in Immune Characteristics and Related Gene Expression in Spleen among Ningxiang, Berkshire Breeds and Their Hybrid Pigs. Genes 2024, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, Y.; Jiang, S.; Sui, M.; Wang, C.; Kang, L.; Sun, Y.; Jiang, Y. Suppression of lymphocyte apoptosis in spleen by CXCL13 after porcine circovirus type 2 infection and regulatory mechanism of CXCL13 expression in pigs. Vet. Res. 2019, 50, 17. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Takahama, Y. XCL1 and XCR1 in the immune system. Microbes Infect. 2012, 14, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Peters, V.A.; Joesting, J.J.; Freund, G.G. IL-1 receptor 2 (IL-1R2) and its role in immune regulation. Brain Behav. Immun. 2013, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Tang, Z.L.; Yang, S.; Cao, J.Y.; Li, K. Molecular characteristics of the porcine TIMD4 gene and its association analysis. Biochem. Genet. 2012, 50, 538–548. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, P.; Xu, Y.; Gao, L.; Wang, H.; Jia, X.; Ma, H.; Liang, X.; Ma, C.; Gao, L. Tim-4 protects mice against lipopolysaccharide-induced endotoxic shock by suppressing the NF-kappaB signaling pathway. Lab. Investig. 2016, 96, 1189–1197. [Google Scholar] [CrossRef]
- Anandasabapathy, N.; Ford, G.S.; Bloom, D.; Holness, C.; Paragas, V.; Seroogy, C.; Skrenta, H.; Hollenhorst, M.; Fathman, C.G.; Soares, L. GRAIL: An E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity 2003, 18, 535–547. [Google Scholar] [CrossRef]
- Song, G.; Liu, B.; Li, Z.; Wu, H.; Wang, P.; Zhao, K.; Jiang, G.; Zhang, L.; Gao, C. E3 ubiquitin ligase RNF128 promotes innate antiviral immunity through K63-linked ubiquitination of TBK1. Nat. Immunol. 2016, 17, 1342–1351. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Shen, A.; Liu, Z.; He, T.S. E3 ubiquitin ligase RNF128 negatively regulates the IL-3/STAT5 signaling pathway by facilitating K27-linked polyubiquitination of IL-3Ralpha. Cell Commun. Signal 2024, 22, 254. [Google Scholar] [CrossRef]
- Hu, J.; Yang, D.; Wang, H.; Li, C.; Zeng, Y.; Chen, W. CpG Oligodeoxynucleotides Induce Differential Cytokine and Chemokine Gene Expression Profiles in Dapulian and Landrace Pigs. Front. Microbiol. 2016, 7, 1992. [Google Scholar] [CrossRef]
- Hu, J.; Yang, D.; Chen, W.; Li, C.; Wang, Y.; Zeng, Y.; Wang, H. Whole Blood Transcriptome Sequencing Reveals Gene Expression Differences between Dapulian and Landrace Piglets. BioMed Res. Int. 2016, 2016, 7907980. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Wang, R.; Qiao, S.; Wan, B.; Wang, Y.; Liu, M.; Shi, X.; Guo, J.; Zhang, G. Antibody-dependent enhancement of PRRSV infection down-modulates TNF-alpha and IFN-beta transcription in macrophages. Vet. Immunol. Immunopathol. 2013, 156, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Meng, X.; Zhen, Y.; Zhang, Y.; Hu, X.; Zhang, Q.; Zhou, X.; Liu, B. Integration of Transcriptome and Proteome in Lymph Nodes Reveal the Different Immune Responses to PRRSV Between PRRSV-Resistant Tongcheng Pigs and PRRSV-Susceptible Large White Pigs. Front. Genet. 2022, 13, 800178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, L.; Li, Y.; Jiang, P.; Wang, Y.; Wang, P.; Kang, L.; Wang, Y.; Sun, Y.; Jiang, Y. Identification and characterization of microRNA in the lung tissue of pigs with different susceptibilities to PCV2 infection. Vet. Res. 2018, 49, 18. [Google Scholar] [CrossRef]
- Li, Q.J.; Chau, J.; Ebert, P.J.; Sylvester, G.; Min, H.; Liu, G.; Braich, R.; Manoharan, M.; Soutschek, J.; Skare, P.; et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell 2007, 129, 147–161. [Google Scholar] [CrossRef]
- Ye, L.; Su, X.; Wu, Z.; Zheng, X.; Wang, J.; Zi, C.; Zhu, G.; Wu, S.; Bao, W. Analysis of differential miRNA expression in the duodenum of Escherichia coli F18-sensitive and -resistant weaned piglets. PLoS ONE 2012, 7, e43741. [Google Scholar] [CrossRef]
- Tang, X.; Lan, T.; Wu, R.; Zhou, Z.; Chen, Y.; Sun, Y.; Zheng, Y.; Ma, J. Analysis of long non-coding RNAs in neonatal piglets at different stages of porcine deltacoronavirus infection. BMC Vet. Res. 2019, 15, 111. [Google Scholar] [CrossRef]
- Jarret, A.; McFarland, A.P.; Horner, S.M.; Kell, A.; Schwerk, J.; Hong, M.; Badil, S.; Joslyn, R.C.; Baker, D.P.; Carrington, M.; et al. Hepatitis-C-virus-induced microRNAs dampen interferon-mediated antiviral signaling. Nat. Med. 2016, 22, 1475–1481. [Google Scholar] [CrossRef]
- Wysocki, M.; Chen, H.; Steibel, J.P.; Kuhar, D.; Petry, D.; Bates, J.; Johnson, R.; Ernst, C.W.; Lunney, J.K. Identifying putative candidate genes and pathways involved in immune responses to porcine reproductive and respiratory syndrome virus (PRRSV) infection. Anim. Genet. 2012, 43, 328–332. [Google Scholar] [CrossRef]
- Cayrol, C.; Girard, J.P. Interleukin-33 (IL-33): A nuclear cytokine from the IL-1 family. Immunol. Rev. 2018, 281, 154–168. [Google Scholar] [CrossRef]
- Ou, R.; Zhang, M.; Huang, L.; Flavell, R.A.; Koni, P.A.; Moskophidis, D. Regulation of immune response and inflammatory reactions against viral infection by VCAM-1. J. Virol. 2008, 82, 2952–2965. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boraschi, D.; Italiani, P.; Weil, S.; Martin, M.U. The family of the interleukin-1 receptors. Immunol. Rev. 2018, 281, 197–232. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.P.; Rohrer, G.A.; Alexander, L.J.; Troyer, D.L.; Kirby-Dobbels, K.R.; Janzen, M.A.; Cornwell, D.L.; Louis, C.F.; Schook, L.B.; Beattie, C.W. Directed integration of the physical and genetic linkage maps of swine chromosome 7 reveals that the SLA spans the centromere. Genome Res. 1995, 5, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Hammer, S.E.; Ho, C.S.; Ando, A.; Rogel-Gaillard, C.; Charles, M.; Tector, M.; Tector, A.J.; Lunney, J.K. Importance of the Major Histocompatibility Complex (Swine Leukocyte Antigen) in Swine Health and Biomedical Research. Annu. Rev. Anim. Biosci. 2020, 8, 171–198. [Google Scholar] [CrossRef]
- Wimmers, K.; Schellander, K.; Ponsuksili, S. BF, HP, DQB and DRB are associated with haemolytic complement activity, acute phase protein reaction and antibody response in the pig. Vet. Immunol. Immunopathol. 2004, 99, 215–228. [Google Scholar] [CrossRef]
- Hewitt, E.W. The MHC class I antigen presentation pathway: Strategies for viral immune evasion. Immunology 2003, 110, 163–169. [Google Scholar] [CrossRef]
- Lunney, J.K.; Murrell, K.D. Immunogenetic analysis of Trichinella spiralis infections in swine. Vet. Parasitol. 1988, 29, 179–193. [Google Scholar] [CrossRef]
- Madden, K.B.; Moeller, R.F., Jr.; Douglass, L.W.; Goldman, T.; Lunney, J.K. Trichinella spiralis: Genetic basis and kinetics of the anti-encysted muscle larval response in miniature swine. Exp. Parasitol. 1993, 77, 23–35. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Tian, Z.; Yu, M.; Zhang, S.; Zhang, M.; Zhai, X.; Shen, W.; Wang, J. Whole-Transcriptome Analysis Reveals the Regulatory Network of Immune Response in Dapulian Pig. Animals 2024, 14, 3546. https://doi.org/10.3390/ani14233546
Wang T, Tian Z, Yu M, Zhang S, Zhang M, Zhai X, Shen W, Wang J. Whole-Transcriptome Analysis Reveals the Regulatory Network of Immune Response in Dapulian Pig. Animals. 2024; 14(23):3546. https://doi.org/10.3390/ani14233546
Chicago/Turabian StyleWang, Tao, Zhe Tian, Mubin Yu, Shuer Zhang, Min Zhang, Xiangwei Zhai, Wei Shen, and Junjie Wang. 2024. "Whole-Transcriptome Analysis Reveals the Regulatory Network of Immune Response in Dapulian Pig" Animals 14, no. 23: 3546. https://doi.org/10.3390/ani14233546
APA StyleWang, T., Tian, Z., Yu, M., Zhang, S., Zhang, M., Zhai, X., Shen, W., & Wang, J. (2024). Whole-Transcriptome Analysis Reveals the Regulatory Network of Immune Response in Dapulian Pig. Animals, 14(23), 3546. https://doi.org/10.3390/ani14233546




