Comparison Study on the Trophic Niche of Red Pandas Using Stable Isotope Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
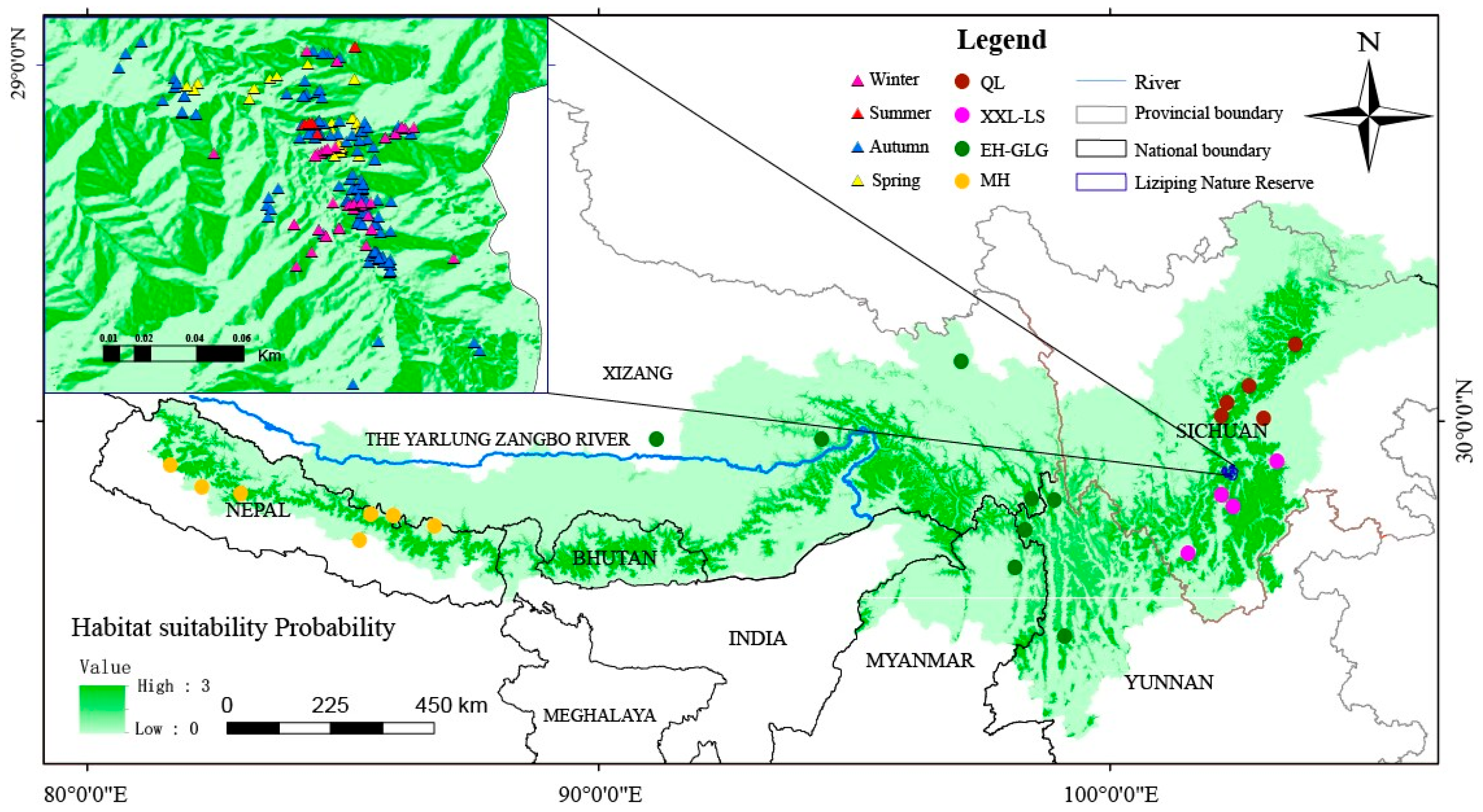
2.1. Sample Collection
2.2. Laboratory Methods
2.3. Stable Isotope Analysis
2.4. Statistical Analysis
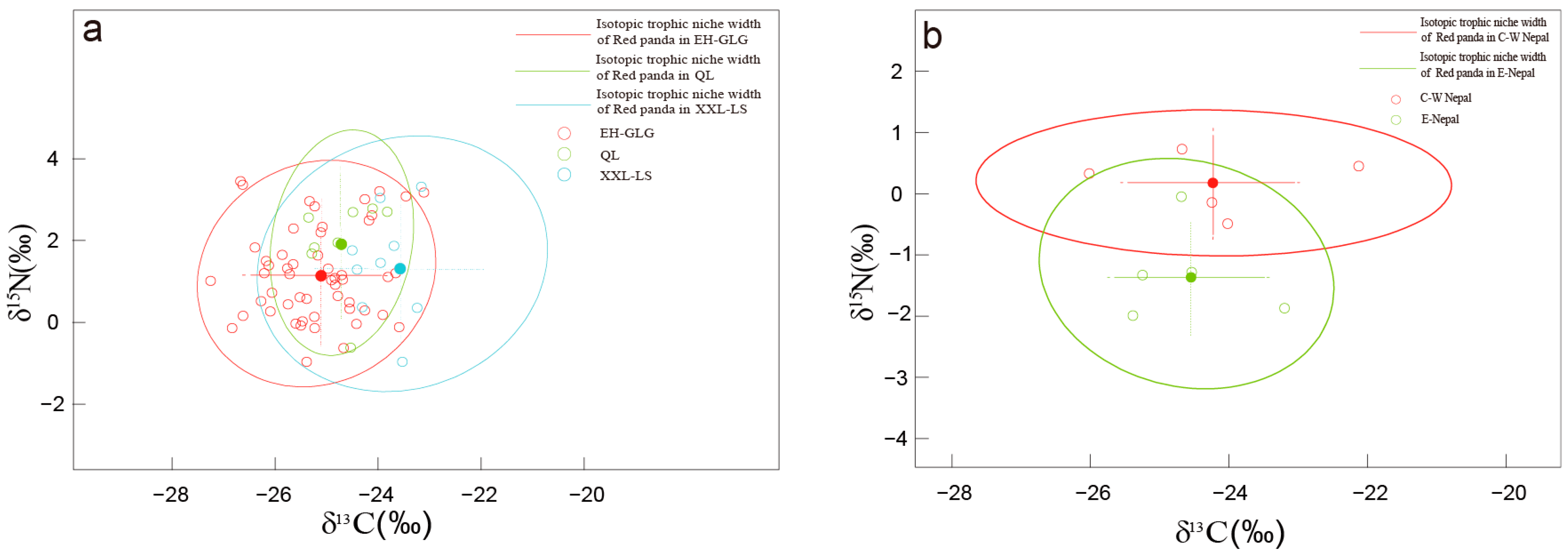
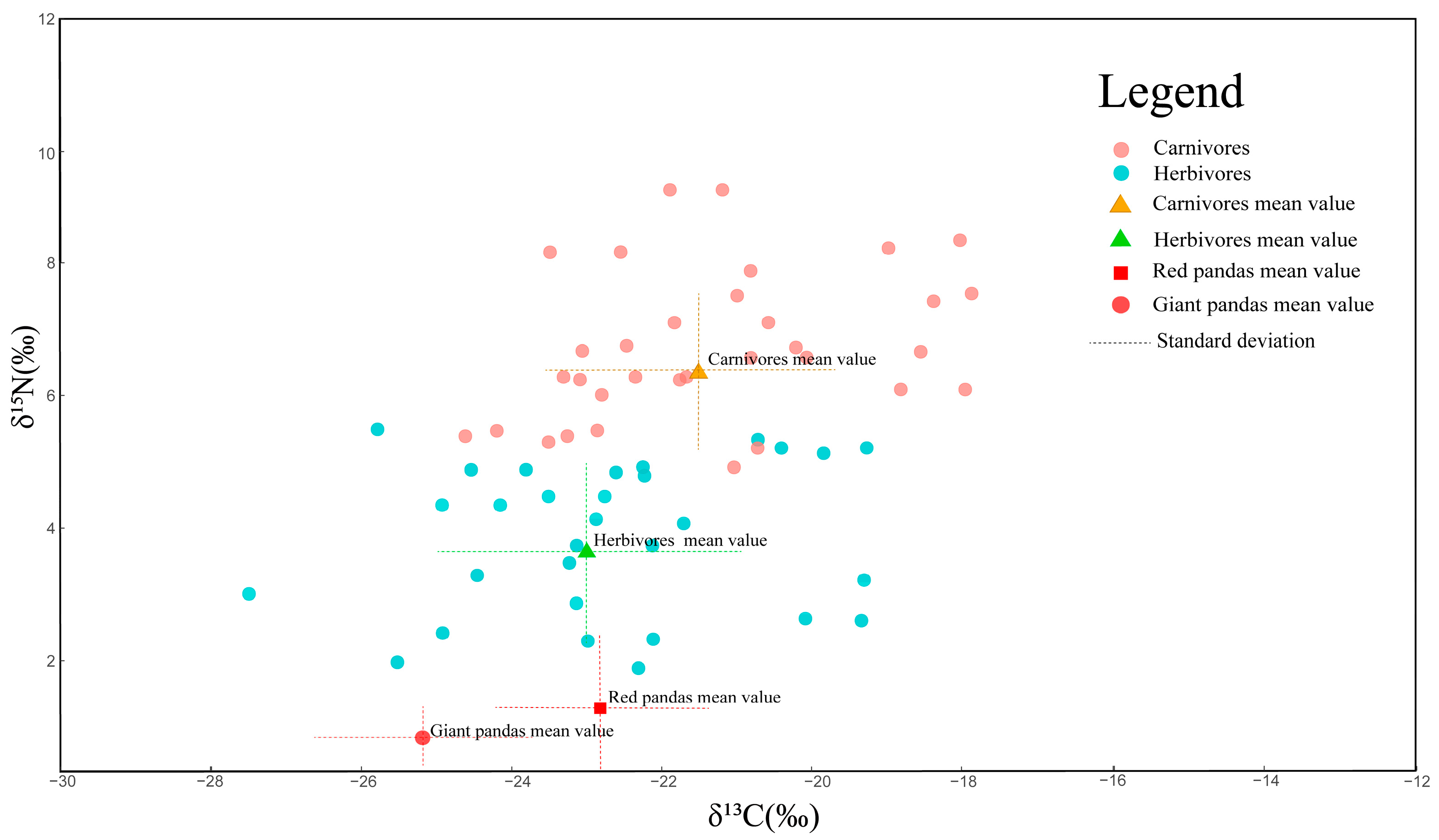
3. Results
3.1. Characterization of Two Red Panda Species
3.2. Different Populations Within the Red Panda Species
3.3. Giant Pandas and Red Pandas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Wu, Q.; Ma, S.; Ning, Z.; Wei, F. Genomic evidence for two phylogenetic species and long-term population bottlenecks in red pandas. Sci. Adv. 2020, 6, eaax5751. [Google Scholar] [CrossRef]
- Dierenfeld, E.; Hintz, H.; Robertson, J.; Van Soest, P.; Oftedal, O. Utilization of bamboo by the giant panda. J. Nutr. 1982, 4, 636–641. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Q.; Ma, S.; Ning, Z.; Wei, F. Comparative genomics reveals convergent evolution between the bamboo-eating giant and red pandas. Proc. Natl. Acad. Sci. USA 2017, 114, 1081–1086. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, Z.; Liu, S.; Li, M.; Hu, J. Genetic and subspecies divergences within the red panda (Carnivora: Procyonidae). Acta Zootaxonom. Sin. 2002, 27, 403–408. [Google Scholar]
- Thapa, A.; Hu, Y.; Wei, F. The endangered red panda (Ailurus fulgens): Ecology and conservation approaches across the entire range. Biol. Conserv. 2018, 220, 112–121. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, Z.; Hu, J. Research advances and perspectives on the ecology of wild giant pandas. Acta Theriol. Sinica. 2011, 31, 412. [Google Scholar]
- Cuvier, F. Histoire Naturelle des Mammifères, Avec des Figures Originales, Colorées, Desinéesd’apres Desanimauxvivants; Tome: Paris, French, 1825; Volume 5, pp. 1–3. [Google Scholar]
- Lowther, D.A. The first painting of the red panda (Ailurus fulgens) in Europe? Natural history and artistic patronage in early nineteenth-century India. Arch. Nat. Hist. 2021, 48, 368–376. [Google Scholar] [CrossRef]
- Roberts, M.S.; Gittleman, J.L. Ailurus Fulgens. In Mammalian Species; The American Society of Mammalogists: West Lafayette, IN, USA, 1984; Volume 222, pp. 1–8. [Google Scholar]
- Li, H.; Zhang, Z.; Liu, S.; Wei, F.; Hu, J. Maxent model-based evaluation of habitat suitability of Chinese red panda in Qionglai mountains. J. Guizhou Norm. Univ. Nat. Sci. 2022, 40, 34–39. [Google Scholar]
- Thapa, A.; Wu, R.; Hu, Y.; Nie, Y.; Singh, P.B.; Khatiwada, J.R.; Yan, L.; Gu, X.; Wei, F. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10554. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, Z.; Hu, J. Current distribution, status and conservation of wild red pandas Ailurus fulgens in China. Biol. Conserv. 1999, 89, 285–291. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, Z.; Hu, J. Habitat use and separation between the giant panda and the red panda. J. Mammal. 2000, 81, 448–455. [Google Scholar] [CrossRef]
- Wei, F.; Wang, Z.; Feng, Z. Comparison of habitats selected by giant and red pandas in Xiangling Mountains, China. Acta Zool. Sin. 2000, 46, 287. [Google Scholar]
- Liu, X.; Zhang, Y.; Li, Q.; Wang, S.; Zhao, Y. Reproductive behavior variations and reproductive strategy in the captive red panda. Acta Ther. Sin. 2004, 24, 173–176. [Google Scholar]
- Zhang, Z.; Wang, Y.; Wei, F.; Liu, Z. Winter microhabitat separation between giant and red pandas in Bashania faberi bamboo forest in Fengtongzhai Nature Reserve. J. Wildl. Manage. 2006, 70, 231–235. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Liu, S.; Wei, F. Food habits and space-use of red pandas Ailurus fulgens in the Fengtongzhai Nature Reserve, China: Food effects and behavioural responses. Acta Theriol. 2009, 54, 225–234. [Google Scholar] [CrossRef]
- Karki, S.; Rijal, S.; Kandel, S.; Dhakal, M.; Shrestha, U.; Neupane, S.; Sharma, R. Reaching over the gap: A review of trends in and status of red panda research over 193 years (1827–2020). Sci. Total Environ. 2021, 781, 146659. [Google Scholar] [CrossRef]
- Bista, D.; Acharya, K.P.; Vaidya, P.; Basnet, S.; Shrestha, U.; Kandel, S.; Aryal, A. Seasonal resource selection of an arboreal habitat specialist in a human-dominated landscape: A case study using red panda. Curr. Zool. 2023, 69, 1–11. [Google Scholar] [CrossRef]
- Ruan, T.; Hu, Y.; Zhang, Z.; Wei, F. Research on the changes in distribution and habitat suitability of the Chinese red panda population. Animals 2024, 14, 424. [Google Scholar] [CrossRef]
- Feng, B.; Li, Y.; Zhao, W.; Wang, Z.; Wei, F. Species coexistence and niche interaction between sympatric giant panda and Chinese red panda: A spatiotemporal approach. Ecol. Evol. 2023, 13, e9937. [Google Scholar] [CrossRef]
- Zhuang, H.; Chen, Z.; Liu, Y.; Wang, Z.; Wei, F. Functional zoning of China’s protected area needs to be optimized for protecting giant panda. Glob. Ecol. Conserv. 2021, 25, e01392. [Google Scholar] [CrossRef]
- Pfeifer, M.; Lefebvre, V.; Peres, C.A.; Banks-Leite, C.; Wearn, O.R.; Marsh, C.J.; Butchart, S.H.M.; Arroyo-Rodríguez, V.; Barlow, J.; Cerezo, A.; et al. Creation of forest edges has a global impact on forest vertebrates. Nature 2017, 551, 187–191. [Google Scholar] [CrossRef]
- Tucker, M.A.; Böhning-Gaese, K.; Fagan, W.F.; Fryxell, J.M.; Van Moorter, B.; Alberts, S.C.; Ali, A.H.; Allen, A.M.; Attias, N.; Avgar, T.; et al. Moving in the Anthropocene: Global reductions in terrestrial mammalian movements. Science 2018, 359, 466–469. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Arends, C.L.; Vander Zanden, H.B.; Lamont, M.M. Isotopic niche partitioning in a multi-species assemblage. Mar. Biol. 2024, 171, 2. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Y.; Zhao, L.; Guo, C.; Li, F. Intra-tooth stable isotope analysis reveals seasonal dietary variability and niche partitioning among bushpigs/red river hogs and warthogs. Curr. Zool. 2024, zoae007. [Google Scholar] [CrossRef]
- Balzani, P.; Haubrock, P.J. Expanding the invasion toolbox: Including stable isotope analysis in risk assessment. NeoBiota 2022, 76, 191–210. [Google Scholar] [CrossRef]
- Shipley, O.N.; Matich, P. Studying animal niches using bulk stable isotope ratios: An updated synthesis. Oecologia 2020, 193, 27–51. [Google Scholar] [CrossRef]
- Raffard, A.; Santoul, F.; Blanchet, S.; Cucherousset, J. Linking intraspecific variability in trophic and functional niches along an environmental gradient. Freshw. Biol. 2020, 65, 1401–1411. [Google Scholar] [CrossRef]
- Newsome, S.D.; Martinez del Rio, C.; Bearhop, S.; Phillips, D.L. A niche for isotopic ecology. Front. Ecol. Environ. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Phillips, D.L. Converting isotope values to diet composition: The use of mixing models. J. Mammal. 2012, 93, 342–352. [Google Scholar] [CrossRef]
- Rader, J.A.; Newsome, S.D.; Sabat, P.; Chesser, R.T.; Dillon, M.E.; Martínez del Rio, C. Isotopic niches support the resource breadth hypothesis. J. Anim. Ecol. 2017, 86, 405–413. [Google Scholar] [CrossRef]
- Gong, Y.; Li, Y.; Chen, X.; Yu, W. Trophic niche and diversity of a pelagic squid (Dosidicus gigas): A comparative study using stable isotope, fatty acid, and feeding apparatuses morphology. Front. Mar. Sci. 2020, 7, 642. [Google Scholar] [CrossRef]
- Hetherington, E.D.; Close, H.G.; Haddock, S.H.; Damian-Serrano, A.; Dunn, C.W.; Wallsgrove, N.J. Vertical trophic structure and niche partitioning of gelatinous predators in a pelagic food web: Insights from stable isotopes of siphonophores. Limnol. Oceanogr. 2024, 69, 902–919. [Google Scholar] [CrossRef]
- Blumenthal, S.A.; Chritz, K.L.; Rothman, J.M.; Cerling, T.E. Detecting intraannual dietary variability in wild mountain gorillas by stable isotope analysis of feces. Proc. Natl. Acad. Sci. USA 2012, 109, 21277–21282. [Google Scholar] [CrossRef]
- Han, H.; Wei, W.; Hu, Y.; Nie, Y.; Ji, X.; Yan, L.; Zhang, Z.; Shi, X.; Zhu, L.; Luo, Y.; et al. Diet evolution and habitat contraction of giant pandas via stable isotope analysis. Curr. Biol. 2019, 29, 664–669. [Google Scholar] [CrossRef]
- Lei, W.; Wei, W.; Pu, D.; Qubi, S.; Zhou, H.; Hong, M.; Tang, J.; Han, H. Comparative analysis of trophic niche using stable isotopes provides insight into resource use of giant pandas. Integr. Zool. 2024, 19, 1151–1162. [Google Scholar] [CrossRef]
- Hu, Y.; Thapa, A.; Wei, F. Ailurus fulgens (Himalayan Red Panda) and Ailurus styani (Chinese Red Panda). Trends Genet. 2020, 36, 624–625. [Google Scholar] [CrossRef]
- Han, H.; Wei, W.; Nie, Y.; Zhou, W.; Hu, Y.; Wu, Q.; Wei, F. Distinctive diet-tissue isotopic discrimination factors derived from the exclusive bamboo-eating giant panda. Integr. Zool. 2016, 11, 447–456. [Google Scholar] [CrossRef]
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research; R package Version 2.4.6; Northwestern University: Evanston, IL, USA, 2024; Available online: https://CRAN.R-project.org/package=psych (accessed on 27 June 2024).
- Suess, H.E. Radiocarbon concentration in modern wood. Science 1955, 122, 415–417. [Google Scholar] [CrossRef]
- Cerling, T.E.; Manthi, F.K.; Mbua, E.N.; Leakey, L.N.; Leakey, M.G.; Leakey, R.E.; Wood, B.A. Stable isotope-based diet reconstructions of Turkana Basin hominins. Proc. Natl. Acad. Sci. USA 2013, 110, 10501–10506. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2012, 80, 595–602. [Google Scholar] [CrossRef]
- Ambrose, S.H. Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeol. Sci. 1990, 17, 431–451. [Google Scholar] [CrossRef]
- Kohn, M.J. You are what you eat. Science 1999, 283, 335–336. [Google Scholar] [CrossRef]
- Tieszen, L.L.; Boutton, T.W.; Tesdahl, K.G.; Slade, N.A. Fractionation and turnover of stable carbon isotopes in animal tissues: Implications for δ13C analysis of diet. Oecologia 1983, 57, 32–37. [Google Scholar] [CrossRef]
- Hobson, K.A.; Clark, R.G. Assessing avian diets using stable isotopes I: Turnover of 13C in tissues. Condor 1992, 94, 181–188. [Google Scholar] [CrossRef]
- Kelly, J.F. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 2000, 78, 1–27. [Google Scholar] [CrossRef]
- Schwarcz, H.P.; Schoeninger, M.J. Stable isotope analyses in human nutritional ecology. Am. J. Phys. Anthropol. 1991, 34, 283–321. [Google Scholar] [CrossRef]
- Gannes, L.Z.; Del Rio, C.M.; Koch, P. Natural abundance variations in stable isotopes and their potential uses in animal physiological ecology. Comp. Biochem. Physiol. A 1998, 119, 725–737. [Google Scholar] [CrossRef]
- Liao, K.; Lai, X.; Zhu, Q. Soil δ15N is a better indicator of ecosystem nitrogen cycling than plant δ15N: A global meta-analysis. Soil 2021, 7, 733–742. [Google Scholar] [CrossRef]
- Sah, S.P.; Brumme, R. Altitudinal gradients of natural abundance of stable isotopes of nitrogen and carbon in the needles and soil of a pine forest in Nepal. J. For. Sci. 2003, 49, 19–26. [Google Scholar] [CrossRef]
- Bista, D.; Shrestha, S.; Sherpa, P.; Thapa, G.J.; Kokh, M.; Lama, S.T.; Khanal, K.; Thapa, A.; Jnawali, S.R. Distribution and habitat use of red panda in the Chitwan-Annapurna Landscape of Nepal. PLoS ONE 2017, 12, e0178797. [Google Scholar] [CrossRef] [PubMed]
- Hu, J. The Beautiful Red Panda; University of Electronic Science and Technology Press: Chengdu, China, 1992. [Google Scholar]
- Kang, L. The Effects of Seasonal Changes in Diets on Gut Microbes of Wild Red Pandas; China West Normal University: Nanchong, China, 2023. [Google Scholar]
- Yang, J.; Zhang, Z.; Hu, J.; Li, M.; Wei, F. Foraging characteristics and nutrition strategy of red pandas in Fengtongzhai Nature Reserve, Sichuan, China. Acta Theriol. Sin. 2007, 27, 249. [Google Scholar]
- Ehleringer, J.R.; Rundel, P.W.; Nagy, K.A. Stable isotopes in physiological ecology and food web research. Trends Ecol. Evol. 1986, 1, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Pu, D. Stable Isotope Technology-Based Study on Trophic Niche and Formation Mechanism of Giant Panda (Ailuropoda melanoleuca); China West Normal University: Nanchong, China, 2022. [Google Scholar]
- Belovsky, G.E. Optimal foraging and community structure: The allometry of herbivore food selection and competition. Evol. Ecol. 1997, 11, 641–672. [Google Scholar] [CrossRef]
- Schaller, G.B.; Hu, J.; Pan, W.; Zhu, J. The Giant Pandas of Wolong; University of Chicago Press: Chicago, IL, USA, 1985. [Google Scholar]
- Wei, F.; Hu, J.; Wang, W.; Wei, Y. Estimation of daily energy intake of giant pandas and energy supply of bamboo resources in Mabian Dafengding Reserve. Acta Theriol. Sin. 1997, 17, 8–12. [Google Scholar]
- Johnson, K.G.; Schaller, G.B.; Hu, J. Comparative behavior of red and giant pandas in the Wolong Reserve, China. J. Mammal. 1988, 69, 552–564. [Google Scholar] [CrossRef]
- Reid, D.G.; Hu, J. Giant panda selection between Bashania faberi habitats in Wolong Reserve, Sichuan, China. J. Appl. Ecol. 1991, 28, 228–243. [Google Scholar] [CrossRef]
- Brown, J.S.; Kotler, B.P.; Porter, W.P. How foraging allometries and resource dynamics could explain Bergmann’s rule and the body-size diet relationship in mammals. Oikos 2016, 126, 224–230. [Google Scholar] [CrossRef]
- Qi, D.; Hu, Y.; Gu, X.; Li, M.; Wei, F. Ecological niche modeling of the sympatric giant and red pandas on a mountain-range scale. Biodivers. Conserv. 2009, 18, 2127–2141. [Google Scholar] [CrossRef]
- Luo, L.; Zhou, H.; Tang, J.; Wei, W.; Han, H.; Zhang, Z.; Hong, M. Niche differentiation in elevation distribution between giant pandas and coexisting animals. Acta Theriol. Sin. 2020, 40, 337–345. [Google Scholar]
- Wang, X.; Xu, W.; Ouyang, Z.; Liu, J.; Yi, X.; Chen, Y.; Huang, J. Application of niche factor analysis in habitat evaluation for giant pandas. Acta Ecol. Sin. 2008, 28, 821–828. [Google Scholar]
- Wei, F.; Feng, Z.; Wang, Z.; Li, M. Feeding strategy and resource partitioning between giant and red pandas. Mammalia 1999, 63, 417–429. [Google Scholar] [CrossRef]
- Wei, F.; Hu, Y.; Yan, L.; Nie, Y.; Wu, Q.; Zhang, Z. Giant pandas are not an evolutionary cul-de-sac: Evidence from multidisciplinary research. Mol. Biol. Evol. 2015, 32, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Feng, Z.; Wang, Z.; Zhou, A.; Hu, J. Use of the nutrients in bamboo by the red panda (Ailurus fulgens). J. Zool. 1999, 248, 535–541. [Google Scholar] [CrossRef]
- Sarmento, W.; Berger, J. Conservation implications of using an imitation carnivore to assess rarely used refuges as critical habitat features in an alpine ungulate. PeerJ 2020, 8, e9296. [Google Scholar] [CrossRef]
- Salandre, J.A.; Beil, R.; Loehr, J.A.; Sundell, J. Foraging decisions of North American beaver (Castor canadensis) are shaped by energy constraints and predation risk. Mammal Res. 2017, 62, 229–239. [Google Scholar] [CrossRef]
- Lowrey, B.; Garrott, R.A.; Miyasaki, H.M.; Fralick, G.; Dewey, S.R. Seasonal resource selection by introduced mountain goats in the southwest Greater Yellowstone Area. Ecosphere 2017, 8, e01769. [Google Scholar] [CrossRef]

| Species | Group | N | δ13C (‰) | δ13C (‰) Range | δ15N (‰) | δ15N (‰) Range |
|---|---|---|---|---|---|---|
| Ailurus styani | EH-GLG | 53 | −25.19 ± 0.95 | −27.25~−23.11 | 1.19 ± 1.13 | −0.97~3.45 |
| XXL-LS | 10 | −23.53 ± 1.15 | −24.50~−20.53 | 1.43 ± 1.28 | −0.97~3.31 | |
| QL | 8 | −24.70 ± 0.57 | −25.34~−23.82 | 1.95 ± 1.13 | −0.62~2.78 | |
| Unclassified | 15 | −24.43 ± 1.20 | −0.10 ± 1.17 | |||
| Total | 86 | −24.73 ± 1.26 | −27.25~−20.41 | 1.30 ± 1.09 | −0.97~3.45 | |
| Ailurus fulgens | C-W Nepal | 5 | −24.22 ± 1.40 | −26.02~−22.13 | 0.18 ± 0.49 | −0.49~0.73 |
| E-Nepal | 5 | −24.61 ± 0.87 | −25.39~−23.20 | −1.30 ± 0.77 | −1.99~0.05 | |
| Unclassified | 6 | −24.31 ± 1.65 | 0.87 ± 0.86 | |||
| Total | 16 | −24.43 ± 1.20 | −26.02~−22.13 | −0.10 ± 1.17 | −1.99~0.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lei, W.; Luo, W.; Dai, Q.; Han, H.; Nie, Y. Comparison Study on the Trophic Niche of Red Pandas Using Stable Isotope Analysis. Animals 2024, 14, 3512. https://doi.org/10.3390/ani14233512
Zhang Y, Lei W, Luo W, Dai Q, Han H, Nie Y. Comparison Study on the Trophic Niche of Red Pandas Using Stable Isotope Analysis. Animals. 2024; 14(23):3512. https://doi.org/10.3390/ani14233512
Chicago/Turabian StyleZhang, Yuyu, Wanxin Lei, Wei Luo, Qinlong Dai, Han Han, and Yonggang Nie. 2024. "Comparison Study on the Trophic Niche of Red Pandas Using Stable Isotope Analysis" Animals 14, no. 23: 3512. https://doi.org/10.3390/ani14233512
APA StyleZhang, Y., Lei, W., Luo, W., Dai, Q., Han, H., & Nie, Y. (2024). Comparison Study on the Trophic Niche of Red Pandas Using Stable Isotope Analysis. Animals, 14(23), 3512. https://doi.org/10.3390/ani14233512





