Simple Summary
This study determined whether cassava bioethanol by-products (CEP) and crude palm oil (CPO) alter fatty acid profiles and lipid quality indices of beef from Thai indigenous crossbred heifers. Supplementation with CPO resulted in fatty acid profiles that differed significantly in loin and round meat compared to diets without CPO; it decreased saturated fatty acids and increased unsaturated fatty acids. The interaction of CEP and CPO has been found to modulate levels of certain fatty acids in subcutaneous fat. CPO-fed beef also improved some lipid quality indices. Successful chemometric analysis discriminated between CPO levels using fatty acid profiles. Based on these findings, dietary manipulation by CEP and CPO can be used to affect the fatty acid composition and nutritional quality of beef for the benefit of consumers.
Abstract
The quality and nutritional value of meat are significantly attributed to the composition of fatty acids (FAs). This investigation used gas chromatography to assess FAs in longissimus et lumborum (LL), semimembranosus (SM), and subcutaneous fat (SC) tissues of 18 heifers feeding low (15%, LCEP) or high (30%, HCEP) cassava bioethanol by-products (CEP) and 0 (CPO-0), 2 (CPO-2), or 4% (CPO-4) crude palm oil (CPO). The experimental diet was provided at 1.75% of body weight, along with free access to rice straw and water for 150 days. The results showed that the highest content of saturated (SFAs, 50.14, 42.76, and 68.76%, mainly C16:0), monounsaturated (MUFAs, 44.89, 49.14, and 30.41%, mainly C18:1n9c), and polyunsaturated fatty acids (PUFAs, 4.96, 8.10, and 0.84%, mainly C18:2n6c and C18:2n6t) were observed in LL, SM, and fat tissues. CPO supplementation significantly affected the FAs in LL and SM meat, with CPO-2 and CPO-4 diets leading to decreased SFAs and increased MUFAs and PUFAs compared to the CPO-0 diet. Multivariate analysis showed the most important FAs that highlight discrimination between different oil supplementation levels (CPO-0 vs. CPO-2, CPO-0 vs. CPO-4, CPO-2 vs. CPO-4) in LL (C18:2n6c, C20:3n3, C13:0), SM (C13:0, C18:0, C13:0), and SC fat (C18:2n6t, none, none) tissues. This data generates key insights into FA profiles resulting from different levels of oil supplements in cattle diets, which could influence future research on precision nutrition in beef production.
1. Introduction
The composition of fatty acids in beef meat greatly influences its nutritional quality, organoleptic properties, and consumer acceptance [1,2,3,4]. With the demand for better beef products increasing in the international markets, there is more incentive to develop better feeding regimes that improve the fatty acid composition of beef meat [5,6,7]. Strategic diet manipulation is crucial for optimizing meat’s fatty acid composition, enhancing health benefits for consumers [7,8]. The type of feed—whether it be forage-based, grain-based, or oil-based—directly alters the fatty acid profile in livestock [9,10,11,12].
Crude palm oil (CPO) serves as a vital component in animal nutrition, providing a high energy content, essential fatty acids, and vitamins that enhance livestock health and productivity [13,14]. Cassava, a starch-rich crop widely used in bioethanol production [15,16], yields cassava bioethanol by-products (CEP) with potential as animal feed [17,18]. Utilizing the bioethanol industry by-products can improve the environmental sustainability and cost-effectiveness of beef production systems. Previous research has examined the effects of CEP and CPO on nutrient digestibility, growth performance [17], carcass characteristics, and meat quality [18] in heifers. However, their impact on fatty acid profiles across different tissues remains unexplored.
This research, therefore, investigates the fatty acid composition of longissimus et lumborum (LL), semimembranosus (SM), and subcutaneous fat (SC) in crossbred beef heifers fed cassava bioethanol by-products and crude palm oil. Additionally, chemometric analysis was used to identify fatty acid biomarkers in each tissue between feeding levels. In this study, we will elucidate how these dietary interventions influence fatty acid profiles in beef meat, which will be useful to the beef industry and researchers looking to optimize beef production systems for quality and sustainability. The study hypothesizes that the fatty acid composition of beef meat and subcutaneous fat tissue from heifers differs from that of beef, affecting their nutritional profiles. It also seeks to identify potential fatty acid biomarkers in response to different levels of dietary oil supplementation.
2. Materials and Methods
All animal procedures followed the National Research Council of Thailand’s Ethical Principles and Guidelines for the Use of Animals and received approval from Khon Kaen University’s Animal Care and Use Committee (approval number: AEKKU 77/2556).
2.1. Animal and Diet Background and Meat Samples
Eighteen yearling Brahman–Thai native crossbred heifers (130 ± 14 kg BW, 1-year-old) were used. They received a concentrate feed at 1.75% BW, with free access to water and rice straw for 5 months. The treatments consisted of two levels of cassava bioethanol by-product (15 or 30%; LCEP or HCEP) each supplemented with three levels of crude palm oil (0, 2, and 4%; CPO-0, CPO-2, and CPO-4), designated as (1) LCEP + CPO-0, (2) LCEP + CPO-2, (3) LCEP + CPO-4, (4) HCEP + CPO-0, (5) HCEP + CPO-2, and (6) HCEP + CPO-4, respectively. Growth performance, nutrient digestibility, carcass traits, and meat quality results have been reported in detail elsewhere [17,18]. In brief, the results demonstrated that feed intake was exclusively influenced by the concentration of CPO, with elevated CPO levels enhancing fat digestibility. Nutrient intake and growth performance were similar. The DM, CP, and EE digestibility of HCEP was lower than LCEP [17]. The lean meat percentage and meat/bone ratio were less than those of LCEP-fed cattle. Carcass fat, fat content, and meat redness were significantly increased with diets with 4% dietary CPO [18]. After measurement of carcass characteristics, around 100 g of each longissimus et lumborum (LL) and semimembranosus (SM) from the right side of the carcass, and 25 g of subcutaneous fat (SC) at the 12th and 13th rib were then collected (within 1 h p.m.), packed in an LDPE vacuum bag, and frozen in the freezer at −25 °C for six months before examining fatty acid profiles.
2.2. Analysis of Fatty Acid Profiles
Total lipids are extracted using chloroform–methanol in feed, LL, SM, and SC fat samples [19,20]. The 15 g of ground samples were extracted with 90 mL of chloroform-methanol (2:1 v/v) and blended for 120 s, then 30 mL of chloroform was added and blended again for 120 s using Nissei AM-8 Homogenizer (Nihon Seiki Kaisha, Ltd., Tokyo, Japan). The homogenates were isolated in the separating funnel, followed by an additional 30 mL of dH2O and 5 mL of 0.58% NaCl, then shaken and set aside before clear separation of the solution. The lower solution was left to the known weight of the evaporate flask. The solvent is extracted from fat by BUCHI Rotavapor R-200 Rotary Evaporator (BUCHI Labortecnnik AG, Flawil, Switzerland), evaporating at 40 °C and then deposited at −20 °C under N2 gas. The extracted lipids were methylated to yield fatty acid methyl esters (FAMEs) [21]. At first, 30 mg of the extracted lipids were transferred to a 15 mL test tube with a screw cap. Next, 1.5 mL of 0.5 N NaOH/MeOH was applied, flushed by N2, covered, heated for 5 min at 100 °C through intermittent shaking, and then kept cool at room temperature. Then, 1 mL of heptadecanoic acid (C17:0), as an internal standard, was added, and 2 mL of 14% BF3/MeOH, heated for 5 min at 100 °C by infrequent trembling, and 10 mL of dH2O then added. Each methylated solution was placed in a centrifuged tube, and the mixture with hexane (5 mL) was then centrifuged at a speed of 5000× g for 15 min. The hexane layer was completely dehydrated by sodium sulfate (Na2SO4), transferred to amber vial, flushed by N Rapid Preparation of Fatty Acid Esters from Lipids for Gas Chromatographic Analysis, and FAMEs was analyzed by gas chromatography (GC) (HP 6890 GC Systems, Agilent Technologies, Inc., Wilmington, DE, USA) fitted with a capillary column (SP-2560, L × I.D. 100 m × 0.25 mm, 0.20 μm film thickness). The settings of the GC conditions were as follows: carrier gas, He, injector temperature, 250 °C, flame ionization detector (FID), 260 °C, split ratio, 100:1, oven temperature, for 5 min at 140 °C, and the temperature was then raised to 240 °C at a rate of 4 °C/min. Each 1 μL of the sample was automatically injected, the fatty acid peaks were defined and compared to established reference methyl esters. All fatty acid values were calculated as a percentage of total fatty acids by weight. Total saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), PUFA/SFA ratio (P/S), n-3 and n-6 fatty acids, and n-6/n-3 ratio were calculated. Other lipid quality indices were also computed.
2.3. Analysis of Healthy Indices of Lipids
The various quality indices of lipids were calculated to determine their atherogenicity index (AI), thrombogenicity index (TI), hypocholesterolemia/hypercholesterolemic ratio (h/H), nutritive value index (NVI), health-promoting index (HPI), Peroxidizability index (PI), and the enzyme activity index were also computed.
2.3.1. Atherogenicity Index (AI)
The AI highlights the balance between pro-atherogenic saturated fatty acids and anti-atherogenic unsaturated fatty acids. These fatty acids contribute to the development of immune cells and the circulatory system. AI was calculated using the following formula [22]:
2.3.2. Thrombogenicity Index (TI)
The TI indicates the tendency of the blood vessel to form clots. It is linked between prothrombogenetic (saturated) and anti-thrombogenetic (unsaturated) fatty acids as per the following formula [23].
2.3.3. Hypocholesterolemic/Hypercholesterolemic Ratio (h/H)
The h/H ratio is a commonly used index for the fatty acid profile of meat and blood plasma. It shows the relationship between the observed hypocholesterolemic (unsaturated) fatty acid and the hypercholesterolemic (saturated) fatty acids as per the following formula [24].
2.3.4. Nutritive Value Index (NVI)
The NVI values were calculated as per the following equation [25]:
2.3.5. Health-Promoting Index (HPI)
The HPI evaluates the nutritional quality of various dietary fats by examining how their composition impacts cardiovascular disease (CVD) [26].
2.3.6. Peroxidizability Index (PI)
The PI values was calculated as following equation [27]:
2.3.7. Enzyme Activity Index
The delta-9 fatty acid desaturation index was used as an indicator of metabolic disease [28].
2.4. Statistical Analysis
Data is presented as least square means ± SD. Fatty acid content, lipid indices, and enzyme indexes were analyzed for analysis of variance (ANOVA) using 2 × 3 factorial arrangements in a completely randomized design (CRD) with a GLM procedure of SAS [29]. The analysis included two levels of CEP and three levels of CPO as main factors, along with their interaction (CPO × CEP). The differences among means were compared using Tukey–Kramer post hoc test (p < 0.05). A Venn diagram was created with a free online tool (https://bioinformatics.psb.ugent.be/webtools/Venn/, accessed on 23 September 2024). Multivariate analysis was performed using the free online tool, MetaboAnalyst 6.0 [30]. Principal components analysis (PCA) was initially used for visualization. Orthogonal partial least squares discriminant analysis (OPLS-DA) was then conducted to identify candidate biomarkers based on variable importance in projection (VIP > 1) values, p-Values (p < 0.05), and false discovery rate (FDR) (p < 0.05) [31,32].
3. Results
3.1. Fatty Acid Profiles
Fatty acid profiles (% of total FA) of experimental diets are shown in Table 1. The percentages of fatty acid profiles in meat and fat tissues obtained from beef heifers fed diets with different levels of CEP and CPO are displayed as least square means and standard deviations in Table 2, Table 3 and Table 4. In both LL and SM meat, the interaction effect between CEP and CPO and the main effect of CEP was not significant (p > 0.05), indicating there were no significant differences for any fatty acid profiles for each factor level combination of CEP and CPO or CEP alone.

Table 1.
Fatty acid profiles (% of total FAs) of cassava bioethanol by-product (LCEP and HCEP), crude palm oil (CPO), and rice straw (RS).

Table 2.
Fatty acid profile (% of total FAs) of LL meat of beef heifer fed with different diets.

Table 3.
Fatty acid profile (% of total FAs) of SM meat of beef heifer fed with different diets.

Table 4.
Fatty acid profile (% of FAs) of subcutaneous fat of beef heifer fed with different diets.
For LL meat, the main effect of CPO was significant (p < 0.05), affecting the levels of several fatty acids, including C12:0, C13:0, C14:0, C14:1, C16:0, C16:1, C18:0, C18:1n9t, C18:1n9c, C18:2n6c, C20:3n6, C20:3n3, C22:6n3, as well as SFAs, MUFAs, PUFAs, omega-3, omega-6, omega-6:3, PUFA:SFA, MUFA:SFA, UFA:SFA, and HFA:SFA. The concentrations of C12:0, C14:0, C14:1, C16:0, C18:0, C18:1n9t, SFAs, omega-6:3, and HFA:SFA were significantly higher in the CPO-0 group compared to the CPO-2 and CPO-4 groups. The levels of C14:1, C18:1n9ct, C18:1n9c, and HFA:SFA were significantly higher in the CPO-2 group than in the CPO-4 group. While the values of C13:0, C16:1, C18:2n6c, C20:3n6, C20:3n3, C22:6n3, MUFAs, PUFAs, omega-3, omega-6, PUFA:SFA, MUFA:SFA, and UFA:SFA for CPO-0 were significantly smaller than those of CPO-2 and CPO-4. There was no significant difference in C18:3n3 across CPO levels.
Including CPO in the heifer diet, notably impacts the fatty acid profiles of SM meat (p < 0.05, Table 3). The CPO-2 diet resulted in lower levels of saturated (C12:0, 14:0, 16:0, 18:0) and some unsaturated (14:1, 18:1n9t, 18:2n6c, 18:3n3) fatty acids, together with total SFAs, PUFAs, omega-6, omega-6:3, and PUFA:SFA than heifers consuming CPO-0 and CPO-4 diets. In opposition, it increased C13:0, C16:1, C18:1n9c, C20:3n3, C22:6n3, total MUFAs, omega-3, MUFA:SFA, UFA:SFA, and HFA:SFA. C20:3n6 remained unaffected by CPO levels.
The levels of C16:1, C18:2n6c, C20:3n6, and omega-6:3 in SC fat (Table 4) displayed a statistically significant interaction between CEP and CPO (p < 0.05). The mix of low CEP (LCEP) and CPO-0 led to lower C16:1, C18:2n6c, and omega-6:3 levels than those of other groups. The LCEP and CPO-4 combination had the lowest C20:3n6. Furthermore, both LCEP and HCEP affected fatty acids C10:0, C9T11, C18:3n3, C9C11, C21:0, C22:6n3, PUFAs, omega-3, omega-6, and PUFA:SFA (p < 0.05), with the LCEP group presenting higher amounts of C10:0 and C18:3n3, and lower values of C9T11, C9C11, C21:0, C22:6n3, PUFAs, omega-3, omega-6, and PUFA:SFA compared to the HCEP group. The SC fat from heifers whose diet included CPO revealed effect on C10:0, C13:0, C16:0, C18:2n6t, C20:3n3, and C22:6n3 (p < 0.05), observing that the CPO-0 group had higher C10:0, C13:0, C18:2n6t, and C20:3n3 levels than those of CPO-2 and CPO-4 groups. In contrast, C16:0 and C22:6n3 in the CPO-4 group were greater than those in the CPO-2 group. No discernible distinctions were found for C12:0, C14:0, C18:0, C18:1n9c, T10C12, C20:2, SFAs, or MUFAs among various CEP or CPO levels.
3.2. Healthy Indices of Lipids
Quality indices of lipids in LL, SM meat, and SC fat under different levels of CEP and CPO are shown in Table 2 and Table 3. For lipid quality indices, there was no significant interaction (p < 0.05) between CEP and CPO in both meats, and the main effect of CEP was not observed. Nevertheless, CPO pronounced impacted various indices in LL meat (p < 0.05) such as AI, TI, h:H, NVI, HPI, PI, SCD-16, SCD-18, EI, and TE. Supplementation with a higher CPO level in the diet resulted in reduced indices of AI, TI, and EI, but increased h:H, NVI, HPI, PI, SCD-16, SCD-18, and TE. Similarly, CPO-2 improved h:H, NVI, HPI, SCD-16, SCD-18, and TE, but had lowered AI, TI, and EI compared to the other dietary treatments in SM meat (Table 2 and Table 3). A significant CEP x CPO interaction was seen for SCD-16 in SC fat (p < 0.05). The HCEP combined with the CPO-4 group containing the highest SCD-16 value. Nonetheless, it was similar to other treatments with the exception of LCEP and CPO-0. There were no significant differences between the LCEP and HCEP groups for any CPO level in the AI parameter. Similarly, the TI, h:H, NVI, HPI, and EI were not significantly different among treatments. The PI and TE index were different between the LCEP and HCEP groups (p < 0.05).
3.3. Multivariate Analysis Using Chemometric Approach
The PCA and OPLS-DA (Figures S1–S3) score plots were used to analyze the percentages of FAs. The principal components of PC1 and PC2 explained 88.7% and 9.9% of the variation in LL, 68.5% and 24.2% in SM, and 74.0% and 11.9% in SC fat, respectively. However, the CPO groups were not entirely separated in the unsupervised PCA of SC fat tissues. Based on OPLS-DA models (Table S1 and Figure S1), LL with CPO-0 versus CPO-2 were discriminated with a model with a R2X of 0.82, R2Y of 1.00, a Q2 of 0.98, and a VIP threshold of 1.0 with p-Value and FDR less than 0.05 (Table S1). In this model, 7 FAs including C18:2n6c, C13:0, C14:0, C20:3n3, C12:0, C22:6n3, and C16:0 were found to carry the class separation. Clear separation of LL with CPO-0 from CPO-4 with a model including 12 FAs such as C20:3n3, C20:3n6, C14:0, C16:0, C12:0, C18:2n6c, C13:0, C22:6n3, C18:1n9t, C18:0, C16:1, and C14:1 (R2X of 0.97, R2Y of 1.00, Q2 of 1.00) were observed. OPLS-DA model also showed a clear separation of CPO-2 vs. CPO-4 with a model including 8 FAs including C13:0, C14:1, C20:3n6, C18:3n3, C16:1, C14:0, C12:0, and C16:0 with a R2X of 0.79, R2Y of 1.00, a Q2 of 0.98.
In SM meat (Table S1 and Figure S2), 8 FAs including C13:0, C14:1, C20:3n6, C18:3n3, C16:1, C14:0, C12:0, and C16:0 were the potential FAs that could distinguish between CPO-0 versus CPO-2 groups. The key parameters of this model, R2X = 0.79, R2Y = 1.00, and Q2 = 0.98, were good predictors of model fit. The OPLS-DA model showed good performance in discriminating between CPO-0 and CPO-4, with 8 FAs including C18:0, C22:6n3, C13:0, C16:1, C18:1n9t, C18:1n9c, C18:3n3, and C20:3n3 were the key discrimination (R2X = 1.00, R2Y = 1.00, and Q2 = 1.00). Lastly, good discrimination was shown between CPO-2 and CPO-4 with C13:0, C18:1n9c, C18:1n9t, C18:3n3, C18:2n6c, C12:0, C14:1, with a R2X = 0.89, R2Y of 1.00, a Q2 of 0.99. In fat tissues (Table S1 and Figure S3), only the single most identifying variable (C18:2n6t) was prioritized for comparing CPO-0 with CPO-2 (R2X = 0.63, R2Y of 1.00, a Q2 of 0.94). While there were no significant findings to identify the key FAs for distinguishing between CPO-0 vs. CPO-4 and CPO-2 vs. CPO-4.
A Venn diagram (Figure 1) is a graphic representation that makes use of concentric circles to highlight the connections between different things or limited sets of things. The characteristics shared by overlapping circles are not shared by nonoverlapping ones. A total of 21 components contributed to this effort, and LL, SM, or fat influenced the overflow at the same time. The 14 parameters listed below were measured at multiple locations: For LL and SM, C14:1 and C18:1n9t were measured on the plane, while the other 12 parameters (C12:0, C13:0, C14:0, C16:0, C16:1, C18:0, C18:1n9c, C18:2n6c, C18:3n3, C20:3n3, C20:3n6, and C22:6n3) were measured on each of the three planes. So, the 7 separate variables (C10:0, C9T11, C9C11, T10C12, C18:2n6t, C20:2, and C21:0) were measured based on fat alone.
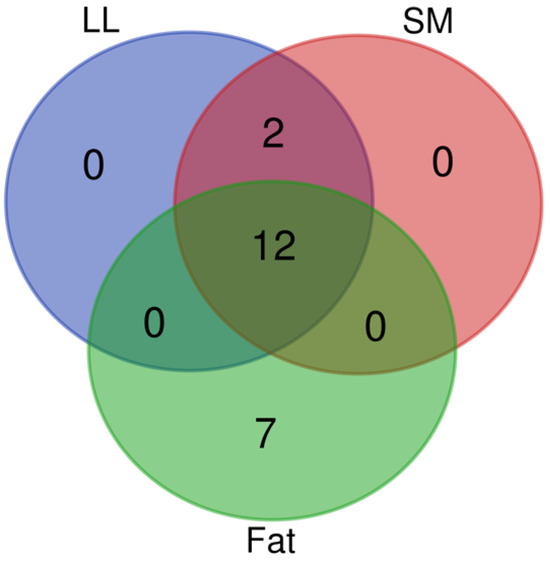
Figure 1.
Venn diagram of fatty acid profiles. The Venn diagram depicts the fatty acids shared by LL meat, SM meat, and SC fat of Brahman crossbred heifers fed varied diets. The overlapping sections denote the number of fatty acids shared by the tissues shown by the circles, which each reflect the total number of fatty acids contained in their respective tissues.
4. Discussion
Concerning human nutrition, red meat is an important source of essential polyunsaturated fatty acids (PUFAs), protein, vitamins, and minerals [6,33]. Nonetheless, feeding regimens practiced in beef cattle production can modify the fatty acid composition of their meat and, thus, it could also influence the content of essential fatty acids [8,12]. For instance, linseed oil supplementation promotes n-3 PUFA and CLA deposition and improves the n-6/n-3 PUFA ratio [34]. The fatty acids found in the intramuscular fat (IMF) of beef typically contain around 45 to 48% saturated fats and between 35 and 45% monounsaturated fats, with a slight percentage of polyunsaturated fats (5%) [6]. Results from our investigation indicate that increasing CPO levels linearly reduced SFAs in LL (55.62, 52.18, and 42.61%). However, in SM, the lowest SFA content was observed in the CPO-2 group. Changes in SFAs are caused by biohydrogenation of dietary unsaturated fat by rumen bacteria [35,36]. With high-concentrate diets, biohydrogenation of PUFA in the rumen is reduced [37] and causes the unsaturated deposition. In LL, higher CPO levels linearly increased in both MUFAs (41.12, 43.60, and 49.95%) and PUFAs (3.25, 4.21, 7.42%). In SM, MUFAs content was higher in CPO-2 (51.66%) compared to CPO-4 (47.90%) and CPO-0 (47.85%). Additionally, SM PUFA content was lower in CPO-supplemented groups (7.93 and 7.86%) than in the CPO-0 (8.49%). Compared to other palm oil studies in ruminants, no changes in the total fatty acid composition were found when Friesian bulls were fed tallow or various hydrogenated palm oils at 5.5% [38] or Friesian steers fed different types of fats at 4% [39]. In SC fat, PUFA content was affected only by CEP level, with HCEP showing a higher value (0.95%) than LCEP (0.73%). The values are consistent with the grain-fed beef report in the US, where SFAs, MUFAs, and PUFAs averaged 43.4, 45.3, and 4.5%, respectively [40]. These fatty acid profiles in meat are important because they alter both its nutritional content and sensory properties, such as juiciness and taste [7].
Among dietary SFAs, myristic (C14:0) and palmitic (C16:0) acids are known to elevate plasma cholesterol, with myristic acid exerting a stronger effect. In contrast, stearic acid (C18:0) has minimal impact on cholesterol levels [41,42]. Our findings reveal a fatty acid profile of C16:0 > C18:0 > C14:0, in which the higher CPO increased C16:0 and C18:0 in SC fat, while the values in LL and SM were opposite. The greater proportion of C16:0 than C18:0 and C14:0 are consistent with previous reports on African beef [11], US beef [40], young bull [43], Brahman steers [44], and Korean native cattle [45]. Regarding MUFAs, our analysis revealed that oleic acid (C18:1n9c) is the predominant fatty acid, constituting 28.5% of total fatty acids, followed by palmitoleic acid (C16:1). Oleic acid proportions differed significantly amongst samples at different CPO levels, where the values in LL and SM reduced with higher dietary fat levels. This agrees with previous studies on Nellore steers, where oleic acid concentrations were lowest in steers fed diets with supplemental lipid sources [12]. This MUFA profile aligns with previous findings in young beef cattle fed with grass or grain diets [11], conventional beef [46], and fat-fed beef [39,44], where oleic acid is consistently the most abundant MUFA. The oleic acid content in food not only contributes to its palatability but also offers potential health benefits [47,48], particularly lowering LDL cholesterol.
PUFAs are recognized in beef for their cholesterol-lowering properties and various health benefits [47]. Our findings indicated that PUFA levels varied significantly in beef samples, spanning from 0.84% in fat to 4.96% in LL and 8.10% in SM meat. The PUFA content in LL increased proportionally with CPO supplementation levels. However, in SM, the content was lower in treatments with higher CPO levels compared to CPO-0. Among PUFAs, the main n-6 fatty acid in all samples was linoleic acid (C18:2n-6c), followed by C20:3n3 (ETE) and C22:6n3 (DHA). Similar PUFAs in loin and SC fat were observed in Nellore steers fed varied lipid sources [12]. However, palm kernel oil supplementation did not affect PUFAs in bulls [43]. These results correlate with investigations from Africa, Argentina, and the UK that identified greater levels of n-6 fatty acids in beef produced by grain-fed cattle than from grass-fed cattle [11,49,50].
The proportion of conjugated linoleic acid (CLA) in beef affects its nutritional quality and health benefits [7]. Dietary supplementation with CLA reduces body fat, increases lean muscle mass, decreases atherosclerosis severity, and inhibits carcinogenesis [51,52,53]. We found that only CLA isomers (C9T11, C9C11, T10C12) existed in SC fat but not in LL and SM meat, and the C9T11 and C9C11 levels increased with HCEP diets. Several studies have shown that cattle fed on a forage-rich diet, such as grass, can increase CLA levels in meat [10,50,54].
Thrombotic and atherogenic indices are managed by omega 3 and omega 6 fatty acids, and their balance affects the hypocholesterolemic index [55]. Decreases in the omega 6 ratio typically decrease inflammation and elevate long-term illness risks [56]. A reduced omega 6 to omega 3 ratio is better for lowering the chances of numerous chronic illnesses prevalent in several nations [57]. In Western diets, the ratio normally stands at around 16:1; however, a 1:1 ratio is viewed as the best option [58,59,60]. In the current study, dietary CPO linearly increased omega 3 (1.47, 2.00, 3.52) and omega 6 (1.79, 2.21, 3.90) but decreased omega 6:3 ratio (1.23, 1.10, 1.11) in LL. However, in SM, the lowest omega 3 was found in CPO-4, while the lowest omega 6 and omega 6:3 ratio was found in CPO-2. In SC fat omega 3 and 6 of HCEP were higher than LCEP. Additionally, heifers fed HCEP and CPO-4 had the lowest omega 6:3 ratio (3.90) compared to other treatments. Previous works have shown that different cattle breeds and dietary fat supplementations have yielded different omega 6:3 ratio. According to Fiorentini et al. [12], Nellore steers fed various lipid sources had ratios varying from 4.50 to 12.20. Supplementation of 5.5% of different fat types to Friesian steers resulted in higher ratios of 16.73–20.57 [39]. Bulls fed palm kernel oil from 0.0–34.6 g/kg diet had lower values of 2.54–2.22 [43]. The ratio was approximately 10.53 for Brahman steers supplemented with 200 g/day palm oil. In comparison, grass-fed beef generally has a more favorable fatty acid profile than grain-fed beef, with higher omega 3 PUFA content and lower omega 6:3 ratios [54]. Maximizing meat quality is challenging because very high PUFA levels may alter the content of these profiles [6]. Some enhancements in the fatty acid profile of beef can be achieved while still maintaining desired meat quality [8,61].
The recommended PUFA to SFA (P:S) ratios in foods is greater than or equal to 0.40 [62]. Levels lower than this threshold are considered undesirable for the human diet [63] because of their potential to increase blood cholesterol. In this study, P:S ratios of LL ranged from 0.08 in CPO-0 to 0.15 in CPO-4, SM ranged from 0.15 in CPO-0 to 0.18 in CPO-4, and SC ranged from 0.01 to 0.02 when comparing CPO-0 to CPO-4. Similar results are also reported by [3], where the P:S ratios in the lean beef from feeding a ruminally-protected lipid was 0.11, indicating a relatively high in SFA content. Also, this proportion in beef is generally minimal, approximating 0.1 [6].
Dietary CPO linearly increased the unsaturated to saturated fatty acid ratio (UFA:SFA) in LL meat (0.80, 0.92, and 1.35). Furthermore, our findings indicate that the CPO-2 diet significantly elevated the UFA:SFA ratio in both the SM meat and SC fat. The present results concord with another study that investigated the ratio of UFA:SFA in porcine meat generation and stated that the UFA:SFA ratio was 1.32 [64]. The primary determinant of meat flavor profiles is the UFA:SFA ratio, in which UFA has important roles in sensory taste enhancement as well as the removal of deleterious free radicals [65].
Potential cardiovascular health impacts are expressed as the ratio of hypercholesterolemic (HFA) to saturated fatty acids (HFA:SFA) in meat. The higher presence of HFA:SFA ratio indicates a greater risk of cardiovascular diseases [8,66]. In this study, beef from CPO-2 and CPO-0 treatment had higher HFA:SFA ratios when compared to CPO-4. The LL, SM, and fat from CPO-fed beef had the mean HFA:SFA ratios of 0.67, 0.67, and 0.69, respectively. The results reported in this work are similar to those in broiler meat (0.62) [67] and organic cow milk (0.74) [68].
Healthy indices of lipids are important tools for evaluating the nutritional quality and potential health impacts of fatty acids in meat. The assessment of lipid profiles in relation to cardiovascular health and overall nutritional quality involves several key indices. The atherogenicity (AI) and thrombogenicity (TI) indices evaluate the potential for atherosclerosis and thrombosis development, respectively [22]. Lower AI and TI indicate a decreased risk of developing cardiovascular diseases. This study reveals that AI (0.85), and TI (1.35) are approximately 3 times less in meat (LL and SM) than in SC fat (2.54 and 4.31). In LL meat, AI and TI were decreased by increasing the CPO levels, but in SM, the lowest values were presented in heifers fed CPO-2 and these values were similar in SC fat. However, the results obtained are greater than those for beef [69].
For the fatty acids in the diet, the hypocholesterolemic to hypercholesterolemic ratio (h:H) provides insight into cholesterol-lowering capacity, with a higher h:H being preferred in human health [27]. The h:H index obtained in the present study ranged from 0.91 to 1.38, which was lower than goose meat (2.7) [27], lamb (1.92) [70], and beef (1.8) [71]. The nutritive value index (NVI) is a ratio of the nutritional value of the meat, with a higher value indicating high nutritional value [25]. The highest value of NVI was noted for the SM meat (1.71) due to the highest proportion of C18:0, C 18:1, and the lowest C16:0 content. Similar results were found in hair and rabbit meat [72].
Regarding the main effect of CPO, HPI, and PI in LL linearly increased with increasing CPO levels. A higher value in the health-promoting index (HPI) indicates an association with a higher health benefit potential for human health [66]. Our study found HPI values of 1.26 for meat and 0.4 for fat. In comparison, other studies reported HPI ranges of 0.16 to 0.68 for milk [66] and 1.59 to 1.81 for poultry [73]. The peroxidizabilityi index (PI) measures the susceptibility of fatty acids to oxidative damage and its role in determining lipid stability as well as potential health impact, but as PI increases, the greater the protective potential against coronary artery disease [27]. The lower PI [11] of this present beef, compared to poultry meat [27], indicates fewer fatty acids autooxidation and longer shelf life.
The Δ9-desaturase enzymes, particularly SCD-16 and SCD-18, are crucial for con-verting SFAs into MUFAs. Higher activity of these enzymes is generally beneficial because it leads to increased production of palmitoleic acid (C16:1) and oleic acid (18:1n-9), which is known to be associated with improved lipid profiles and less inflammation [65,74]. In contrast, some research indicates that food with lower amounts of SCD-16 and SCD-18 tends to improve health outcomes because it decreases risks linked to metabolic syndrome and obesity [75,76,77,78]. In this experiment, the SCD-16 (25.42, 28.61, and 46.00) and SCD-18 (61.08, 64.04, and 66.74) values of LL meat rose as the CPO levels increased. But in SM, SCD-16 (41.95, 48.80, and 44.52) and SCD-18 (67.92, 68.91, and 65.01) showed the highest values for cattle-fed CPO-2 compared to others. The SCD-16 value was the lowest for SC from the LCEP and CPO-0 treatment compared to other groups. Among tissues, these enzymes were most abundant in SM > LL > fat. Compared to Nellore bulls fed 0–30% crude glycerin [79], where SCD-16 ranged from 8.5–13.6 and SCD-18 from 63.6–74.4 in loin, and SCD-16 from 9.4–14.1 and SCD-18 from 70.0–74.9 in fat, our results exhibit that CPO-fed cattle have nearly equal SCD-18 content in meat and fat, but higher SCD-16 in meat and lower SCD-16 in fat.
The elongase index (EI) and thioesterase index (TE) are also central to fatty acid metabolism. A higher EI may reflect an increased ability to desaturate fatty acids elongated via the elongase pathway [73], which is advantageous in generating long-chain FAs required for multiple physiological processes [28,76]. On the other hand, an excess of this elongation can be associated with the rise in SFAs and some negative effects on human health [80], so a moderate EI is preferable. The TE, depending on the activity of enzymes related to fatty acid synthesis, should also be optimal; low activity may be beneficial in protecting from excessive fat deposition, while high activity can lead to metabolic imbalances and health issues [81,82]. The reduced EI and TE indices of meat observed in this study may be explained by the increased conversion of palmitic and stearic acids to their respective unsaturated substances, possibly as a result of increased SCD-1 expression [67].
The OPLS-DA models constructed indicate an alteration in the fatty acid content of LL, SM, and SC fat because of oil supplementation. The clear classification between groups with different CPO content (0%, 2%, and 4%) demonstrates the effect of dietary fats on meat fatty acids with likely effects on its quality and nutrition. However, in terms of fat samples, evident separation was only achieved with 0 and 2% of CPO. The analysis highlighted some key FAs as important in the discrimination of treatment groups. Four FAs in LL (C12:0, C13:0, C14:0, and C16:0) and two FAs in SM (C13:0 and C18:3n3) were identified as key differentiators between 0, 2, and 4% of CPO. These key FAs can be useful in revealing the reasons behind the meat and health quality of animals with different diets. An OPLS-DA model with 7, 12, and 8 FAs, and 8, 8, and 7 FAs succeeded in separating LL and SM meat with 0 vs. 2, 0 vs. 4, and 0 vs. 4 CPO. Of these, Q2 values around 1 (higher than 0.9) indicate that the model was excellent [32,83]. Interestingly, while several key FAs distinguished CPO-0 and CPO-2 groups in meat, only one FA (C18:2n6t) was significant in fat tissues. The proposed 0 vs. 4 and 2 vs. 4 observed the same low oil restriction, indicating that more oil levels may not distinguish fatty acid compositions on top of what has already been achieved with lower levels.
5. Conclusions
It could be concluded that crude palm oil (CPO) supplementation in heifer diets greatly improves beef fatty acid profiles and nutritional quality. Both longissimus lumborum (CPO-4) and semimembranosus (CPO-2) muscles reduced saturated (SFAs) and increased unsaturated fatty acids (UFAs) from CPO supplementation. Supplementation of CPO improved some health indices, especially the AI, TI, and h:H indices. Fatty acid profiles from beef from heifers fed different CPO levels were successfully differentiated by chemometric analysis. Fatty acid composition alterations suggest consumer health benefits. These results show that CPO in the feed can make beef more nutritious by altering its fatty acid content. Further research, however, would be necessary to optimize CPO supplementation levels and to assess, if any, possible negative effects on meat quality or production efficiency for a long-term study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani14233478/s1, Figures S1–S3: PCA, OPLS-DA, and permutation test of CPO-0 vs. CPO-2, CPO-0 vs. CPO-4, and CPO-2 vs. CPO-4 in LL, SM, and fat; Table S1: Possible biomarker for separating fatty acid profiles from different levels of crude palm oil.
Author Contributions
Conceptualization, C.P. and S.U.; methodology, C.P. and S.U.; validation, C.P., S.U., R.P. and T.T.; formal analysis, C.P.; investigation, C.P., S.U. and T.T.; resources, C.P. and S.U.; data curation, C.P. and S.U.; writing—original draft preparation, C.P.; writing—review and editing, C.P., S.U., R.P. and T.T.; visualization, C.P.; supervision, S.U. and T.T.; project administration, S.U.; funding acquisition, S.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Increased Production Efficiency and Meat Quality of Native Beef and Buffalo Research Group, Khon Kaen University.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of Khon Kaen University (protocol code: AEKKU 77/2556) for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hocquette, J.F.; Botreau, R.; Picard, B.; Jacquet, A.; Pethick, D.W.; Scollan, N.D. Opportunities for predicting and manipulating beef quality. Meat Sci. 2012, 92, 197–209. [Google Scholar] [CrossRef]
- Ponnampalam, E.; Priyashantha, H.; Vidanarachchi, J.; Kiani, A.; Holman, B. Effects of nutritional factors on fat content, fatty acid composition, and sensorial properties of meat and milk from domesticated ruminants: An overview. Animals 2024, 14, 840. [Google Scholar] [CrossRef] [PubMed]
- Aldai, N.; Dugan, M.E.R.; Rolland, D.C.; Kramer, J.K.G. Survey of the fatty acid composition of canadian beef: Backfat and longissimus lumborum muscle. Can. J. Anim. Sci. 2009, 89, 315–329. [Google Scholar] [CrossRef]
- Arshad, M.S.; Sohaib, M.; Ahmad, R.S.; Nadeem, M.T.; Imran, A.; Arshad, M.U.; Kwon, J.-H.; Amjad, Z. Ruminant meat flavor influenced by different factors with special reference to fatty acids. Lipids Health Dis. 2018, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.D.; Price, E.M.; Morgan, S.A.; Huws, S.A.; Shingfield, K.J. Can we improve the nutritional quality of meat? Proc. Nutr. Soc. 2017, 76, 603–618. [Google Scholar] [CrossRef]
- Scollan, N.; Hocquette, J.-F.F.; Nuernberg, K.; Dannenberger, D.; Richardson, I.; Moloney, A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006, 74, 17–33. [Google Scholar] [CrossRef]
- Scollan, N.D.; Dannenberger, D.; Nuernberg, K.; Richardson, I.; MacKintosh, S.; Hocquette, J.-F.F.; Moloney, A.P. Enhancing the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2014, 97, 384–394. [Google Scholar] [CrossRef]
- Vahmani, P.; Mapiye, C.; Prieto, N.; Rolland, D.C.; McAllister, T.A.; Aalhus, J.L.; Dugan, M.E.R. The scope for manipulating the polyunsaturated fatty acid content of beef: A review. J. Anim. Sci. Biotechnol. 2015, 6, 29. [Google Scholar] [CrossRef]
- Realini, C.E.; Duckett, S.K.; Brito, G.W.; Dalla Rizza, M.; De Mattos, D. Effect of Pasture vs. concentrate feeding with or without antioxidants on carcass characteristics, fatty acid composition, and quality of Uruguayan beef. Meat Sci. 2004, 66, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Dannenberger, D.; Nuernberg, K.; Nuernberg, G.; Scollan, N.; Steinhart, H.; Ender, K. Effect of pasture vs. concentrate diet on CLA isomer distribution in different tissue lipids of beef cattle. Lipids 2005, 40, 589–598. [Google Scholar] [CrossRef]
- Hall, N.; Schönfeldt, H.; Pretorius, B. Fatty acids in beef from grain- and grass-fed cattle: The unique South African scenario. S. Afr. J. Clin. Nutr. 2016, 29, 55–62. [Google Scholar] [CrossRef]
- Fiorentini, G.; Lage, J.F.; Carvalho, I.P.C.; Messana, J.D.; Canesin, R.C.; Reis, R.A.; Berchielli, T.T. Lipid sources with different fatty acid profile alters the fatty acid profile and quality of beef from confined Nellore steers. Asian-Australas. J. Anim. Sci. 2015, 28, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and nutritional properties of palm oil and palmitic acid: Effects on health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef] [PubMed]
- Lai, O.-M.; Phuah, E.-T.; Lee, Y.-Y.; Basiron, Y. Palm Oil. In Bailey’s Industrial Oil and Fat Products, 7th ed.; Shahidi, F., Ed.; John Wiley & Sons Inc: Hoboken, NJ, USA, 2020; pp. 385–485. [Google Scholar]
- Ogbonna, C.; Okoli, E. Evaluation of the potentials of some cassava varieties in nigeria for bio-ethanol production. Bio-Res. 2011, 8, 674–678. [Google Scholar] [CrossRef]
- Sriroth, K.; Piyachomkwan, K.; Wanlapatit, S.; Nivitchanyong, S. The promise of a technology revolution in cassava bioethanol: From thai practice to the world practice. Fuel 2010, 89, 1333–1338. [Google Scholar] [CrossRef]
- Phoemchalard, C.; Uriyapongson, S.; Berg, E.P. Effect of cassava bioethanol by-product and crude palm oil in Brahman x Thai native yearling heifer cattle diets: I. Nutrient digestibility and growth performance. Trop. Anim. Health Prod. 2014, 46, 663–668. [Google Scholar] [CrossRef]
- Phoemchalard, C.; Uriyapongson, S. Effect of cassava bioethanol by-product and crude palm oil in Brahman x Thai native yearling heifer cattle diets: II. Carcass characteristics and meat quality. Trop. Anim. Health Prod. 2015, 47, 1629–1631. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloanee Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Metcalfe, L.D.; Schmitz, A.A.; Pelka, J.R. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal. Chem. 1966, 38, 514–515. [Google Scholar] [CrossRef]
- Ostrowska, E.; Dunshea, F.R.; Muralitharan, M.; Cross, R.F. Comparison of silver-ion high-performance liquid chromatographic quantification of free and methylated conjugated linoleic acids. Lipids 2000, 35, 1147–1153. [Google Scholar] [CrossRef]
- Ulbricht, T.L.; Southgate, D.A. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Abrami, G.; Natiello, F.; Bronzi, P.; McKenzie, D.; Bolis, L.; Agradi, E. A comparison of highly unsaturated fatty acid levels in wild and farmed eels (Anguilla Anguilla). Comp. Biochem. Physiol. Part B Comp. Biochem. 1992, 101, 79–81. [Google Scholar] [CrossRef]
- Fernandes, C.E.; Vasconcelos, M.A.d.S.; de Almeida Ribeiro, M.; Sarubbo, L.A.; Andrade, S.A.C.; Filho, A.B.d.M. Nutritional and lipid profiles in marine fish species from Brazil. Food Chem. 2014, 160, 67–71. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Y.; Xiao, Y.; Chen, H.; Zhao, L.; Huang, M.; Zhou, G. Differences in physicochemical and nutritional properties of breast and thigh meat from crossbred chickens, commercial broilers, and spent hens. Asian-Australas. J. Anim. Sci. 2015, 29, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and sensory properties of dairy products from cows with various milk fatty acid compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef] [PubMed]
- Wołoszyn, J.; Haraf, G.; Okruszek, A.; Wereńska, M.; Goluch, Z.; Teleszko, M. Fatty acid profiles and health lipid indices in the breast muscles of local Polish Goose varieties. Poult. Sci. 2020, 99, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Malau-Aduli, A.E.O.; Siebert, B.D.; Bottema, C.D.K.; Pitchford, W.S. A Comparison of the fatty acid composition of triacylglycerols in adipose tissue from Limousin and Jersey cattle. Aust. J. Agric. Res. 1997, 48, 715. [Google Scholar] [CrossRef]
- SAS. SAS/STAT® 14.1 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Phoemchalard, C.; Tathong, T. Chemometric approach to characterizing and comparing the quality of buffalo meat from Nakhon Phanom and Khammouane Provinces. Int. J. Agric. Technol. 2023, 19, 2589–2604. [Google Scholar]
- Phoemchalard, C.; Uriyapongson, S.; Tathong, T.; Pornanek, P. 1H NMR metabolic profiling and meat quality in three beef cattle breeds from northeastern Thailand. Foods 2022, 11, 3821. [Google Scholar] [CrossRef]
- Biesalski, H.-K. Meat as a component of a healthy diet—Are There any risks or benefits if meat is avoided in the diet? Meat Sci. 2005, 70, 509–524. [Google Scholar] [CrossRef]
- Conte, G.; Serra, A.; Casarosa, L.; Ciucci, F.; Cappucci, A.; Bulleri, E.; Corrales-Retana, L.; Buccioni, A.; Mele, M. Effect of linseed supplementation on total longissimus muscle lipid composition and shelf-life of beef from young Maremmana bulls. Front. Vet. Sci. 2019, 5, 1–15. [Google Scholar] [CrossRef]
- Nuernberg, K. 14-Optimising the Nutritional Profile of Beef. In Improving the Sensory and Nutritional Quality of Fresh Meat; Kerry, J.P., Ledward, D., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2009; pp. 321–341. ISBN 978-1-84569-343-5. [Google Scholar]
- Tomkins, T.; Drackley, J.K. Applications of palm oil in animal nutrition. J. Oil Palm Res. 2010, 22, 835–845. [Google Scholar]
- Doreau, M.; Ferlay, A. Digestion and utilisation of fatty acids by ruminants. Anim. Feed Sci. Technol. 1994, 45, 379–396. [Google Scholar] [CrossRef]
- Partida, J.A.; Olleta, J.L.; Sañudo, C.; Albertí, P.; Campo, M.M. Fatty acid composition and sensory traits of beef fed palm oil supplements. Meat Sci. 2007, 76, 444–454. [Google Scholar] [CrossRef]
- Guerrero, A.; Muela, E.; Valero, M.V.; Prado, I.N.; Campo, M.M.; Olleta, J.L.; Catalán, O.; Sañudo, C. Effect of the type of dietary fat when added as an energy source on animal performance, carcass characteristics and meat quality of intensively reared Friesian steers. Anim. Prod. Sci. 2016, 56, 1144. [Google Scholar] [CrossRef]
- Duckett, S.K.; Neel, J.P.S.; Fontenot, J.P.; Clapham, W.M. Effects of winter stocker growth rate and finishing system on: III. Tissue proximate, fatty acid, vitamin, and cholesterol content. J. Anim. Sci. 2009, 87, 2961–2970. [Google Scholar] [CrossRef]
- FAO. Fats and Fatty Acids in Human Nutrition. Rep. Expert Consult. 2008, 10, 14. [Google Scholar]
- Shramko, V.S.; Polonskaya, Y.V.; Kashtanova, E.V.; Stakhneva, E.M.; Ragino, Y.I. The short overview on the relevance of fatty acids for human cardiovascular disorders. Biomolecules 2020, 10, 1127. [Google Scholar] [CrossRef]
- dos Santos, N.J.A.; Bezerra, L.R.; Castro, D.P.V.; Marcelino, P.D.R.; Virgínio Júnior, G.F.; da Silva Júnior, J.M.; Pereira, E.S.; de Andrade, E.A.; Silva, T.M.; Barbosa, A.M.; et al. Effect of dietary palm kernel oil on the quality, fatty acid profile, and sensorial attributes of young bull meat. Foods 2022, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Noosen, P.; Lounglawan, P.; Suksombat, W. Linseed Oil supplemented concentrate fed to Brahman crossbred fattening steers on carcass quality traits and intramuscular fatty acid profiles. Songklanakarin J. Sci. Technol. 2017, 39, 1–10. [Google Scholar] [CrossRef]
- Park, S.; Yan, Z.; Choi, C.; Kim, K.; Lee, H.; Oh, Y.; Jeong, J.; Lee, J.; Smith, S.B.; Choi, S. Carcass and meat characteristics and gene expression in intramuscular adipose tissue of korean native cattle fed finishing diets supplemented with 5% palm oil. Korean J. Food Sci. Anim. Resour. 2017, 37, 168–174. [Google Scholar] [CrossRef]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M. Monounsaturated fatty acids and risk of cardiovascular disease. Circulation 1999, 100, 1253–1258. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids and risk of cardiovascular disease: Synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef]
- Enser, M.; Hallett, K.; Hewitt, B.; Fursey, G.A.J.; Wood, J.D. Fatty acid content and composition of English beef, lamb and pork at retail. Meat Sci. 1996, 42, 443–456. [Google Scholar] [CrossRef]
- Garcia, P.T.; Pensel, N.A.; Sancho, A.M.; Latimori, N.J.; Kloster, A.M.; Amigone, M.A.; Casal, J.J. Beef lipids in relation to animal breed and nutrition in Argentina. Meat Sci. 2008, 79, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Alfaia, C.P.M.; Alves, S.P.; Martins, S.I.V.; Costa, A.S.H.; Fontes, C.M.G.A.; Lemos, J.P.C.; Bessa, R.J.B.; Prates, J.A.M. Effect of the feeding system on intramuscular fatty acids and conjugated linoleic acid isomers of beef cattle, with emphasis on their nutritional value and discriminatory ability. Food Chem. 2009, 114, 939–946. [Google Scholar] [CrossRef]
- Park, Y.; Pariza, M.W. Mechanisms of body fat modulation by conjugated linoleic acid (CLA). Food Res. Int. 2007, 40, 311–323. [Google Scholar] [CrossRef]
- Whigham, L.D.; Cook, M.E.; Atkinson, R.L. Conjugated linoleic acid: Implications for human health. Pharmacol. Res. 2000, 42, 503–510. [Google Scholar] [CrossRef]
- Nuernberg, K.; Dannenberger, D.; Nuernberg, G.; Ender, K.; Voigt, J.; Scollan, N.D.; Wood, J.D.; Nute, G.R.; Richardson, R.I. Effect of a grass-based and a concentrate feeding system on meat quality characteristics and fatty acid composition of longissimus muscle in different cattle breeds. Livest. Prod. Sci. 2005, 94, 137–147. [Google Scholar] [CrossRef]
- Kratz, M. Dietary Cholesterol, Atherosclerosis and Coronary Heart Disease. In Atherosclerosis: Diet and Drugs; von Eckardstein, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 195–213. ISBN 978-3-540-27661-6. [Google Scholar]
- Djuricic, I.; Calder, P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids on human health: An update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. OCL-Oléagineux Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P.; Stanton, C. Health implications of high dietary omega-6 polyunsaturated fatty acids. J. Nutr. Metab. 2012, 2012. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed. Pharmacother. 2006, 60, 502–507. [Google Scholar] [CrossRef]
- Verbeke, W. Functional foods: Consumer willingness to compromise on taste for health? Food Qual. Prefer. 2006, 17, 126–131. [Google Scholar] [CrossRef]
- Department of Health and Social Security Diet and Cardiovascular Disease; Report on Health and Social Subjects; Her Majesty’s Stationery Office (HMSO): London, UK, 1984; Volume 28.
- Williams, C.M. Dietary Fatty Acids and Human Health. Ann. Zootech. 2000, 49, 165–180. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Liu, X.; He, Y. Effects of dietary fat saturation level on growth performance, carcass traits, blood lipid parameters, tissue fatty acid composition and meat quality of finishing pigs. Anim. Biosci. 2021, 34, 895–903. [Google Scholar] [CrossRef]
- Smith, S.B. Oleic acid concentration in bovine adipose tissues: Impact on human health, sensory attributes, and genetic regulation. Front. Anim. Sci. 2024, 5, 1332861. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional indices for assessing fatty acids: A mini-review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Dev, K.; Begum, J.; Biswas, A.; Mir, N.A.; Singh, J.; Prakash, R.; Sonowal, J.; Bharali, K.; Tomar, S.; Kant, R.; et al. Hepatic transcriptome analysis reveals altered lipid metabolism and consequent health indices in chicken supplemented with dietary bifidobacterium bifidum and mannan-oligosaccharides. Sci. Rep. 2021, 11, 17895. [Google Scholar] [CrossRef] [PubMed]
- Pilarczyk, R.; Wójcik, J.; Sablik, P.; Czerniak, P. fatty acid profile and health lipid indices in the raw milk of Simmental and Holstein-Friesian cows from an organic farm. S. Afr. J. Anim. Sci. 2015, 45, 30. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Cavallo, M.; Menchetti, L.; Angelucci, E.; Cartoni Mancinelli, A.; Vaudo, G.; Marconi, S.; Camilli, E.; Galli, F.; Castellini, C.; et al. The healthy fatty index allows for deeper insights into the lipid composition of foods of animal origin when compared with the atherogenic and thrombogenicity indexes. Foods 2024, 13, 1568. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.; Santos-Silva, F. Effect of genotype, feeding system and slaughter weight on the quality of light lambs. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Mapiye, C.; Chimonyo, M.; Dzama, K.; Hugo, A.; Strydom, P.E.; Muchenje, V. Fatty acid composition of beef from Nguni steers supplemented with Acacia Karroo leaf-meal. J. Food Compos. Anal. 2011, 24, 523–528. [Google Scholar] [CrossRef]
- Frunză, G.; Murariu, O.C.; Ciobanu, M.-M.; Radu-Rusu, R.-M.; Simeanu, D.; Boișteanu, P.-C. Meat quality in rabbit (Oryctolagus Cuniculus) and hare (Lepus Europaeus Pallas)—A nutritional and technological perspective. Agriculture 2023, 13, 126. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Cartoni Mancinelli, A.; Vaudo, G.; Cavallo, M.; Castellini, C.; Mattioli, S. Indexing of Fatty acids in poultry meat for its characterization in healthy human nutrition: A comprehensive application of the scientific literature and new proposals. Nutrients 2022, 14, 3110. [Google Scholar] [CrossRef] [PubMed]
- Rezamand, P.; Watts, J.S.; Yavah, K.M.; Mosley, E.E.; Ma, L.; Corl, B.A.; McGuire, M.A. Relationship between Stearoyl-CoA desaturase 1 gene expression, relative protein abundance, and its fatty acid products in bovine tissues. J. Dairy Res. 2014, 81, 333–339. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Arancibia-Riveros, C.; Tresserra-Rimbau, A.; Castro-Barquero, S.; Casas, R.; Vázquez-Ruiz, Z.; Ros, E.; Fitó, M.; Estruch, R.; López-Sabater, M.C.; et al. Relationship between estimated desaturase enzyme activity and metabolic syndrome in a longitudinal study. Front. Nutr. 2022, 9, 1–9. [Google Scholar] [CrossRef]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K.; Watanabe, T.; Kohri, T.; Yamasaki, M.; Watanabe, R.; Baba, K.; Shibata, K.; et al. Lower estimates of δ-5 desaturase and elongase activity are related to adverse profiles for several metabolic risk factors in young Japanese women. Nutr. Res. 2008, 28, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Hyun Joo, D.; Chung, H.K.; Moon, J.; Shin, M.-J. Relationship between the estimates of desaturase activities and cardiometabolic phenotypes in koreans. J. Clin. Biochem. Nutr. 2011, 49, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Bonafini, S.; Giontella, A.; Tagetti, A.; Bresadola, I.; Gaudino, R.; Cavarzere, P.; Ramaroli, D.A.; Branz, L.; Marcon, D.; Pietrobelli, A.; et al. Fatty acid profile and desaturase activities in 7–10-year-old children attending primary school in Verona South District: Association between palmitoleic acid, SCD-16, indices of adiposity, and blood pressure. Int. J. Mol. Sci. 2020, 21, 3899. [Google Scholar] [CrossRef] [PubMed]
- van Cleef, E.H.C.B.; D’Áurea, A.P.; Fávaro, V.R.; van Cleef, F.O.S.; Barducci, R.S.; Almeida, M.T.C.; Machado Neto, O.R.; Ezequiel, J.M.B. Effects of dietary inclusion of high concentrations of crude glycerin on meat quality and fatty acid profile of feedlot fed Nellore bulls. PLoS ONE 2017, 12, e0179830. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Gao, R.; Liu, M.; Xie, W. A Comprehensive review of the family of very-long-chain fatty acid elongases: Structure, function, and implications in physiology and pathology. Eur. J. Med. Res. 2023, 28, 532. [Google Scholar] [CrossRef]
- Tillander, V.; Alexson, S.E.H.; Cohen, D.E. Deactivating Fatty Acids: Acyl-CoA Thioesterase-mediated control of lipid metabolism. Trends Endocrinol. Metab. 2017, 28, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Caswell, B.T.; de Carvalho, C.C.; Nguyen, H.; Roy, M.; Nguyen, T.; Cantu, D.C. Thioesterase enzyme families: Functions, structures, and mechanisms. Protein Sci. 2022, 31, 652–676. [Google Scholar] [CrossRef]
- Xia, X.; Chen, C.; Yang, L.; Wang, Y.; Duan, A.; Wang, D. Analysis of metabolites in young and mature Docynia Delavayi (Franch.) Schneid leaves using UPLC-ESI-MS/MS. PeerJ 2022, 10, e12844. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).