Sericin-Enriched Rabbit Semen Preservation: Implications for Short-Term Storage Quality and Fertility at 4 or 15 °C
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Extender Preparation
2.2. Animal Selection and Husbandry Practices
2.3. Semen Collection and Evaluation
2.4. Sperm Treatment and Processing
2.5. Sperm Motility and Kinematics
2.6. Evaluation of Sperm Viability
2.7. Assessment of Sperm Membrane Integrity
2.8. Evaluation of Acrosome Integrity
2.9. Assessment of Bacterial Growth
2.10. Evaluation of In Vivo Fertility
2.11. Statistical Analyses
3. Results
3.1. Experiment 1
3.1.1. Sperm Motility Characteristics (Motility and Kinematics)
3.1.2. Sperm Viability, Plasma Membrane Integrity, and Acrosome Integrity
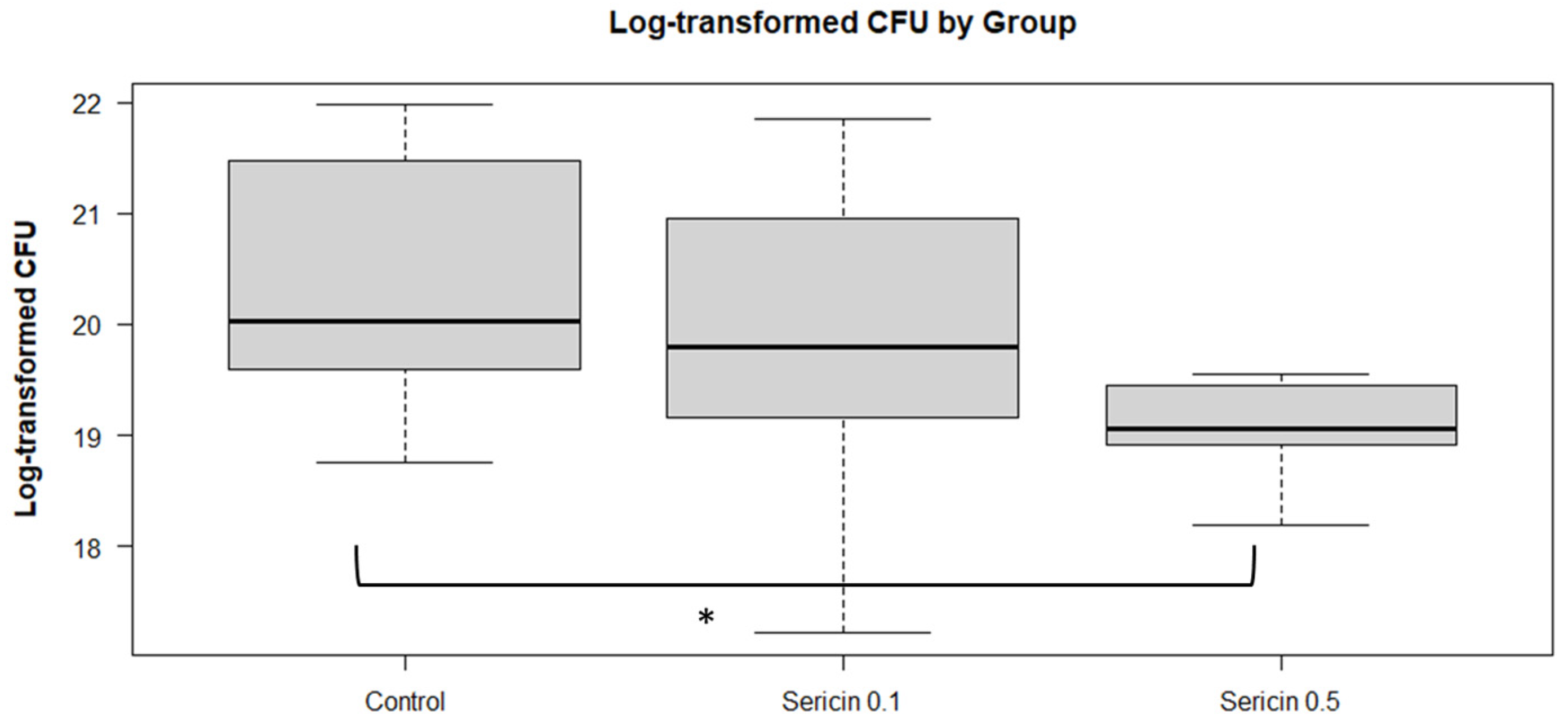
3.1.3. Bacterial Load
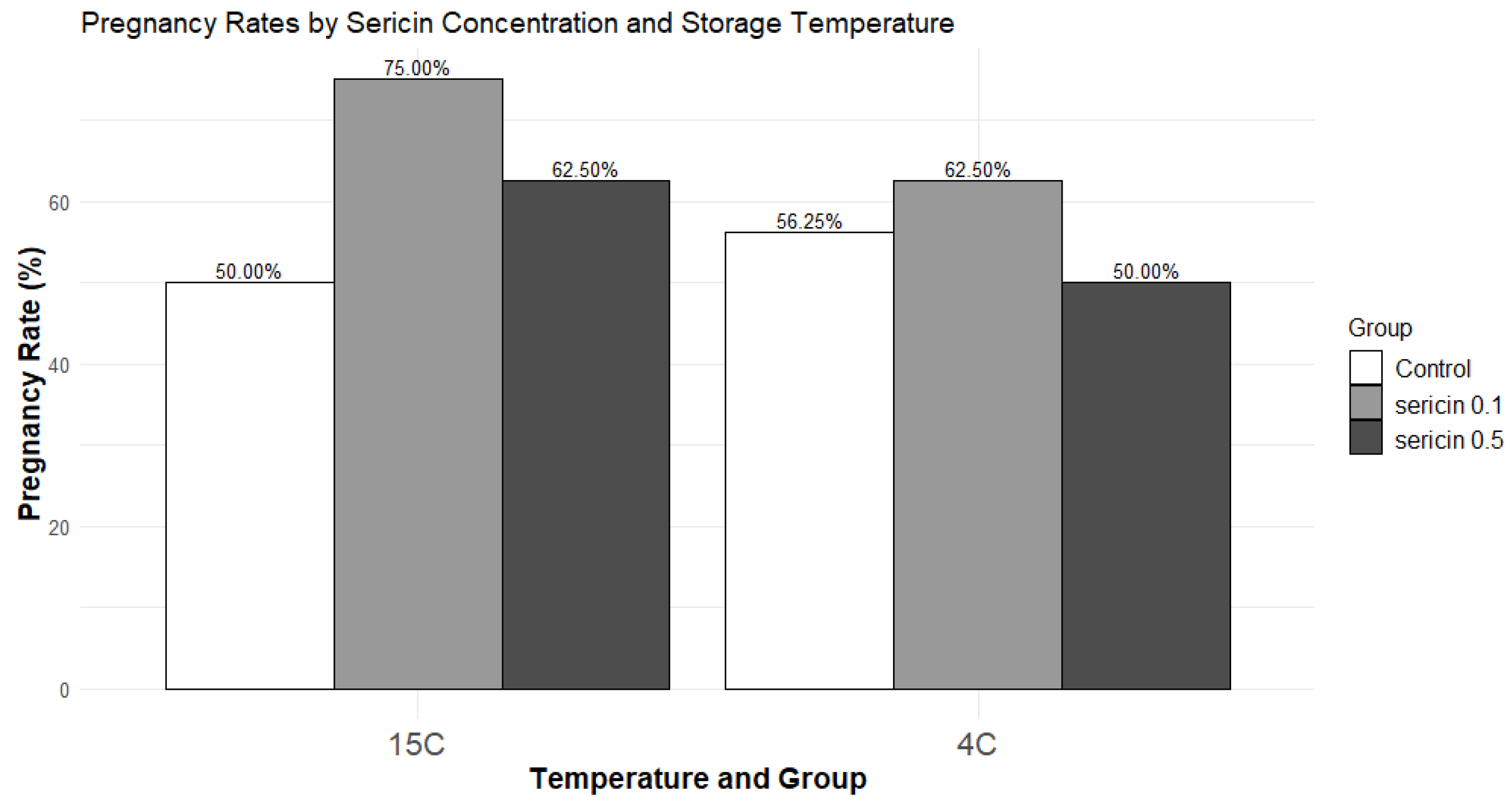
3.1.4. In Vivo Fertility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Küçük, N.; Raza, S.; Matsumura, K.; Uçan, U.; Serin, İ.; Ceylan, A.; Aksoy, M. Effect of different carboxylated poly l-lysine and dimethyl sulfoxide combinations on post thaw rabbit sperm functionality and fertility. Cryobiology 2021, 102, 127–132. [Google Scholar] [CrossRef]
- Martínez-Pastor, F.; Lacalle, E.; Martínez-Martínez, S.; Fernández-Alegre, E.; Álvarez-Fernández, L.; Martinez-Alborcia, M.-J.; Bolarin, A.; Morrell, J. Low density Porcicoll separates spermatozoa from bacteria and retains sperm quality. Theriogenology 2021, 165, 28–36. [Google Scholar] [CrossRef]
- Akarsu, S.; Acısu, T.; Güngör, İ.; Çakır Cihangiroğlu, A.; Koca, R.; Türk, G.; Sönmez, M.; Gür, S.; Fırat, F.; Esmer Duruel, H. The effect of luteolin on spermatological parameters, apoptosis, oxidative stress rate in freezing rabbit semen. Pol. J. Vet. Sci. 2023, 26, 91–98. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Hassan, M.A.; Mohammed, A.K.; Alhimaidi, A.R.; Al-Gabri, N.; Al-Khaldi, K.O.; Swelum, A.A. The effect of adding different levels of curcumin and its nanoparticles to extender on post-thaw quality of cryopreserved rabbit sperm. Animals 2020, 10, 1508. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Simkin, M.; Yang, X.; Foote, R. Fertility of Fresh and Frozen Rabbit Semen Inseminated at Different Times is Indicative of Male Differences in Capacitatlon Time. Biol. Reprod. 1989, 41, 848–853. [Google Scholar] [CrossRef]
- Daniel, N.; Renard, J.-P. Artificial insemination in rabbits. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5358. [Google Scholar] [CrossRef]
- Di Iorio, M.; Rusco, G.; Colonna, M.A.; Schiavitto, M.; D’Andrea, M.S.; Cerolini, S.; Iaffaldano, N. Improving the rabbit semen cryopreservation protocol: Comparison between two extenders and inseminating doses. Ann. Anim. Sci. 2020, 20, 887–898. [Google Scholar] [CrossRef]
- Di Iorio, M.; Manchisi, A.; Rocco, M.; Chrenek, P.; Iaffaldano, N. Comparison of different extenders on the preservability of rabbit semen stored at 5 C for 72 hours. Ital. J. Anim. Sci. 2014, 13, 3444. [Google Scholar] [CrossRef]
- Mocé, E.; Lavara, R.; Vicente, J.S. Effect of cooling rate to 5 C, straw size and farm on fertilizing ability of cryopreserved rabbit sperm. Reprod. Domest. Anim. 2010, 45, e1–e7. [Google Scholar] [CrossRef]
- Viudes-de-Castro, M.P.; Marco-Jimenez, F.; Vicente, J.S.; Marin, C. Antibacterial activity of some molecules added to rabbit semen extender as alternative to antibiotics. Animals 2021, 11, 1178. [Google Scholar] [CrossRef]
- Sarıözkan, S.; Özdamar, S.; Türk, G.; Cantürk, F.; Yay, A. In vitro effects of l-carnitine and glutamine on motility, acrosomal abnormality, and plasma membrane integrity of rabbit sperm during liquid-storage. Cryobiology 2014, 68, 349–353. [Google Scholar] [CrossRef]
- Gardela, J.; Ruiz-Conca, M.; Palomares, A.; Olvera-Maneu, S.; García-Calvo, L.; López-Béjar, M.; Martínez-Pastor, F.; Álvarez-Rodríguez, M. Effect of Honey, Coenzyme Q10, and β-Carotene/α-Tocopherol as Novel Additives in Rabbit-Sperm Cryopreservation Extender. Animals 2023, 13, 2392. [Google Scholar] [CrossRef]
- Fadl, A.M.; Ghallab, A.R.M.; Abou-Ahmed, M.M.; Moawad, A.R. Melatonin can improve viability and functional integrity of cooled and frozen/thawed rabbit spermatozoa. Reprod. Domest. Anim. 2021, 56, 103–111. [Google Scholar] [CrossRef]
- Kubovicova, E.; Makarevich, A.V.; Balazi, A.; Vasicek, J.; Chrenek, P. Factors affecting rabbit sperm cryopreservation: A mini-review. Zygote 2022, 30, 1–8. [Google Scholar] [CrossRef]
- Hozbor, F.; Ledesma, A.; Manes, J.; Rios, G.L.; Kaiser, G.; Cano, A.; Luciano, C.; Alberio, R. Improve intra-uterine insemination in rabbits using ultra-high temperature skim milk as extender to keep semen at room temperature. Andrologia 2016, 48, 231–234. [Google Scholar] [CrossRef]
- Zhang, Y.-Q. Applications of natural silk protein sericin in biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Raza, S.; Uçan, U.; Aksoy, M.; Erdoğan, G.; Ceylan, A.; Serin, I. Silk protein sericin pretreatment enhances osmotic tolerance and post-thaw sperm quality but reduces the ability of sperm cells to undergo in vitro induced acrosome reaction in rabbit. Cryobiology 2019, 90, 1–7. [Google Scholar] [CrossRef]
- Sasaki, M.; Kato, Y.; Yamada, H.; Terada, S. Development of a novel serum-free freezing medium for mammalian cells using the silk protein sericin. Biotechnol. Appl. Biochem. 2005, 42, 183–188. [Google Scholar] [CrossRef]
- Reddy, V.S.; Yadav, B.; Yadav, C.L.; Anand, M.; Swain, D.K.; Kumar, D.; Kritania, D.; Madan, A.K.; Kumar, J.; Yadav, S. Effect of sericin supplementation on heat shock protein 70 (HSP70) expression, redox status and post thaw semen quality in goat. Cryobiology 2018, 84, 33–39. [Google Scholar] [CrossRef]
- Aghaz, F.; Khazaei, M.; Vaisi-Raygani, A.; Bakhtiyari, M. Cryoprotective effect of sericin supplementation in freezing and thawing media on the outcome of cryopreservation in human sperm. Aging Male 2018, 23, 469–476. [Google Scholar] [CrossRef]
- Ratchamak, R.; Ratsiri, T.; Kheawkanha, T.; Vongpralub, T.; Boonkum, W.; Chankitisakul, V. Evaluation of cryopreserved boar semen after supplementation sericin form silkworm (Bombyx mori) in semen extender. Anim. Sci. J. 2020, 91, e13428. [Google Scholar] [CrossRef] [PubMed]
- Ratchamak, R.; Authaida, S.; Boonkum, W.; Chankitisakul, V. Improvement of rooster semen freezability and fertility rate after sericin supplementation in freezing semen extender. Anim. Biosci. 2023, 36, 1530. [Google Scholar] [CrossRef] [PubMed]
- Naseer, Z.; Ahmad, E.; Şahiner, H.S.; Epikmen, E.T.; Fiaz, M.; Yousuf, M.R.; Khan, S.A.; Serin, İ.; Ceylan, A.; Aksoy, M. Dietary quercetin maintains the semen quality in rabbits under summer heat stress. Theriogenology 2018, 122, 88–93. [Google Scholar] [CrossRef]
- Larson, J.L.; Miller, D.J. Simple histochemical stain for acrosomes on sperm from several species. Mol. Reprod. Dev. 1999, 52, 445–449. [Google Scholar] [CrossRef]
- Dollinger, P.; Brack, M.; Furley, C.; Moisson, P.; Schoenbaum, M.; Whitney, R.; Witt, C. Development of an OIE international animal health code recommendation on zoonoses transmissible from non-hu man primates. In Proceedings of the Proc. EAZWV and BVZS II Scientific Meeting, Chester, UK, 21–24 May 1998; p. 1120. [Google Scholar]
- Gączarzewicz, D.; Udała, J.; Piasecka, M.; Błaszczyk, B.; Stankiewicz, T. Bacterial Contamination of Boar Semen and its Relationship to Sperm Quality Preserved in Commercial Extender Containing Gentamicin Sulfate. Pol. J. Vet. Sci. 2016, 19, 451–459. [Google Scholar] [CrossRef]
- Roca, J.; Martınez, S.; Vázquez, J.; Lucas, X.; Parrilla, I.; Martınez, E. Viability and fertility of rabbit spermatozoa diluted in Tris-buffer extenders and stored at 15 C. Anim. Reprod. Sci. 2000, 64, 103–112. [Google Scholar] [CrossRef]
- Rosato, M.; Iaffaldano, N. Effect of chilling temperature on the long-term survival of rabbit spermatozoa held either in a tris-based or a jellified extender. Reprod. Domest. Anim. 2011, 46, 301–308. [Google Scholar] [CrossRef]
- Johinke, D.; de Graaf, S.; Bathgate, R. The Effect of Sperm Concentration and Storage Vessel on Quercetin-Supplemented Rabbit Semen During Chilled Storage. Reprod. Domest. Anim. 2015, 50, 567–573. [Google Scholar] [CrossRef]
- Johinke, D.; De Graaf, S.; Bathgate, R. Quercetin reduces the in vitro production of H2O2 during chilled storage of rabbit spermatozoa. Anim. Reprod. Sci. 2014, 151, 208–219. [Google Scholar] [CrossRef]
- Nagy, S.; Sinkovics, G.; Kovács, A. Viability and acrosome integrity of rabbit spermatozoa processed in a gelatin-supplemented extender. Anim. Reprod. Sci. 2002, 70, 283–286. [Google Scholar] [CrossRef]
- López, F.; Alvariño, J. Effects of added caffeine on results following artificial insemination with fresh and refrigerated rabbit semen. Anim. Reprod. Sci. 2000, 58, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Aad, R.; Dragojlov, I.; Vesentini, S. Sericin Protein: Structure, Properties, and Applications. J. Funct. Biomater. 2024, 15, 322. [Google Scholar] [CrossRef] [PubMed]
- Merati, Z.; Farshad, A.; Farzinpour, A.; Rostamzadeh, J.; Sharafi, M. Anti-apoptotic effects of minocycline on ram epididymal spermatozoa exposed to oxidative stress. Theriogenology 2018, 114, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, M.; Cankat Lehimcioglu, N.; Akman, O. Effect of seminal plasma on functional integrity of rabbit sperm membranes during storage at 4ºC or freezing. World Rabbit Sci. 2010, 16. [Google Scholar] [CrossRef][Green Version]
- Di Iorio, M.; Lauriola, F.; Rusco, G.; Antenucci, E.; Schiavitto, M.; Iaffaldano, N. Cryopreserving Rabbit Semen: Impact of Varying Sperm Concentrations on Quality and the Standardization of Protocol. Vet. Sci. 2023, 11, 9. [Google Scholar] [CrossRef]
- Aksoy, D.; Turan, D.N.; Bayraktar, Z.B. Cholesterol and Sericin as First Aid for Damaged Cells. J. Biosci. Med. 2024, 12, 79–88. [Google Scholar] [CrossRef]
- Yangnam, Y.; Chapanya, S.; Vongpralub, T.; Boonkum, W.; Chankitisakul, V. Effect of semen extender supplementation with sericin on post-thaw dairy bull sperm quality and lipid peroxidation. Czech J. Anim. Sci. 2021, 66, 13–20. [Google Scholar] [CrossRef]
- Xue, R.; Liu, Y.; Zhang, Q.; Liang, C.; Qin, H.; Liu, P.; Wang, K.; Zhang, X.; Chen, L.; Wei, Y. Shape changes and interaction mechanism of Escherichia coli cells treated with sericin and use of a sericin-based hydrogel for wound healing. Appl. Environ. Microbiol. 2016, 82, 4663–4672. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Mao, C.; Jin, L.; Wu, S.; Zheng, Y.; Cui, Z.; Li, Z.; Zhang, Y.; Zhu, S. Natural Extracts for Antibacterial Applications. Small 2023, 20, 2306553. [Google Scholar] [CrossRef]
- Isobe, T.; Ikebata, Y.; Do, L.T.K.; Tanihara, F.; Taniguchi, M.; Otoi, T. In vitro development of OPU-derived bovine embryos cultured either individually or in groups with the silk protein sericin and the viability of frozen-thawed embryos after transfer. Anim. Sci. J. 2015, 86, 661–665. [Google Scholar] [CrossRef]
- Yasmin, C.; Otoi, T.; Setiadi, M.; Karja, N. Maturation and fertilisation of sheep oocytes cultured in serum-free medium containing silk protein sericin. Acta Vet. Hung. 2015, 63, 110–117. [Google Scholar] [CrossRef][Green Version]

| Parameters | Treatment | Time of Storage | Main Effects (p-Value) | Interactions (p-Value) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 72 h | P SEM | Sericin Dose | Storage Duration | Storage Temperature | 1 | 2 | 3 | 4 | ||||||
| 4 °C | 15 °C | 4 °C | 15 °C | 4 °C | 15 °C | 4 °C | 15 °C | ||||||||||
| Progressive motility (%) | Control | 53.4 | 52.0 | 35.2 | 36.1 | 16.4 | 30.1 | 5.8 | 10.4 | 2.10 | 0.00 | 0.00 | 0.00 | 0.20 | 0.48 | 0.00 | 0.73 |
| Sericin 0.1 | 55.2 | 53.2 | 38.8 | 45.8 | 17.8 | 34.6 | 8.7 | 19.2 | |||||||||
| Sericin 0.5 | 54.4 | 52.2 | 38.9 | 43.6 | 18.6 | 33.2 | 12.9 | 15.1 | |||||||||
| Total motility (%) | Control | 85.4 | 84.4 | 56.2 | 62.4 | 48.0 | 56.0 | 17.6 | 32.0 | 2.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.16 | 0.00 | 0.73 |
| Sericin 0.1 | 86.2 | 86.0 | 62.8 | 72.0 | 53.6 | 63.6 | 28.0 | 41.8 | |||||||||
| Sericin 0.5 | 87.1 | 85.0 | 65.8 | 64.8 | 57.8 | 58.0 | 31.8 | 35.6 | |||||||||
| VCL (μm/s) | Control | 102.8 | 94.4 | 85.6 | 89.0 | 77.6 | 82.6 | 74.6 | 59.8 | 3.20 | 0.00 | 0.00 | 0.02 | 0.45 | 0.06 | 0.00 | 0.68 |
| Sericin 0.1 | 101.8 | 95.4 | 97.4 | 102 | 87.4 | 85.8 | 79.6 | 65.1 | |||||||||
| Sericin 0.5 | 103.4 | 106.6 | 100.8 | 105.1 | 96 2 | 97.8 | 81.6 | 69.6 | |||||||||
| VSL (μm/s) | Control | 50.1 | 49.5 | 36.1 | 34.4 | 26.2 | 31.5 | 21.7 | 19.2 | 1.99 | 0.00 | 0.00 | 0.25 | 0.17 | 0.91 | 0.06 | 0.58 |
| Sericin 0.1 | 52.4 | 51.9 | 40.5 | 38.9 | 29.2 | 33 | 23.8 | 22.7 | |||||||||
| Sericin 0.5 | 52.1 | 50.5 | 43.8 | 35 | 33.4 | 31.5 | 23.2 | 23 | |||||||||
| VAP (μm/s) | Control | 63.0 | 61.8 | 52.0 | 41.0 | 35.4 | 38.3 | 27.0 | 21.8 | 2.07 | 0.05 | 0.00 | 0.00 | 0.24 | 0.36 | 0.00 | 0.75 |
| Sericin 0.1 | 63.2 | 59.1 | 54.8 | 47.0 | 39.0 | 39.1 | 29.4 | 25.2 | |||||||||
| Sericin 0.5 | 63.2 | 60.8 | 56.8 | 40.7 | 41.6 | 40.6 | 30.4 | 22.4 | |||||||||
| LIN (%) | Control | 41.7 | 41.2 | 31.2 | 32.7 | 25.6 | 26.9 | 24.3 | 21.8 | 1.78 | 0.23 | 0.00 | 0.09 | 0.40 | 0.79 | 0.71 | 0.99 |
| Sericin 0.1 | 42.6 | 41.1 | 32.2 | 32.5 | 29 | 27.7 | 25.3 | 23.1 | |||||||||
| Sericin 0.5 | 42.7 | 40.5 | 33.4 | 31.1 | 30.9 | 28.3 | 25.6 | 22.8 | |||||||||
| STR (%) | Control | 72.3 | 65.2 | 72.2 | 70.9 | 66.7 | 66.0 | 64.2 | 56.2 | 2.19 | 0.98 | 0.00 | 0.00 | 0.87 | 0.98 | 0.24 | 0.73 |
| Sericin 0.1 | 71.2 | 68.5 | 71.5 | 70.3 | 65.9 | 62.8 | 63.4 | 56.4 | |||||||||
| Sericin 0.5 | 70.3 | 70.2 | 71.5 | 68.1 | 67.6 | 63.7 | 62.6 | 57.4 | |||||||||
| WOB (%) | Control | 46.4 | 42.7 | 43.7 | 45.9 | 39.1 | 44.9 | 33.0 | 38.8 | 1.80 | 0.37 | 0.00 | 0.03 | 0.57 | 0.65 | 0.93 | 0.00 |
| Sericin 0.1 | 48.9 | 44.8 | 43.3 | 45.8 | 39.5 | 43.5 | 35.2 | 39.7 | |||||||||
| Sericin 0.5 | 49.9 | 46.7 | 41.9 | 44.1 | 42.1 | 42.5 | 36.2 | 39.2 | |||||||||
| ALH (μm) | Control | 4.1 | 4.3 | 3.5 | 3.5 | 2.9 | 2.8 | 2.2 | 2.7 | 0.39 | 0.03 | 0.00 | 0.47 | 0.21 | 0.36 | 0.92 | 0.99 |
| Sericin 0.1 | 4.3 | 4.1 | 3.8 | 3.7 | 3.3 | 3.4 | 2.7 | 2.9 | |||||||||
| Sericin 0.5 | 4.4 | 4.1 | 4.1 | 3.4 | 3.6 | 3.2 | 3.1 | 2.9 | |||||||||
| BCF (Hz) | Control | 11.5 | 11.6 | 9.9 | 10.6 | 8.4 | 5.8 | 4.7 | 4.5 | 0.54 | 0.00 | 0.00 | 0.00 | 0.35 | 0.28 | 0.00 | 0.08 |
| Sericin 0.1 | 12.7 | 12.1 | 11.3 | 12.8 | 9.8 | 8.6 | 8.5 | 5.2 | |||||||||
| Sericin 0.5 | 12.8 | 12.5 | 11.1 | 13.2 | 10.1 | 8.9 | 9.2 | 6.5 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, S.; Uçan, U.; Aksoy, M.; Erdoğan, G.; Naseer, Z.; Khan, K. Sericin-Enriched Rabbit Semen Preservation: Implications for Short-Term Storage Quality and Fertility at 4 or 15 °C. Animals 2024, 14, 3429. https://doi.org/10.3390/ani14233429
Raza S, Uçan U, Aksoy M, Erdoğan G, Naseer Z, Khan K. Sericin-Enriched Rabbit Semen Preservation: Implications for Short-Term Storage Quality and Fertility at 4 or 15 °C. Animals. 2024; 14(23):3429. https://doi.org/10.3390/ani14233429
Chicago/Turabian StyleRaza, Sanan, Uğur Uçan, Melih Aksoy, Güneş Erdoğan, Zahid Naseer, and Komal Khan. 2024. "Sericin-Enriched Rabbit Semen Preservation: Implications for Short-Term Storage Quality and Fertility at 4 or 15 °C" Animals 14, no. 23: 3429. https://doi.org/10.3390/ani14233429
APA StyleRaza, S., Uçan, U., Aksoy, M., Erdoğan, G., Naseer, Z., & Khan, K. (2024). Sericin-Enriched Rabbit Semen Preservation: Implications for Short-Term Storage Quality and Fertility at 4 or 15 °C. Animals, 14(23), 3429. https://doi.org/10.3390/ani14233429





