The Effect of Ketoconazole and Quinestrol Combination on Reproductive Physiology in Male Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Drugs
2.2. Experimental Treatment
2.2.1. Sperm Density and Motility
2.2.2. Sperm Abnormality Rate
2.2.3. LH and T Concentrations
2.2.4. CYP3A4 Contents in Small Intestine and Liver
2.3. Statistical Analysis
3. Results
3.1. Internal Organs of Male Mice After 10 d and 30 d
3.2. Reproductive Organs and Sperm Density of Male Mice After 10 d and 30 d
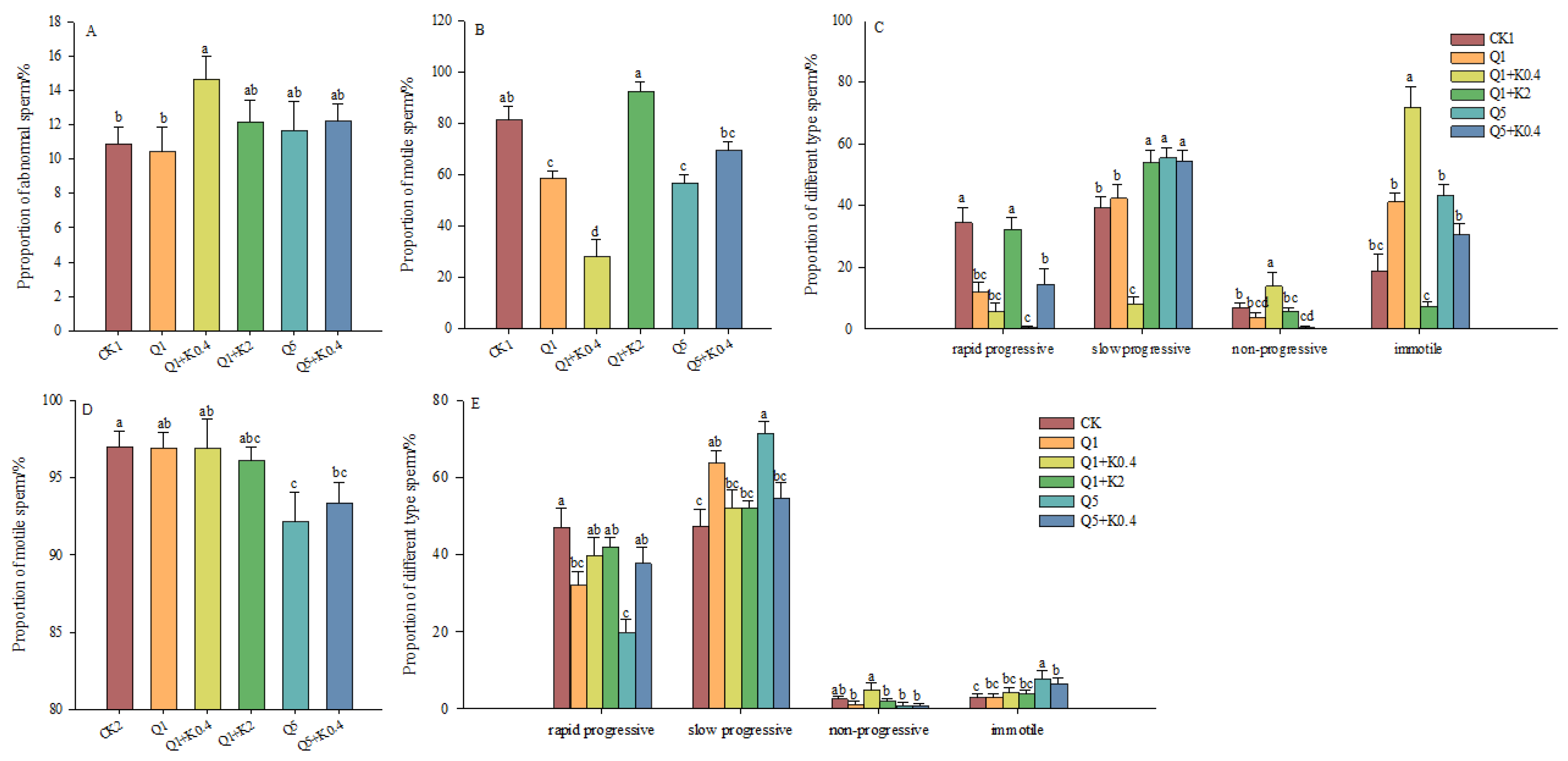
3.3. Sperm Abnormality Rate and Vitality of Male Mice After 10 d and 30 d
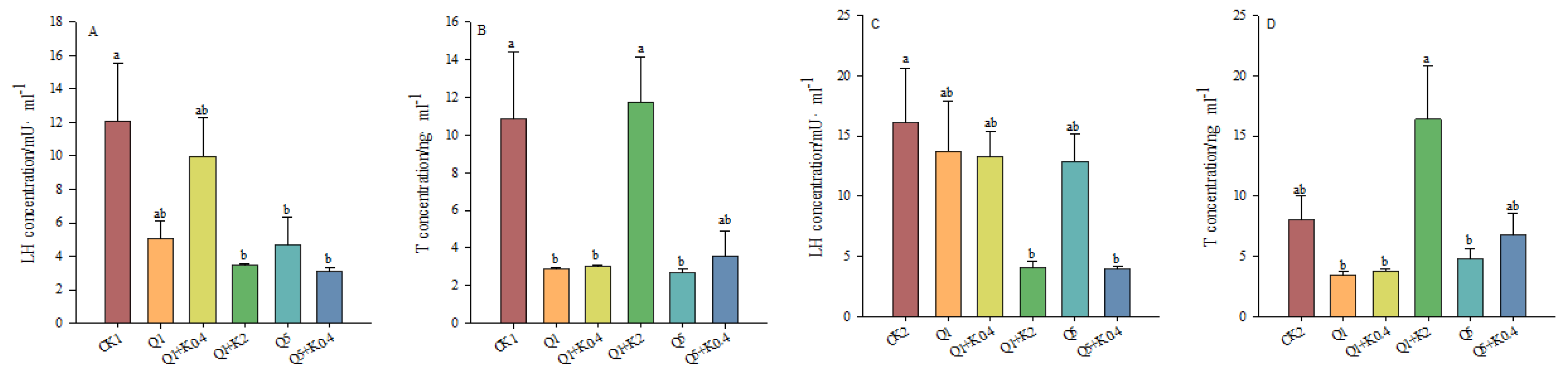
3.4. LH and T Concentrations in Serum of Male Mice After 10 d and 30 d
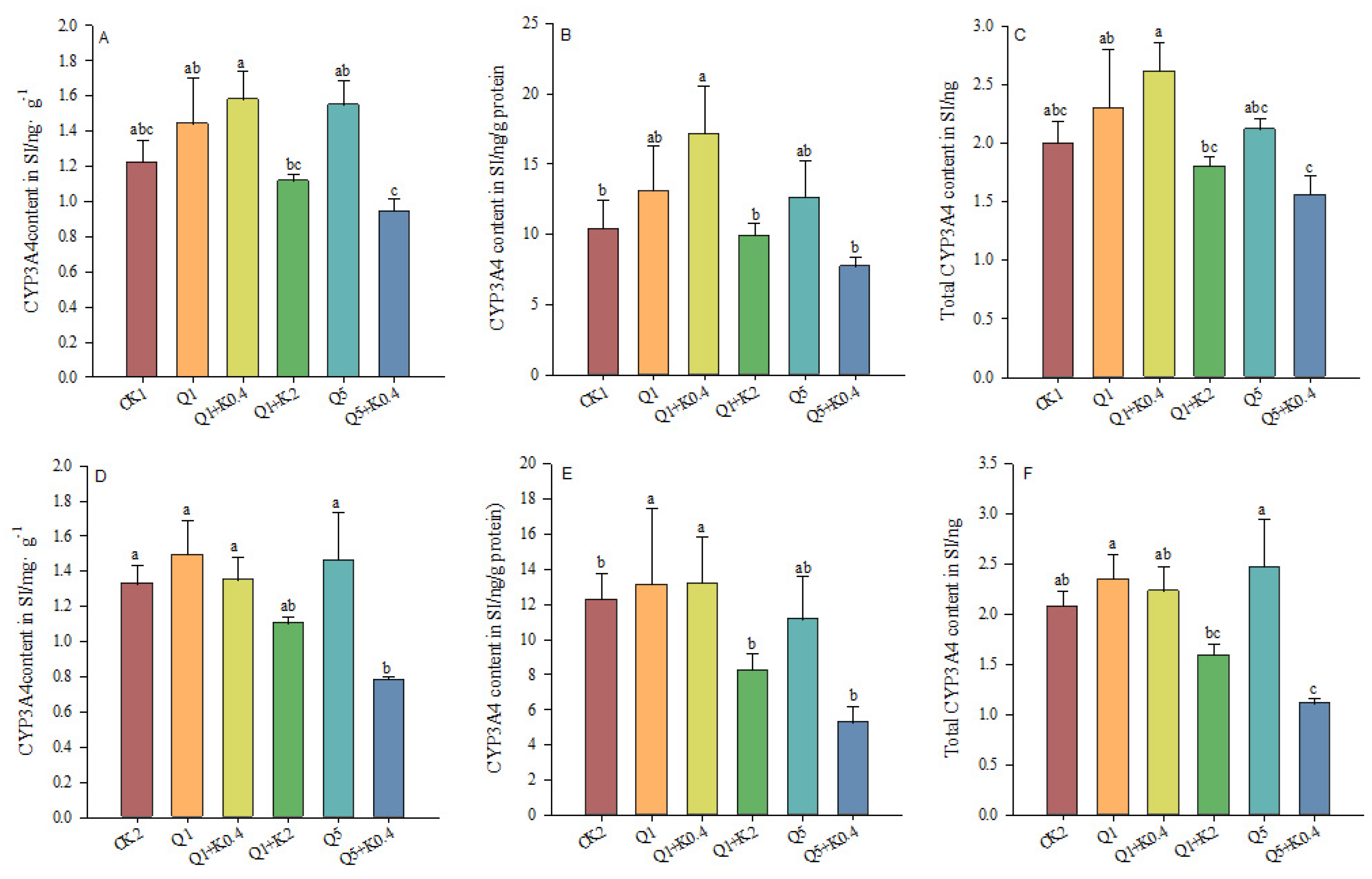
3.5. CYP3A4 Content in Small Intestine of Male Mice After 10 d and 30 d
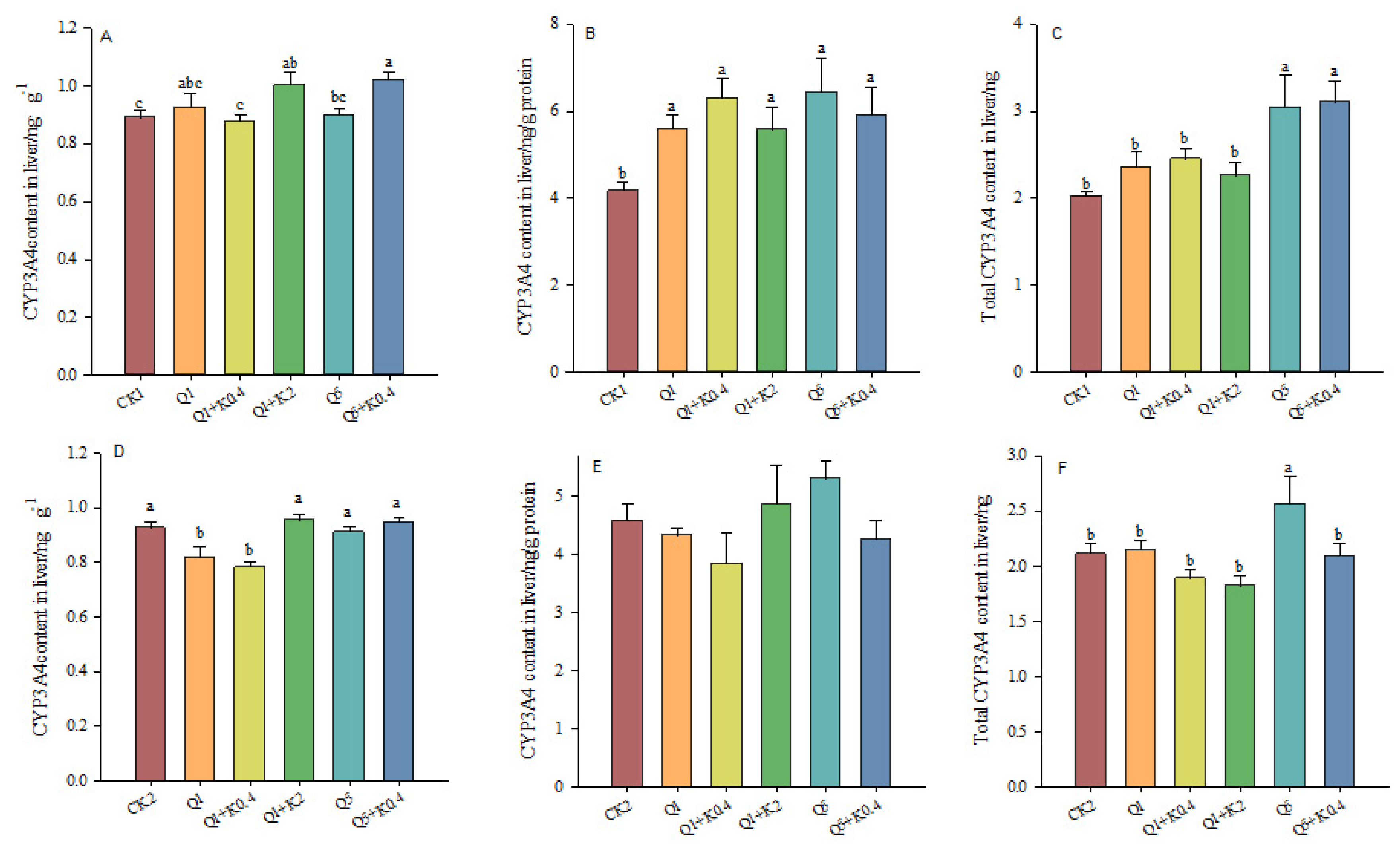
3.6. CYP3A4 Contents in Liver of Male Mice After 10 d and 30 d
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacoblinnert, K.; Jacob, J.; Zhang, Z.; Hinds, L.A. The Status of Fertility Control for Rodents-Recent Achievements and Future Directions. Integr. Zool. 2022, 17, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Bordes, F.; Blasdll, K.; Morand, S. Transmission Ecology of Rodent-borne Diseases: New Frontiers. Integr. Zool. 2015, 10, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Witmer, G. Rodents in Agriculture: A Broad Perspective. Agronomy 2022, 12, 1458. [Google Scholar] [CrossRef]
- Dahmana, H.; Granjon, L.; Diagne, C.; Davoust, B.; Fenollar, F.; Mediannikov, O. Rodents as Hosts of Pathogens and Related Zoonotic Disease Risk. Pathogens 2020, 9, 202. [Google Scholar] [CrossRef]
- Blažić, T.; Stojnić, B.; Milanović, S.; Jokić, G. A Strategy to Improve Rodent Control While Reducing Rodenticide Release into the Environment. Heliyon 2024, 10, e29471. [Google Scholar] [CrossRef]
- MasseiI, G.; Jacob, J.; Hinds, L.A. Developing Fertility Control for Rodents: A Framework for Researchers and Practitioners. Integr. Zool. 2024, 19, 87–107. [Google Scholar] [CrossRef]
- Massei, G. Fertility Control for Wildlife: A European Perspective. Animals 2023, 13, 428. [Google Scholar] [CrossRef]
- Flor, R.J.; Maat, H.; Stuart, A.; Then, R.; Choun, S.; Chhun, S.; Hadi, B.A.R. Introducing an Ecologically-Based Pest Management Approach in Cambodia through Adaptive Learning Networks. Outlook Agric. 2023, 52, 200–211. [Google Scholar] [CrossRef]
- Krijger, I.M.; Belmain, S.R.; Singleton, G.R.; Groot Koerkamp, P.W.; Meerburg, B.G. The Need to Implement the Landscape of Fear within Rodent Pest Management Strategies. Pest Manag. Sci. 2017, 73, 2397–2402. [Google Scholar] [CrossRef]
- Singleton, G.R.; Lorica, R.P.; Htwe, N.M.; Stuart, A.M. Rodent Management and Cereal Production in Asia: Balancing Food Security and Conservation. Pest Manag. Sci. 2021, 77, 4249–4261. [Google Scholar] [CrossRef]
- Montagnini, B.G.; Forcato, S.; Pernoncine, K.V.; Monteiro, M.C.; Pereira, M.R.F.; Costa, N.O.; Moreira, E.G.; Anselmo-Franci, J.A.; Gerardin, D.C.C. Developmental and Reproductive Outcomes in Male Rats Exposed to Triclosan: Two-Generation Study. Front. Endocrinol. 2021, 12, 738980. [Google Scholar] [CrossRef] [PubMed]
- Ducroq, S.; Grange-Messent, V.; Mhaouty-Kodja, S. Exposure to Low Doses of Phthalates in Male Rodents: Effects on Reproductive and Cognitive Behaviors. Neuroendocrinology 2023, 113, 1215–1231. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, W.; Wei, Y.; Zhang, T.; Geng, W.; Zhai, J. Effects of Perinatal Exposure to Endocrine-Disrupting Chemicals on the Reproductive System of F3 Generation Male Rodents: A Meta-Analysis. Environ. Sci. Pollut. Res. 2022, 29, 33218–33229. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Herath, C.B.; Jin, W.; Watanabe, G.; Arai, K.; Suzuki, A.K.; Taya, K. Adverse Effects of Environmental Toxicants, Octylphenol and Bisphenol A, on Male Reproductive Functions in Pubertal Rats. Endocrine 2004, 25, 163–172. [Google Scholar] [CrossRef]
- Liu, M.; Wan, X.; Yin, Y.; Li, Y.; Sun, F.; Zhang, Z.; Wang, Y. Subfertile Effects of Quinestrol and Levonorgestrel in Male Rats. Reprod. Fertil. Dev. 2012, 24, 297. [Google Scholar] [CrossRef]
- Kang, Y.; Tan, Y.; Wang, C.; Yao, B.; An, K.; Liu, M.; Su, J. Antifertility Effects of Levonorgestrel, Quinestrol, and Their Mixture (EP-1) on Plateau Zokor in the Qinghai–Tibetan Plateau. Integr. Zool. 2022, 17, 1002–1016. [Google Scholar] [CrossRef]
- Chen, X.; Hou, X.; Feng, T.; Han, N.; Wang, J.; Chang, G. Anti-fertility Effect of Levonorgestrel and/or Quinestrol on Striped Field Mouse (Apodemus agrarius): Evidence from Both Laboratory and Field Experiments. Integr. Zool. 2022, 17, 1041–1052. [Google Scholar] [CrossRef]
- Sidhu, A.; Singla, N.; Lonare, M.; Mahal, A.K. Effect of Quinestrol on Body Weight, Vital Organs, Biochemicals and Genotoxicity in Adult Male Lesser Bandicoot Rat, Bandicota bengalensis. Pestic. Biochem. Physiol. 2020, 165, 104544. [Google Scholar] [CrossRef]
- Su, Q.; Chen, Y.; Qin, J.; Wang, T.; Wang, D.; Liu, Q. Responses in Reproductive Organs, Steroid Hormones and CYP450 Enzymes in Female Mongolian Gerbil (Meriones unguiculatus) over Time after Quinestrol Treatment. Pestic. Biochem. Physiol. 2017, 143, 122–126. [Google Scholar] [CrossRef]
- Gioia, I.A.; Lencioni, L.J.; Koch, O.R. Effects of Quinestrol on Hepatic Function in the Rat. Contraception 1978, 18, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qin, J.; Chen, Q.; Wang, D.; Shi, D. Fertility Control of Rattus nitidus Using Quinestrol: Effects on Reproductive Organs and Social Behavior. Integr. Zool. 2013, 8, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.H. Quinestrol: A Potential Contraceptive Agent. Fertil. Steril. 1969, 20, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Falconi, G.; Ercoli, A. 3-Cyclopentyl Ether of 17α-Ethynylestradiol: A Potent Anti-Gonadotrophic and Contraceptive Agent in Rodents. Experientia 1963, 19, 249–250. [Google Scholar] [CrossRef]
- Hoffmann, J.C. Effect of Photoperiod on Estrous Cycle Length in the Rat. Endocrinology 1968, 83, 1355–1357. [Google Scholar] [CrossRef]
- Lv, X.; Shi, D. The Effects of Quinestrol as a Contraceptive in Mongolian Gerbils (Meriones unguiculatus). Exp. Anim. 2011, 60, 489–496. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, M.; Li, D.; Wan, X.; Hinds, L.A.; Wang, Y.; Zhang, Z. Anti-fertility Effect of Levonorgestrel and Quinestrol in Brandt’s Voles (Lasiopodomys brandtii). Integr. Zool. 2007, 2, 260–268. [Google Scholar] [CrossRef]
- Lv, X.; Guo, Y.; Shi, D. Effects of Quinestrol on Reproductive Hormone Expression, Secretion, and Receptor Levels in Female Mongolian Gerbils (Meriones unguiculatus). Theriogenology 2012, 77, 1223–1231. [Google Scholar] [CrossRef]
- Shen, W.; Shi, D.; Wang, D.; Guo, Y. Inhibitive Effects of Quinestrol on Male Testes in Mongolian Gerbils (Meriones unguiculatus). Res. Vet. Sci. 2012, 93, 907–913. [Google Scholar] [CrossRef]
- Li, J.; Chen, F.; Li, C.; Chen, Y. Quinestrol Induces Spermatogenic Apoptosis in Vivo via Increasing Pro-Apoptotic Proteins in Adult Male Mice. Tissue Cell 2014, 46, 318–325. [Google Scholar] [CrossRef]
- Guo, M.; Lin, Q.; Xu, Z.; Zhang, C.; Zhao, X.; Tang, T. Adsorption–Desorption Behavior of the Endocrine-Disrupting Chemical Quinestrol in Soils. Sci. Rep. 2020, 10, 13273. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.M.; Herawati, N.A.; Risnelli; Sudarmaji; Liu, M.; Zhang, Z.; Li, H.; Singleton, G.R.; Hinds, L.A. Reproductive Responses of Rice Field Rats (Rattus argentiventer) Following Treatment with the Contraceptive Hormones, Quinestrol and Levonorgestrol. Integr. Zool. 2022, 17, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, H. Pharmacokinetics of Oestrogens and Progestogens. Maturitas 1990, 12, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, F.Z.; Archer, D.F.; Bhavnani, B.R. Ethinyl Estradiol and 17β-Estradiol in Combined Oral Contraceptives: Pharmacokinetics, Pharmacodynamics and Risk Assessment. Contraception 2013, 87, 706–727. [Google Scholar] [CrossRef]
- Diaz-Thomas, A.M.; Golden, S.H.; Dabelea, D.M.; Grimberg, A.; Magge, S.N.; Safer, J.D.; Shumer, D.E.; Stanford, F.C. Endocrine Health and Health Care Disparities in the Pediatric and Sexual and Gender Minority Populations: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2023, 108, 1533–1584. [Google Scholar] [CrossRef]
- Cappola, A.R.; Auchus, R.J.; El-Hajj Fuleihan, G.; Handelsman, D.J.; Kalyani, R.R.; McClung, M.; Stuenkel, C.A.; Thorner, M.O.; Verbalis, J.G. Hormones and Aging: An Endocrine Society Scientific Statement. J. Clin. Endocrinol. Metab. 2023, 108, 1835–1874. [Google Scholar] [CrossRef]
- Anzenbacher, P.; Anzenbacherová, E. Cytochromes P450 and Metabolism of Xenobiotics. Cell. Mol. Life Sci. 2001, 58, 737–747. [Google Scholar] [CrossRef]
- Usmani, K.A.; Cho, T.M.; Rose, R.L.; Hodgson, E. Inhibition of the Human Liver Microsomal and Human Cytochrome P450 1A2 and 3A4 Metabolism of Estradiol by Deployment-Related and Other Chemicals. Drug Metab. Dispos. 2006, 34, 1606–1614. [Google Scholar] [CrossRef]
- Peter Guengerich, F. Metabolism of 17 α-Ethynylestradiol in Humans. Life Sci. 1990, 47, 1981–1988. [Google Scholar] [CrossRef]
- Su, Q.; Chen, Y.; Qin, J.; Li, H.; Liu, M.; Zhang, Z.; Liu, Q. Ratio-Dependent Effects of Quinestrol and Levonorgestrel Compounds (EP-1) on Reproductive Parameters of Adult Male Swiss Mice. Pestic. Biochem. Physiol. 2019, 160, 181–186. [Google Scholar] [CrossRef]
- Atkins, W.M. Non-Michaelis-Menten Kinetics in Cytochrome P450-Catalyzed Reactions. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Hakkola, J.; Hukkanen, J.; Turpeinen, M.; Pelkonen, O. Inhibition and Induction of CYP Enzymes in Humans: An Update. Arch. Toxicol. 2020, 94, 3671–3722. [Google Scholar] [CrossRef]
- Deodhar, M.; Al Rihani, S.B.; Arwood, M.J.; Darakjian, L.; Dow, P.; Turgeon, J.; Michaud, V. Mechanisms of CYP450 Inhibition: Understanding Drug-Drug Interactions Due to Mechanism-Based Inhibition in Clinical Practice. Pharmaceutics 2020, 12, 846. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Luo, X.; Cheng, Z. In Vitro Inhibition and Induction of Human Liver Cytochrome P450 Enzymes by a Novel Anti-Fibrotic Drug Fluorofenidone. Xenobiotica 2021, 51, 745–751. [Google Scholar] [CrossRef]
- Becker, C.; Frey, R.; Unger, S.; Thomas, D.; Reber, M.; Weimann, G.; Dietrich, H.; Arens, E.R.; Mück, W. Pharmacokinetic Interaction of Riociguat with Ketoconazole, Clarithromycin, and Midazolam. Pulm. Circ. 2016, 6, S49–S57. [Google Scholar] [CrossRef]
- Vermeer, L.M.M.; Isringhausen, C.D.; Ogilvie, B.W.; Buckley, D.B. Evaluation of Ketoconazole and Its Alternative Clinical CYP3A4/5 Inhibitors as Inhibitors of Drug Transporters: The In Vitro Effects of Ketoconazole, Ritonavir, Clarithromycin, and Itraconazole on 13 Clinically-Relevant Drug Transporters. Drug Metab. Dispos. 2016, 44, 453–459. [Google Scholar] [CrossRef]
- Chen, J.; He, X.; Li, F.; Wu, J.; Guo, Y. Experimental Research on Antifertility Funetion of Sasanqua Saponin Sterilant Against Rodent. Chin. J. Vector Biol. Control. 2006, 17, 11–13. [Google Scholar]
- Zhang, F.; Li, J.; Jin, G.; Wang, H.; Zhou, B.; Liu, Y.; Huang, C.; Zhao, W. Effects of Quinestrol on the Testes Development and Expression of PCNA and Caspase-3 in Juvenile Mice. China Anim. Husb. Vet. Med. 2014, 41, 199–202. [Google Scholar]
- Shi, L.; Li, X.; Ji, Z.; Wang, Z.; Shi, Y.; Tian, X.; Wang, Z. The Reproductive Inhibitory Effects of Levonorgestrel, Quinestrol, and EP-1 in Brandt’s Vole (Lasiopodomys brandtii). PeerJ 2020, 8, e9140. [Google Scholar] [CrossRef]
- Wang, T.; Guo, Y.; Wang, D.; Hai, S. Suppression Effects of Quinestrol on the Reproduction of Brandt’s Voles (Lasiopodomys brandtii). Acta Theriol. Sin. 2015, 35, 87–94. [Google Scholar] [CrossRef]
- Liu, M.; Luo, R.; Wang, H.; Cao, G.; Wang, Y. Recovery of Fertility in Quinestrol-treated or Diethylstilbestrol-treated Mice: Implications for Rodent Management. Integr. Zool. 2017, 12, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Su, Q.; Liu, Q. Effects of Cabergoline on Germ Cells and Enzymes in Testis of Male Rattus losea. Acta Theriol. Sin. 2015, 35, 184–189. [Google Scholar]
- World Health Organization (WHO). WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Wang, Y.L.; Liu, Y.; Qin, J.; Liu, Y.; Nie, H.; Liu, Q. Effect of Different Doses of Quinestrol on Organs, Sperm Density, Reproductive Hormones and CYP3A4 Enzyme Levels of Male and Female Mice. Acta Theriol. Sin. 2021, 41, 202–213. [Google Scholar]
- Liu, J.; Tu, F.; Liu, M.; Wang, J.; Zhang, Z. Antifertility Effects of EP-1 (Quinestrol and Levonorgestrel) on Pacific Rats (Rattus exulans). Integr. Zool. 2024, 19, 127–142. [Google Scholar] [CrossRef]
- Sidhu, A.; Singla, N. Antifertility Effects of Quinestrol in Male Lesser Bandicoot Rat, Bandicota bengalensis, and Potential in Managing Rodent Population under Field Conditions. Integr. Zool. 2024, 19, 108–126. [Google Scholar] [CrossRef]
- Zhang, J.; Hai, S.; Guo, Y.; Wu, X.; Shi, D. Anti-Fertility Effect of EP-1 Contraceptive Compound on Male Mongolia Gerbils Meriones unguiculatus. Acta Phytophylacica Sin. 2011, 38, 86–90. [Google Scholar] [CrossRef]
- Oyeyemi, W.; Shittu, S.; Kolawole, T.; Ubanecheand, P.; Akinola, A. Protective Effect of Vitamin E on Nicotine Induced Reproductive Toxicity in Male Rats. Niger. J. Basic Appl. Sci. 2015, 23, 7. [Google Scholar] [CrossRef]
- Hinton, B.T.; Palladino, M.A. Epididymal Epithelium: Its Contribution to the Formation of a Luminal Fluid Microenvironment. Microsc. Res. Tech. 1995, 30, 67–81. [Google Scholar] [CrossRef]
- Toragall, M.; Satapathy, S.; Kadadevaru, G.; Hiremath, M. Evaluation of Seminal Fructose and Citric Acid Levels in Men with Fertility Problem. J. Hum. Reprod. Sci. 2019, 12, 199. [Google Scholar] [CrossRef]
- Ma, J.; Chen, H.; Chen, X.; Jin, T. Effect of Different Doses of Quinestrol on Reproductive Physiology of Male Mice. Genom. Appl. Biol. 2018, 37, 5258–5262. [Google Scholar]


| Total Days | Group | Components 1 | Components 2 | Number | Gavage Time |
|---|---|---|---|---|---|
| 10 d | CK1 | Sunflower seed oil | Sunflower seed oil | 15 | 3 d |
| Q1 | Quinestrol 1.0 mg/kg | Sunflower seed oil | 7 | 3 d | |
| Q1 + K0.4 | Quinestrol 1.0 mg/kg | Ketoconazole 0.4 mg/kg | 7 | 3 d | |
| Q1 + K2 | Quinestrol 1.0 mg/kg | Ketoconazole 2.0 mg/kg | 8 | 3 d | |
| Q5 | Quinestrol 5.0 mg/kg | Sunflower seed oil | 7 | 3 d | |
| Q5 + K0.4 | Quinestrol 5.0 mg/kg | Ketoconazole 0.4 mg/kg | 8 | 3 d | |
| 30 d | CK2 | Sunflower seed oil | Sunflower seed oil | 15 | 3 d |
| Q1 | Quinestrol 1.0 mg/kg | Sunflower seed oil | 7 | 3 d | |
| Q1 + K0.4 | Quinestrol 1.0 mg/kg | Ketoconazole 0.4 mg/kg | 7 | 3 d | |
| Q1 + K2 | Quinestrol 1.0 mg/kg | Ketoconazole 2.0 mg/kg | 8 | 3 d | |
| Q5 | Quinestrol 5.0 mg/kg | Sunflower seed oil | 7 | 3 d | |
| Q5 + K0.4 | Quinestrol 5.0 mg/kg | Ketoconazole 0.4 mg/kg | 8 | 3 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Wang, Y.; Liu, Y.; Liu, Y.; Qin, J.; Yuan, D.; Liu, Q. The Effect of Ketoconazole and Quinestrol Combination on Reproductive Physiology in Male Mice. Animals 2024, 14, 3240. https://doi.org/10.3390/ani14223240
Ji Y, Wang Y, Liu Y, Liu Y, Qin J, Yuan D, Liu Q. The Effect of Ketoconazole and Quinestrol Combination on Reproductive Physiology in Male Mice. Animals. 2024; 14(22):3240. https://doi.org/10.3390/ani14223240
Chicago/Turabian StyleJi, Yu, Yujie Wang, Yuhang Liu, Yutong Liu, Jiao Qin, Daohuan Yuan, and Quansheng Liu. 2024. "The Effect of Ketoconazole and Quinestrol Combination on Reproductive Physiology in Male Mice" Animals 14, no. 22: 3240. https://doi.org/10.3390/ani14223240
APA StyleJi, Y., Wang, Y., Liu, Y., Liu, Y., Qin, J., Yuan, D., & Liu, Q. (2024). The Effect of Ketoconazole and Quinestrol Combination on Reproductive Physiology in Male Mice. Animals, 14(22), 3240. https://doi.org/10.3390/ani14223240




