Development of Microsatellite Markers from Transcriptome of Eriocheir sinensis and Their Application in Multiplex PCR Panels
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Tissue Collection
2.2. Transcriptome Sequencing, Assembly, and Microsatellite Identification
2.3. Multiplex PCR Panel Optimization
2.4. Parentage Assignment
2.5. Data Analysis
3. Results
3.1. Primer Design and Validation
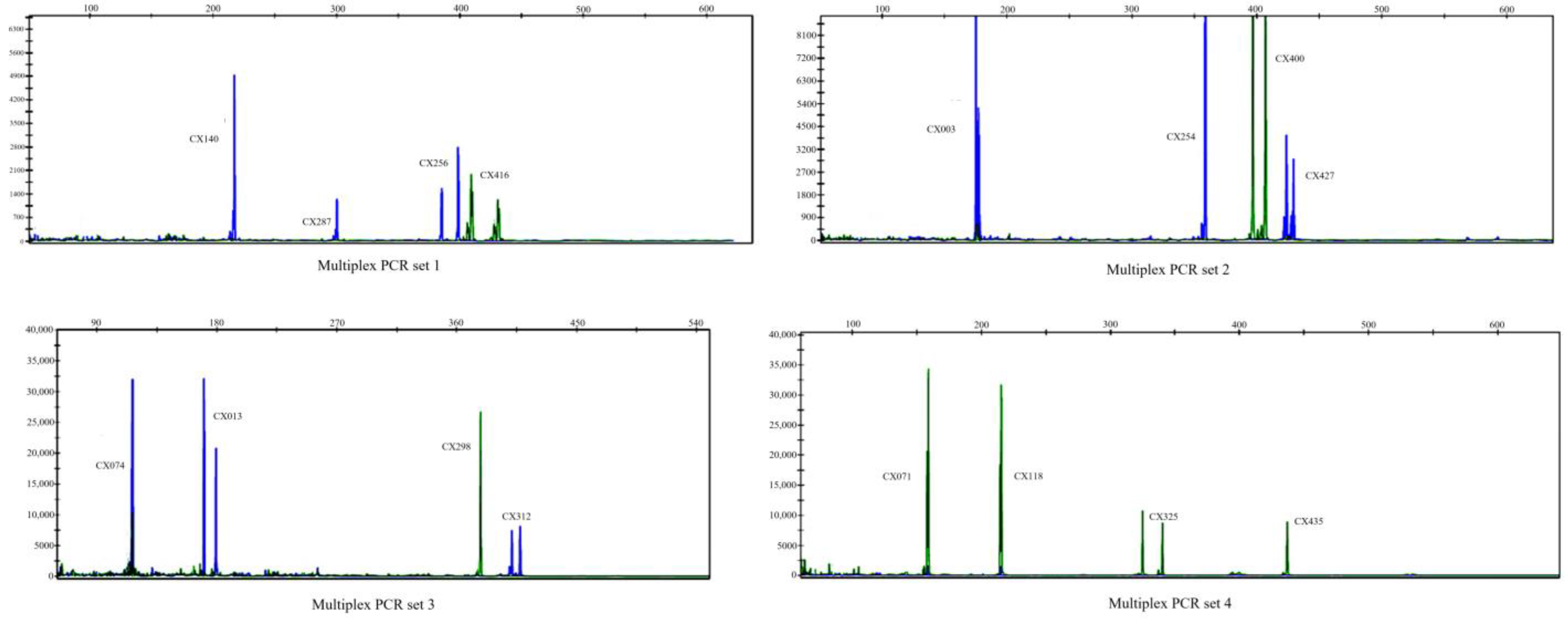
3.2. Established SSR Multiplex PCR Panels
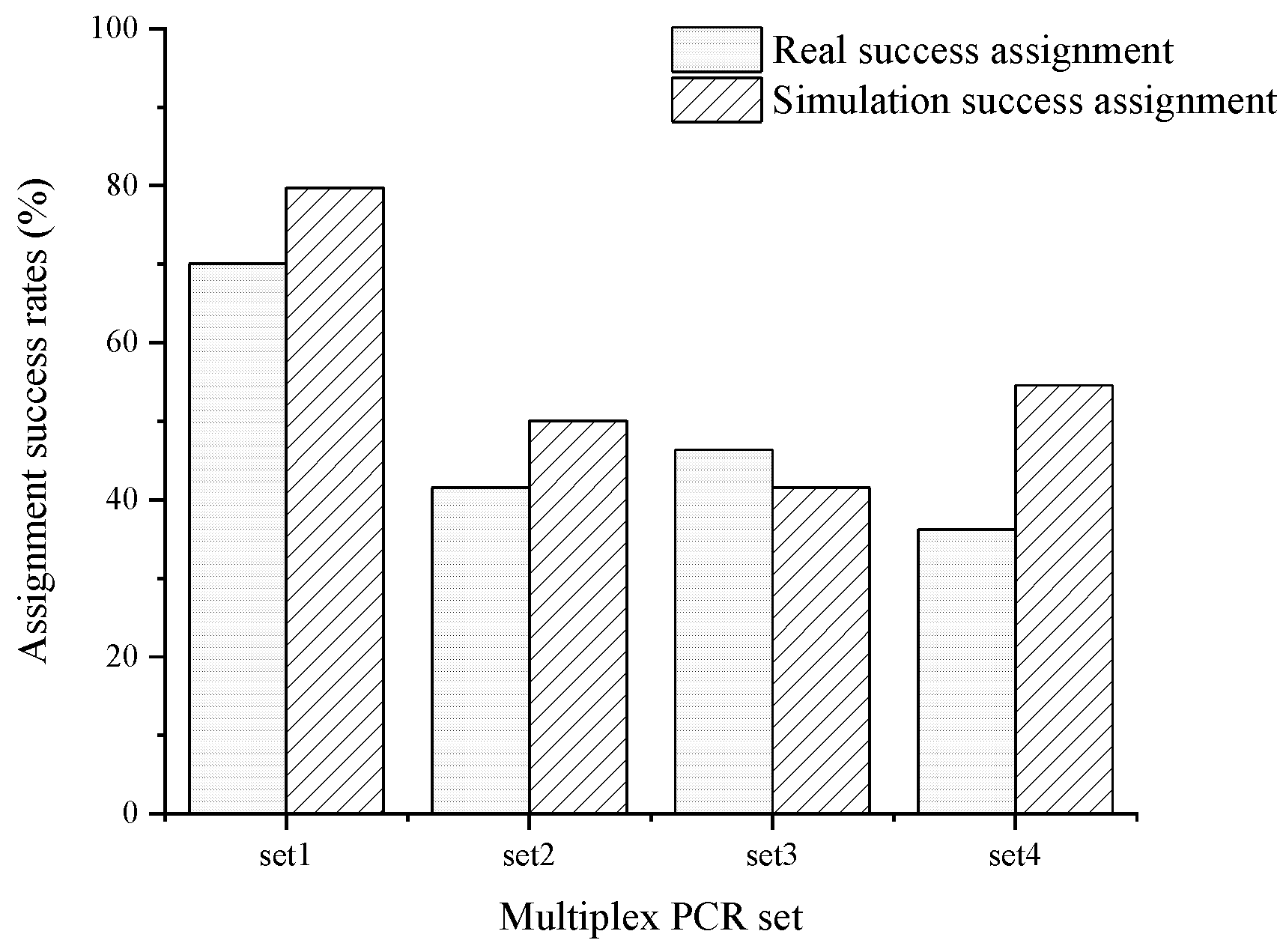
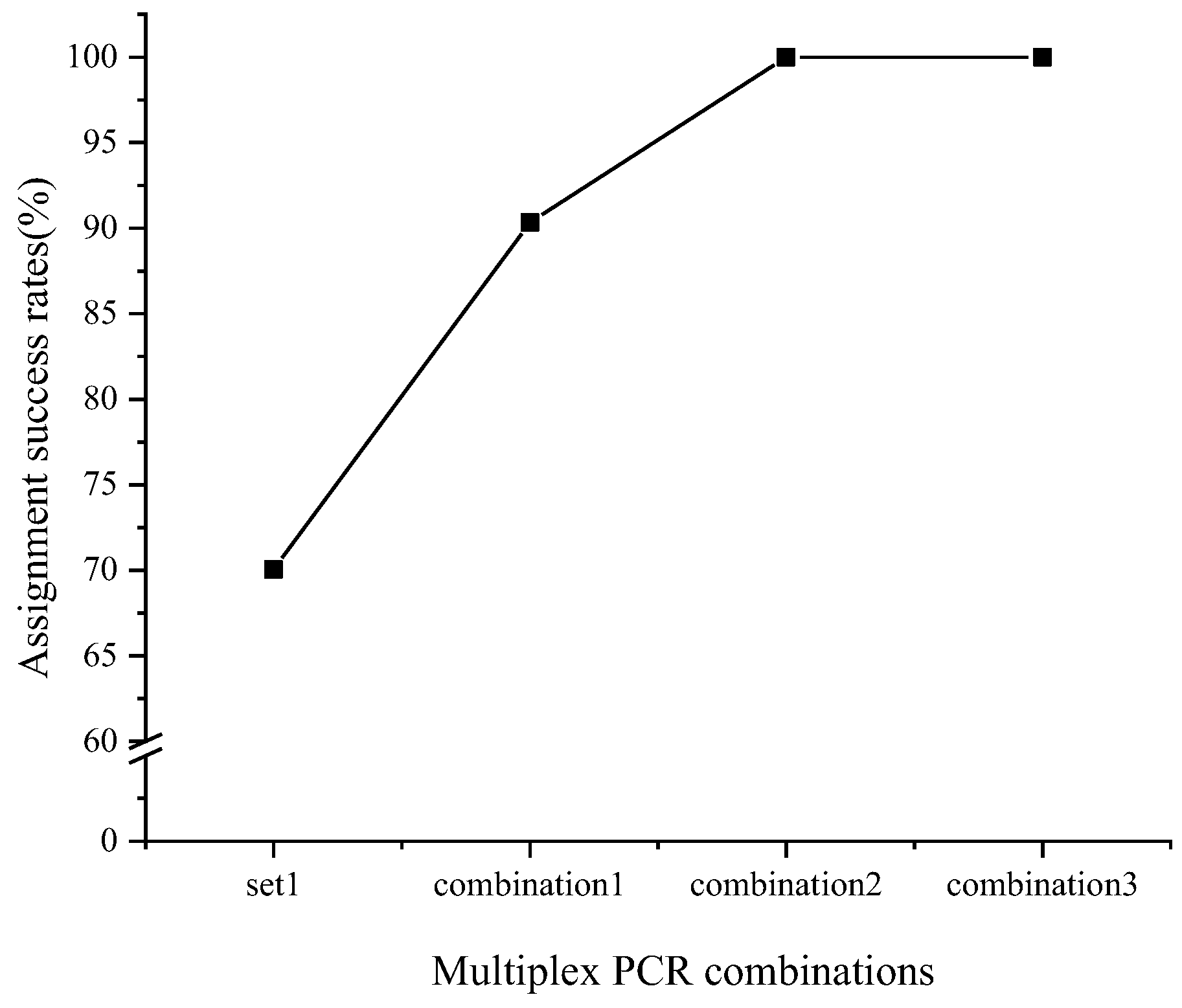
3.3. Result of Parentage Assignment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bureau of Fisheries and Fishery Administration; Ministry of Agriculture and Rural Affairs. 2024 China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2024; p. 24. [Google Scholar]
- Chang, G.; Wu, X.; Cheng, Y.; Zeng, C.; Yu, Z. Reproductive Performance, Offspring Quality, Proximate and Fatty Acid Composition of Normal and Precocious Chinese Mitten Crab Eriocheir sinensis. Aquaculture 2017, 469, 137–143. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Liu, J.; De Silva, S.S. Advances in Precocity Research of the Chinese Mitten Crab Eriocheir sinensis. Aquacult. Int. 2011, 19, 251–267. [Google Scholar] [CrossRef]
- Ding, Z.; Meng, Q.; Liu, H.; Yuan, S.; Zhang, F.; Sun, M.; Zhao, Y.; Shen, M.; Zhou, G.; Pan, J.; et al. First Case of Hepatopancreatic Necrosis Disease in Pond-reared Chinese Mitten Crab, Eriocheir sinensis, Associated with Microsporidian. J. Fish Dis. 2016, 39, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, P.; Zhou, G.; Li, X.; Lu, Q.; Liu, X.; Zhou, J.; Wang, C. Genetic Improvement and Breeding Practices for Chinese Mitten Crab, Eriocheir sinensis. J. World Aquacult. Soc. 2018, 49, 292–301. [Google Scholar] [CrossRef]
- Gjedrem, T. The First Family-based Breeding Program in Aquaculture. Rev. Aquacult. 2010, 2, 2–15. [Google Scholar] [CrossRef]
- Allen, S.K.; Small, J.M.; Kube, P.D. Genetic Parameters for Crassostrea Virginica and Their Application to Family-Based Breeding in the Mid-Atlantic, USA. Aquaculture 2021, 538, 736578. [Google Scholar] [CrossRef]
- Ruohonen, K. Individual Measurements and Nested Designs in Aquaculture Experiments: A Simulation Study. Aquaculture 1998, 165, 149–157. [Google Scholar] [CrossRef]
- Song, C.; Zhong, L.; Chen, X.; Bian, W.; Qiu, L.; Ming, J.; Chen, J. Variation Analysis and Sample Size Estimation for Growth Indicators during PIT-Tag-Assisted Family Construction of Channel Catfish (Ictalurus punctatus). Aquacult. Int. 2014, 22, 821–831. [Google Scholar] [CrossRef]
- Soula, M.; Navarro, A.; Hildebrandt, S.; Zamorano, M.J.; Roo, J.; Hernández-Cruz, C.M.; Afonso, J.M. Evaluation of VIE (Visible Implant Elastomer) and PIT (Passive Integrated Transponder) Physical Tagging Systems for the Identification of Red Porgy Fingerlings (Pagrus pagrus). Aquacult. Int. 2012, 20, 571–583. [Google Scholar] [CrossRef]
- Chauhan, T.; Rajiv, K. Molecular Markers and Their Applications in Fisheries and Aquaculture. Adv. Biosci. Biotechnol. 2010, 1, 281–291. [Google Scholar] [CrossRef]
- Napora-Rutkowski, Ł.; Rakus, K.; Nowak, Z.; Szczygieł, J.; Pilarczyk, A.; Ostaszewska, T.; Irnazarow, I. Genetic Diversity of Common Carp (Cyprinus carpio L.) Strains Breed in Poland Based on Microsatellite, AFLP, and mtDNA Genotype Data. Aquaculture 2017, 473, 433–442. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, Z.; Wang, Q.; Zhang, Q.; Zhang, X.; Xiang, J.; Li, F. Development of High Throughput SNP Genotyping Approach Using Target Sequencing in Pacific White Shrimp and Its Application for Genetic Study. Aquaculture 2020, 528, 735549. [Google Scholar] [CrossRef]
- Silva, N.M.L.; Ianella, P.; Yamagishi, M.E.B.; Rocha, J.L.; Teixeira, A.K.; Farias, F.G.; Guerrelhas, A.C.; Caetano, A.R. Development and Validation of a Low-Density SNP Panel for Paternity and Kinship Analysis and Evaluation of Genetic Variability and Structure of Commercial Pacific White Shrimp (Litopenaeus vannamei) Populations from Brazil. Aquaculture 2022, 560, 738540. [Google Scholar] [CrossRef]
- Ye, S.; Zhou, X.; Ouyang, M.; Cui, W.; Xiang, Z.; Zhang, Y.; Yuan, Y.; Ikhwanuddin, M.; Li, S.; Zheng, H.; et al. Development and Validation of a 40 K Liquid SNP Array for the Mud Crab (Scylla paramamosain). Aquaculture 2025, 594, 741394. [Google Scholar] [CrossRef]
- Duan, B.; Mu, S.; Guan, Y.; Li, S.; Yu, Y.; Liu, W.; Li, Z.; Ji, X.; Kang, X. Genetic Diversity and Population Structure of the Swimming Crab (Portunus trituberculatus) in China Seas Determined by Genotyping-by-Sequencing (GBS). Aquaculture 2022, 555, 738233. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, C.; Lin, K.; You, W.; Yang, Z. Genetic Diversity and Genetic Structure among Four Selected Strains of Whiteleg Shrimp (Litopenaeus vannamei) Using SSR Markers. Fishes 2023, 8, 544. [Google Scholar] [CrossRef]
- Liu, X.; Tang, X.; Chen, M.; Ni, G.; Yang, Y. Development and Application of Molecular Markers in Fisheries, Aquaculture, and Industry of Representative Temperate and Tropical Sea Cucumbers: A Review. Front. Mar. Sci. 2024, 11, 1423096. [Google Scholar] [CrossRef]
- Han, Z.; Han, Q.; Xia, Y.; Geng, X.; Du, K.; Yang, J.; Kang, X. Construction of a Breeding Parent Population of Populus Tomentosa Based on SSR Genetic Distance Analysis. Sci. Rep. 2020, 10, 18573. [Google Scholar] [CrossRef]
- Chen, B.; Long, J.; Liu, J.; Wang, P.; Ma, Z.; Lan, Z.; Liang, Z.; Fu, Q.; Zhang, Z.; Zhang, Y.; et al. The Development of Novel Genome-SSRs, Multiplex PCR Panels, and Allelic Ladders for Parentage Identification in Tachypleus tridentatus. Aquaculture 2024, 592, 741262. [Google Scholar] [CrossRef]
- Sigang, F.; Hao, H.; Yong, L.; Pengfei, W.; Chao, Z.; Lulu, Y.; Xiuting, Q.; Qiu, L. Genome-Wide Identification of Microsatellite and Development of Polymorphic SSR Markers for Spotted Sea Bass (Lateolabrax maculatus). Aquacult. Rep. 2021, 20, 100677. [Google Scholar] [CrossRef]
- Sundaray, J.K.; Rasal, K.D.; Chakrapani, V.; Swain, P.; Kumar, D.; Ninawe, A.S.; Nandi, S.; Jayasankar, P. Simple Sequence Repeats (SSRs) Markers in Fish Genomic Research and Their Acceleration via next-Generation Sequencing and Computational Approaches. Aquacult. Int. 2016, 24, 1089–1102. [Google Scholar] [CrossRef]
- Jerry, D.R.; Evans, B.S.; Kenway, M.; Wilson, K. Development of a Microsatellite DNA Parentage Marker Suite for Black Tiger Shrimp Penaeus monodon. Aquaculture 2006, 255, 542–547. [Google Scholar] [CrossRef]
- Qiu, G.F.; Xiong, L.W.; Liu, Z.Q.; Yan, Y.L.; Shen, H. A First Generation Microsatellite-Based Linkage Map of the Chinese Mitten Crab Eriocheir sinensis and Its Application in Quantitative Trait Loci (QTL) Detection. Aquaculture 2016, 451, 223–231. [Google Scholar] [CrossRef]
- Qiu, G.F.; Xiong, L.W.; Han, Z.K.; Liu, Z.Q.; Feng, J.B.; Wu, X.G.; Yan, Y.L.; Shen, H.; Huang, L.; Chen, L. A Second Generation SNP and SSR Integrated Linkage Map and QTL Mapping for the Chinese Mitten Crab Eriocheir sinensis. Sci. Rep. 2017, 7, 39826. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.G.; Li, H.J.; Li, Y.F.; Sui, L.J.; Zhu, B.; Liang, Y.; Liu, W.D.; He, C.B. Sixteen Polymorphic Simple Sequence Repeat Markers from Expressed Sequence Tags of the Chinese Mitten Crab Eriocheir sinensis. Int. J. Mol. Sci. 2010, 11, 3035–3038. [Google Scholar] [CrossRef]
- Chang, Y.; Liang, L.; Ma, H.; He, J.; Sun, X. Microsatellite Analysis of Genetic Diversity and Population Structure of Chinese Mitten Crab (Eriocheir sinensis). J. Genet. Genom. 2008, 35, 171–176. [Google Scholar] [CrossRef]
- Xiong, L.W.; Wang, Q.; Qiu, G.F. LargeScale Isolation of Microsatellites from Chinese Mitten Crab Eriocheir sinensis via a Solexa Genomic Survey. Int. J. Mol. Sci. 2012, 13, 16333–16345. [Google Scholar] [CrossRef]
- Wang, S.H.; Zhang, C.; Shang, M.; Wu, X.G.; Cheng, Y.X. Genetic Diversity and Population Structure of Native Mitten Crab (Eriocheir sensu stricto) by Microsatellite Markers and Mitochondrial COI Gene Sequence. Gene 2019, 693, 101–113. [Google Scholar] [CrossRef]
- Wang, S.; Luo, L.; Zhang, R.; Guo, K.; Xu, W.; Zhao, Z. Population Genetic Diversity and Differentiation of Mitten Crab, Genus Eriocheir, Based on Microsatellite Markers. Fishes 2022, 7, 182. [Google Scholar] [CrossRef]
- Porta, J.; María Porta, J.; Martínez-Rodríguez, G.; Del Carmen Alvarez, M. Development of a Microsatellite Multiplex PCR for Senegalese Sole (Solea senegalensis) and Its Application to Broodstock Management. Aquaculture 2006, 256, 159–166. [Google Scholar] [CrossRef]
- Li, D.; Wang, S.; Shen, Y.; Meng, X.; Xu, X.; Wang, R.; Li, J. A Multiplex Microsatellite PCR Method for Evaluating Genetic Diversity in Grass Carp (Ctenopharyngodon idellus). Aquac. Fish. 2018, 3, 238–245. [Google Scholar] [CrossRef]
- Popa, O.P.; Bartáková, V.; Bryja, J.; Reichard, M.; Popa, L.O. Characterization of Nine Microsatellite Markers and Development of Multiplex PCRs for the Chinese Huge Mussel Anodonta (Sinanodonta) Woodiana Lea, 1834 (Mollusca, Bivalvia). Biochem. Syst. Ecol. 2015, 60, 234–237. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, D.H.; Yoon, S.J.; Kim, D.H.; Kim, S.G.; Hyun, Y.S.; Min, G.S.; Chung, K.W. Characterization of 20 Microsatellite Loci by Multiplex PCR in Swimming Crab, Portunus trituberculatus. Genes Genom. 2013, 35, 77–85. [Google Scholar] [CrossRef]
- Wu, X.; Liu, M.; Pan, J.; Chen, H.; Zeng, C.; Cheng, Y. The Ovarian Development Pattern of Pond-Reared Chinese Mitten Crab, Eriocheir sinensis H. Milne-Edwards, 1853. Crustaceana 2017, 90, 449–470. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J.; Sambrook, J. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012; ISBN 978-1-936113-41-5. [Google Scholar]
- Feng, Q.; Liu, M.; Cheng, Y.; Wu, X. Comparative Transcriptome Analysis Reveals the Process of Ovarian Development and Nutrition Metabolism in Chinese Mitten Crab, Eriocheir sinensis. Front. Genet. 2022, 13, 910682. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-Level Expression Analysis of RNA-Seq Experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v5: User Manual/Tutorial; Primer-E Ltd.: Plymouth, UK, 2001. [Google Scholar]
- Holleley, C.E.; Geerts, P.G. Multiplex Manager 1.0: A Cross-Platform Computer Program That Plans and Optimizes Multiplex PCR. BioTechniques 2009, 46, 511–517. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising How the Computer Program cervus Accommodates Genotyping Error Increases Success in Paternity Assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. Micro-Checker: Software for Identifying and Correcting Genotyping Errors in Microsatellite Data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Yeh, F.C.; Yang, R.C.; Boyle, T.; Ye, Z.H. PopGene, the User-Friendly Shareware for Population Genetic Analysis; Molecular Biology and Biotechnology Centre, University of Alberta: Edmonton, AB, Canada, 1997. [Google Scholar]
- Nagy, S.; Poczai, P.; Cernák, I.; Gorji, A.M.; Hegedűs, G.; Taller, J. PICcalc: An Online Program to Calculate Polymorphic Information Content for Molecular Genetic Studies. Biochem. Genet. 2012, 50, 670–672. [Google Scholar] [CrossRef]
- Arthofer, W.; Steiner, F.M.; Schlick-Steiner, B.C. Rapid and Cost-Effective Screening of Newly Identified Microsatellite Loci by High-Resolution Melting Analysis. Mol. Genet. Genom. 2011, 286, 225. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, P.; Choi, Y.A.; Huang, S.; Gmitter, F.G. Mining and Characterizing Microsatellites from Citrus ESTs. Theor. Appl. Genet. 2006, 112, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Lyons, R.E.; Dinh, H.; Hurwood, D.A.; McWilliam, S.; Mather, P.B. Transcriptomics of a Giant Freshwater Prawn (Macrobrachium rosenbergii): De Novo Assembly, Annotation and Marker Discovery. PLoS ONE 2011, 6, e27938. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Graner, A.; Sorrells, M.E. Genic Microsatellite Markers in Plants: Features and Applications. Trends Biotechnol. 2005, 23, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Mu, S.; Guan, Y.; Liu, W.; Kang, T.; Cheng, Y.; Li, Z.; Tian, Y.; Kang, X. Development of Microsatellite Markers Based on Transcriptome Sequencing and Evaluation of Genetic Diversity in Swimming Crab (Portunus trituberculatus). Front. Genet. 2022, 13, 932173. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, X.; Li, Z.; Xu, W.; Dong, J.; Wei, H.; Li, Y.; Li, X. Genetic Diversity and Structure of Chinese Grass Shrimp, Palaemonetes sinensis, Inferred from Transcriptome-Derived Microsatellite Markers. BMC Genet. 2019, 20, 75. [Google Scholar] [CrossRef]
- Lerceteau-Köhler, E.; Weiss, S. Development of a Multiplex PCR Microsatellite Assay in Brown Trout Salmo trutta, and Its Potential Application for the Genus. Aquaculture 2006, 258, 641–645. [Google Scholar] [CrossRef]
- Sekino, M.; Kurokawa, T.; Sasaki, K. Multiplex PCR Panels of Novel Microsatellites for the Ark Shell Scapharca Broughtonii (Pteriomorphia, arcoida). Conserv. Genet. Resour. 2010, 2, 39–42. [Google Scholar] [CrossRef]
- Scribner, K.T.; Pearce, J.M. Microsatellites: Evolutionary and Methodological Background and Empirical Applications at Individual, Population, and Phylogenetic Levels. In Molecular Methods in Ecology; Baker, A.J., Ed.; Blackwell Science: Hoboken, NJ, USA, 2000; pp. 235–273. [Google Scholar]
- Yu, J.; Liao, S.; Liu, H.; Wang, W.; Cao, X. The Development of Novel EST-SSRs and Construction of Multiplex PCR Panels for Parentage Assignment in Macrobrachium rosenbergii. Aquaculture 2023, 570, 739440. [Google Scholar] [CrossRef]
- Ding, Q.; Shi, M.; Ji, P.; Qin, L.; Gao, X.; Zhang, X.; Jiang, Q. Genetic Diversity and Differentiation of Cultured Macrobrachium rosenbergii in China Using Newly Developed Microsatellite Multiplex PCR Panels. Aquacult. Int. 2024, 32, 7895–7910. [Google Scholar] [CrossRef]
- Wang, X.; Hua, Q.; Wu, L.; Weng, Z.; Huang, W.; Meng, Z. Development of a Multiplex Microsatellite Assay for Parentage Assignment in Orange-spotted Groupers (Epinephelus coioides). J. World Aquac. Soc. 2021, 52, 435–444. [Google Scholar] [CrossRef]
- Yılmaz, D.; Atasaral, Ş. Microsatellite-Based Parentage Assignment in Tiger Trout (Salmo trutta × Salvelinus fontinalis). Turk. J. Fish. Aquat. Sci. 2024, 24. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Q.; Yang, S.; Li, Y. Isolation of Tetrameric Microsatellite Markers and Its Application on Parentage Identification in Procambarus clarkii. Aquacult. Int. 2023, 31, 2099–2111. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, W.; Wang, W.; Gui, J.-F.; Mei, J. Parentage Determination of Yellow Catfish (Pelteobagrus fulvidraco) Based on Microsatellite DNA Markers. Aquacult. Int. 2016, 24, 567–576. [Google Scholar] [CrossRef]
- Satyanarayana, Y.; Babu, P.G.; Jahageerdar, S. Parentage Determination in the Freshwater Prawn Macrobrachium rosenbergii de Man, 1879 Using Microsatellite Markers. Indian J. Fish. 2015, 62, 29–32. [Google Scholar]
- Yang, M.; Tian, C.; Liang, X.-F.; Lv, L.; Zheng, H.; Yuan, Y.; Zhao, C.; Huang, W. Parentage Determination of Mandarin Fish (Siniperca chuatsi) Based on Microsatellite DNA Markers. Biochem. Syst. Ecol. 2014, 54, 285–291. [Google Scholar] [CrossRef]
- Weinman, L.R.; Solomon, J.W.; Rubenstein, D.R. A Comparison of Single Nucleotide Polymorphism and Microsatellite Markers for Analysis of Parentage and Kinship in a Cooperatively Breeding Bird. Mol. Ecol. Resour. 2015, 15, 502–511. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Tong, G.-X.; Kuang, Y.-Y.; Zhang, C.; Yin, J.-S. Applicability of Microsatellite DNA Markers to the Parental Identification of Hucho taimen (Pallas). Zool. Res. 2010, 31, 395–400. [Google Scholar] [CrossRef]
- Wesmajervi, M.S.; Westgaard, J.-I.; Delghandi, M. Evaluation of a Novel Pentaplex Microsatellite Marker System for Paternity Studies in Atlantic Cod (Gadus morhua L.). Aquac. Res. 2006, 37, 1195–1201. [Google Scholar] [CrossRef]
- Jones, A.G.; Small, C.M.; Paczolt, K.A.; Ratterman, N.L. A Practical Guide to Methods of Parentage Analysis. Mol. Ecol. Resour. 2010, 10, 6–30. [Google Scholar] [CrossRef]
- Hedgecock, D.; Li, G.; Hubert, S.; Bucklin, K.; Ribes, V. Widespread Null Alleles and Poor Cross-Species Amplification of Microsatellite Dna Loci Cloned from the Pacific Oyster, Crassostrea gigas. J. Shellfish Res. 2004, 23, 379–385. [Google Scholar]
| Families | Number of Females | Number of Males |
|---|---|---|
| C1 | 8 | 4 |
| C2 | 15 | 15 |
| C3 | 13 | 25 |
| C4 | 14 | 25 |
| C5 | 15 | 15 |
| C6 | 41 | 17 |
| Locus | Repeat Motif | Primer Sequences (5′−3′) a | Annealing Temperature | Size Range (bp) | Concentration in Multiplex-PCR (µmol·L−1) | (GenBanK Accession No.) |
|---|---|---|---|---|---|---|
| PCR multiplex set 1 | 60 (°C) | |||||
| CX140 | (CAA)4 | F: FAM-TCACCACCATCAGCAACA | 196–223 | 0.2 | MF686621 | |
| R: AGAAAGGACAGGGATAACC | ||||||
| CX287 | (GGC)5 | F: FAM-TCACTAGGATAGGTAACGGA | 292–327 | 0.4 | MF686652 | |
| R: CACTCAACACACGACAGGA | ||||||
| CX256 | (CCT)4 | F: HEX-ATGGCTCTCTCTGGTGGCT | 359–429 | 0.28 | MF686639 | |
| R: TGCTGAAATCGGAAGGTT | ||||||
| CX416 | (TGG)4 | F: FAM-GTAGTGTGAAGCAAGGAGGA | 381–445 | 0.36 | MF686673 | |
| R: GGAGTTTGAGGTGAGCGA | ||||||
| PCR multiplex set 2 | 60 (°C) | |||||
| CX003 | (TATT)4 | F: FAM-CCCTCAACCTGAACCTCT | 119–187 | 0.2 | MF686585 | |
| R: ACTCTTTTTTGCTGCCCT | ||||||
| CX254 | (TCC)9 | F: FAM-CTATCCATCTCTCTACCTCCC | 357–374 | 0.24 | MF686638 | |
| R: GCAAAAACAAACCACTAACC | ||||||
| CX400 | (GAG)7 | F: HEX-ACCCTATCAAACTATTACGACC | 401–411 | 0.24 | MF754123 | |
| R: ATGAACACCGAGACCACG | ||||||
| CX427 | (GTG)4 | F: FAM-GGCATTCAATCTTAAACCC | 415–435 | 0.36 | MF686679 | |
| R: ATCTCCTCCTTCCCTCGT | ||||||
| PCR multiplex set 3 | 55 (°C) | |||||
| CX074 | (CA)6 | F: FAM-GCAAAACTTGGCAAACGA | 110–119 | 0.2 | MF686606 | |
| R: ACCTCCACCCAGCACACT | ||||||
| CX013 | (GGA)4 | F: FAM-ACTACGACTTTTCTACTTACCC | 167–179 | 0.2 | MF686590 | |
| R: CCTCCTTATCCTCTTGCTC | ||||||
| CX298 | (CAC)4 | F: HEX-ACCCAAGCACACCTTCTCC | 332–381 | 0.2 | MF686657 | |
| R: CCAACCCTCCCAACCTAAT | ||||||
| CX312 | (TGG)6 | F: FAM-ATCACCAACACTGCTCTCG | 399–411 | 0.24 | MF686661 | |
| R: CTCAATGCTGCTGCCTCT | ||||||
| PCR multiplex set 4 | 55 (°C) | |||||
| CX071 | (TGG)8 | F: HEX-GCTGGAGTCAGGGCTAAA | 148–171 | 0.24 | MF686604 | |
| R: CAAAACACACACGGAAGAA | ||||||
| CX118 | (AAG)4 | F: HEX-CCACAACTGCTGCTGATG | 214–264 | 0.2 | MF686612 | |
| R: TGTAGACGAAGGAAGGCT | ||||||
| CX325 | (CTC)5 | F: HEX-GAGCGATTAGTTACGGGA | 322–357 | 0.4 | MF686664 | |
| R: GAGGAAGGTGTGTTTGGA | ||||||
| CX435 | (CTC)5 | F: HEX-CATCATCTCCATCTCCTCTG | 405–447 | 0.4 | MF686683 | |
| R: ATCTGCACCCCTTCTCAC |
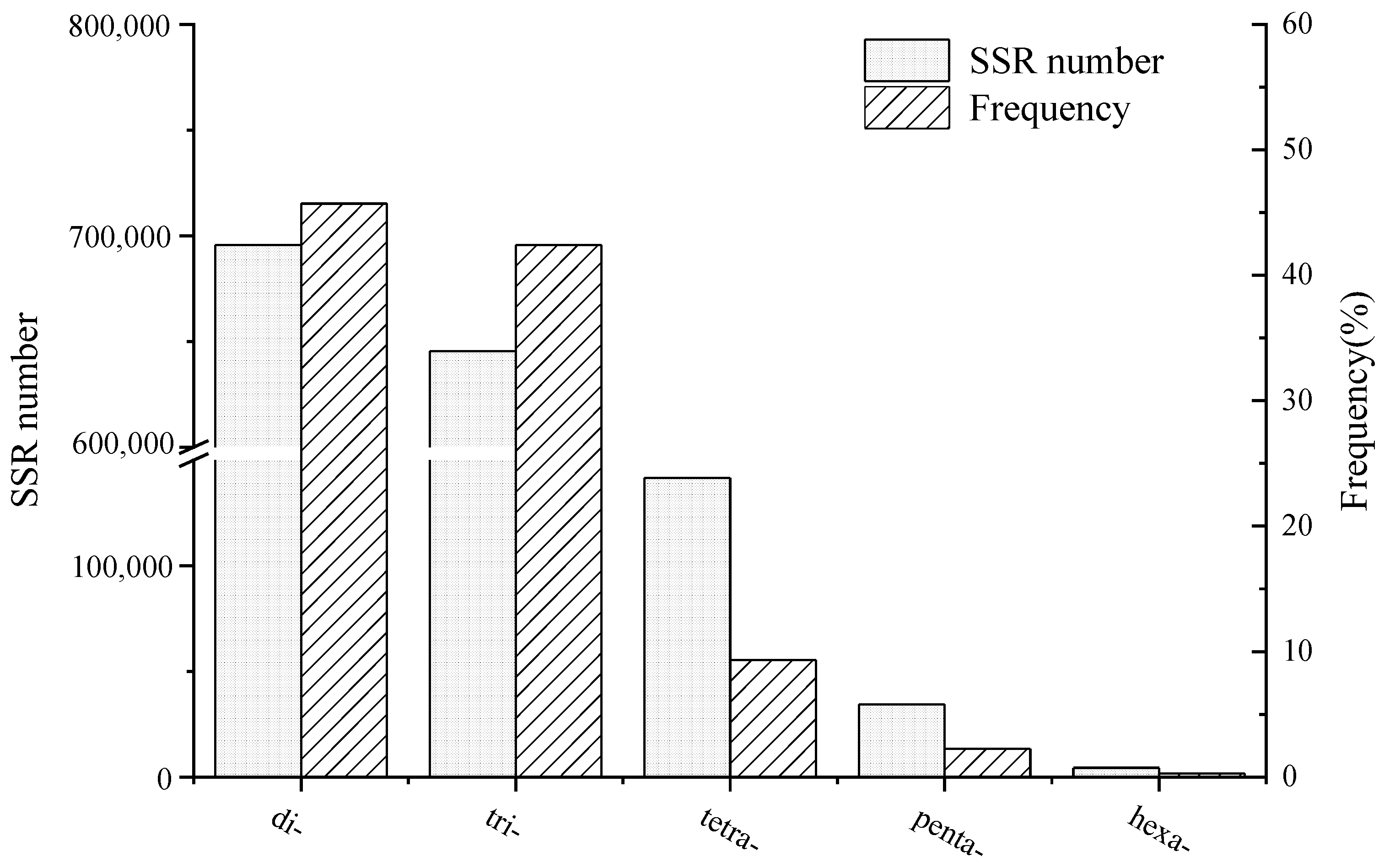
| Type of SSR | Number of SSR | Universality | Polymorphism | ||
|---|---|---|---|---|---|
| Number | Probability (%) | Number | Probability (%) | ||
| Di-nucleotide | 5 | 3 | 60.0 | 3 | 100 |
| Tri-nucleotide | 259 | 197 | 76.1 | 93 | 47.2 |
| Tetra-nucleotide | 4 | 3 | 75.0 | 3 | 100 |
| Penta-nucleotide | 3 | 1 | 33.3 | 0 | 0.0 |
| Hexa-nucleotide | 1 | 1 | 100 | 1 | 100 |
| Total | 272 | 205 | 75.4 | 100 | 48.8 |
| Locus | Na | Ho | He | PIC | HW | F(Null) |
|---|---|---|---|---|---|---|
| CX140 | 15 | 0.435 | 0.724 | 0.697 | *** | 0.2385 |
| CX287 | 9 | 0.822 | 0.867 | 0.850 | *** | 0.0261 |
| CX256 | 21 | 0.935 | 0.909 | 0.900 | NS | −0.0182 |
| CX416 | 10 | 0.488 | 0.767 | 0.736 | *** | 0.2296 |
| CX003 | 8 | 0.356 | 0.758 | 0.731 | *** | 0.3725 |
| CX254 | 15 | 0.877 | 0.852 | 0.833 | *** | −0.0179 |
| CX400 | 9 | 0.849 | 0.792 | 0.762 | *** | −0.0389 |
| CX427 | 9 | 0.617 | 0.838 | 0.816 | *** | 0.1503 |
| CX074 | 9 | 0.621 | 0.858 | 0.840 | *** | 0.1583 |
| CX013 | 7 | 0.656 | 0.810 | 0.785 | *** | 0.0957 |
| CX298 | 7 | 0.660 | 0.778 | 0.746 | *** | 0.0876 |
| CX312 | 7 | 0.757 | 0.808 | 0.780 | *** | 0.0395 |
| CX071 | 9 | 0.626 | 0.734 | 0.701 | *** | 0.0928 |
| CX118 | 11 | 0.587 | 0.656 | 0.633 | NS | 0.0334 |
| CX325 | 16 | 0.951 | 0.898 | 0.887 | NS | −0.0314 |
| CX435 | 11 | 0.681 | 0.829 | 0.804 | ** | 0.0929 |
| Mean value | 10.81 | 0.682 | 0.805 | 0.781 | 0.0944 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Q.; Tang, M.; Li, Q.; Dong, P.; Cheng, Y.; Deng, D.; Wu, X. Development of Microsatellite Markers from Transcriptome of Eriocheir sinensis and Their Application in Multiplex PCR Panels. Animals 2024, 14, 3200. https://doi.org/10.3390/ani14223200
Xiao Q, Tang M, Li Q, Dong P, Cheng Y, Deng D, Wu X. Development of Microsatellite Markers from Transcriptome of Eriocheir sinensis and Their Application in Multiplex PCR Panels. Animals. 2024; 14(22):3200. https://doi.org/10.3390/ani14223200
Chicago/Turabian StyleXiao, Qizhen, Meijun Tang, Qingqing Li, Pengsheng Dong, Yongxu Cheng, Deng Deng, and Xugan Wu. 2024. "Development of Microsatellite Markers from Transcriptome of Eriocheir sinensis and Their Application in Multiplex PCR Panels" Animals 14, no. 22: 3200. https://doi.org/10.3390/ani14223200
APA StyleXiao, Q., Tang, M., Li, Q., Dong, P., Cheng, Y., Deng, D., & Wu, X. (2024). Development of Microsatellite Markers from Transcriptome of Eriocheir sinensis and Their Application in Multiplex PCR Panels. Animals, 14(22), 3200. https://doi.org/10.3390/ani14223200







