An Integrated Profiling of Liver Metabolome and Transcriptome of Pigs Fed Diets with Different Starch Sources
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiment, Management, and Diets
2.2. Sample Collection
2.3. Metabolome Approach and Data Analysis
2.4. RNA Isolation, Sequencing and Data Analysis
2.5. cDNA Synthesis, Primers, and Quantitative Real-Time PCR (qRT-PCR)
2.6. Statistical Analysis
3. Results
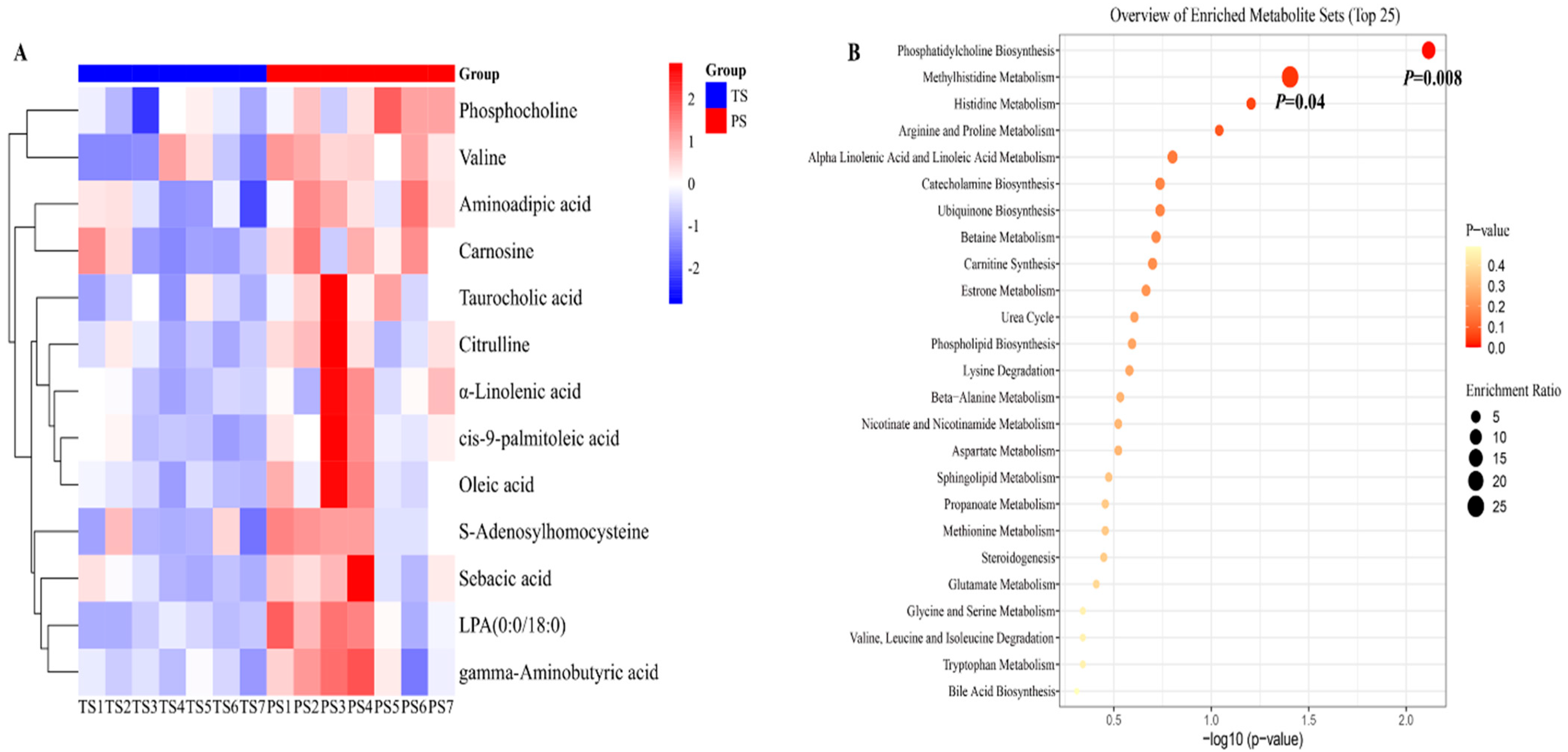
3.1. Variations in Liver Metabolites in Pigs After Different Dietary Treatments
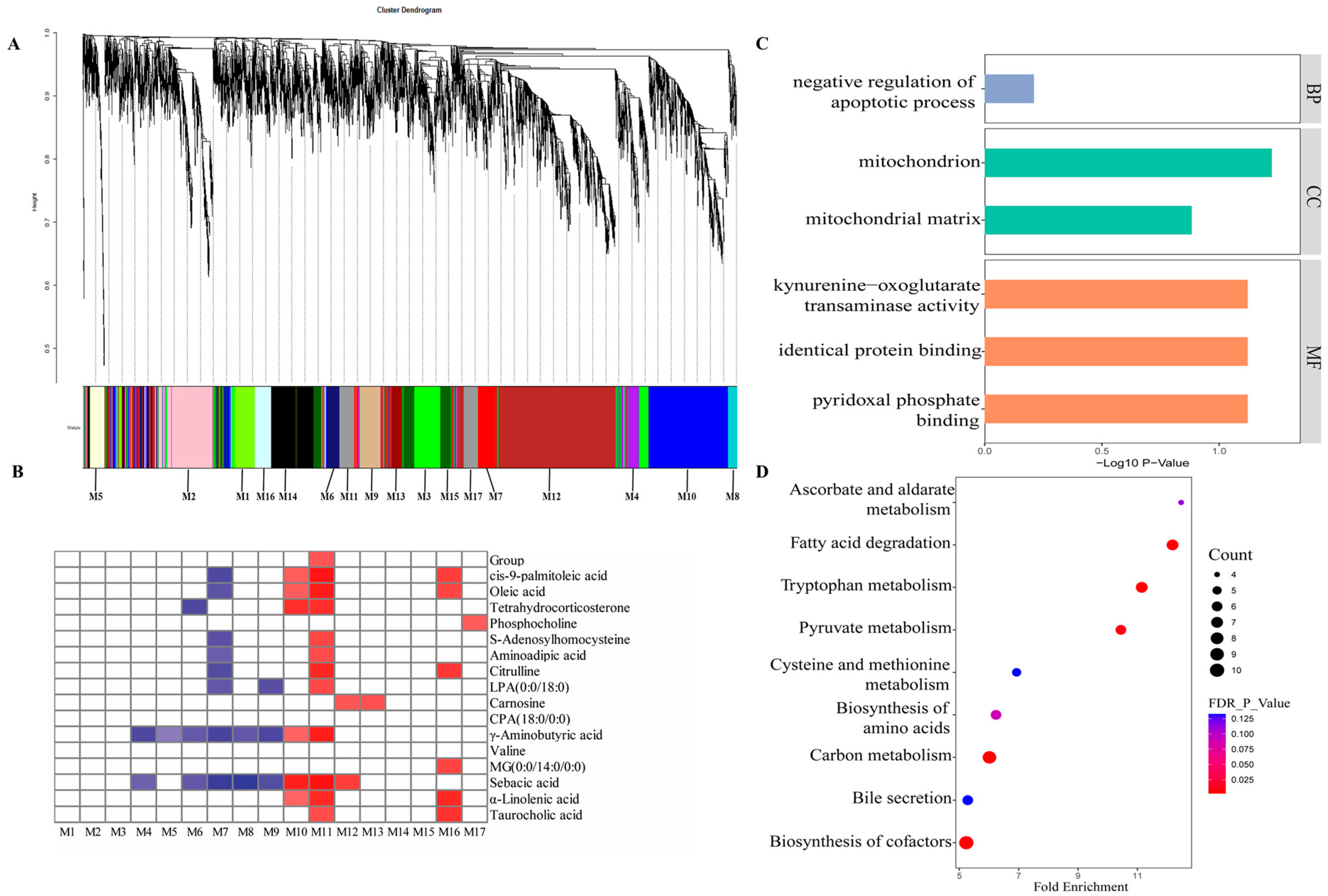
3.2. Gene Co-Expression Network of Host Transcriptome, Diets, and Liver Metabolites
3.3. Functional Profiles of Host Genes in the M11 Module
3.4. Expression Pattern of M11 Hub Genes Between Two Dietary Treatments
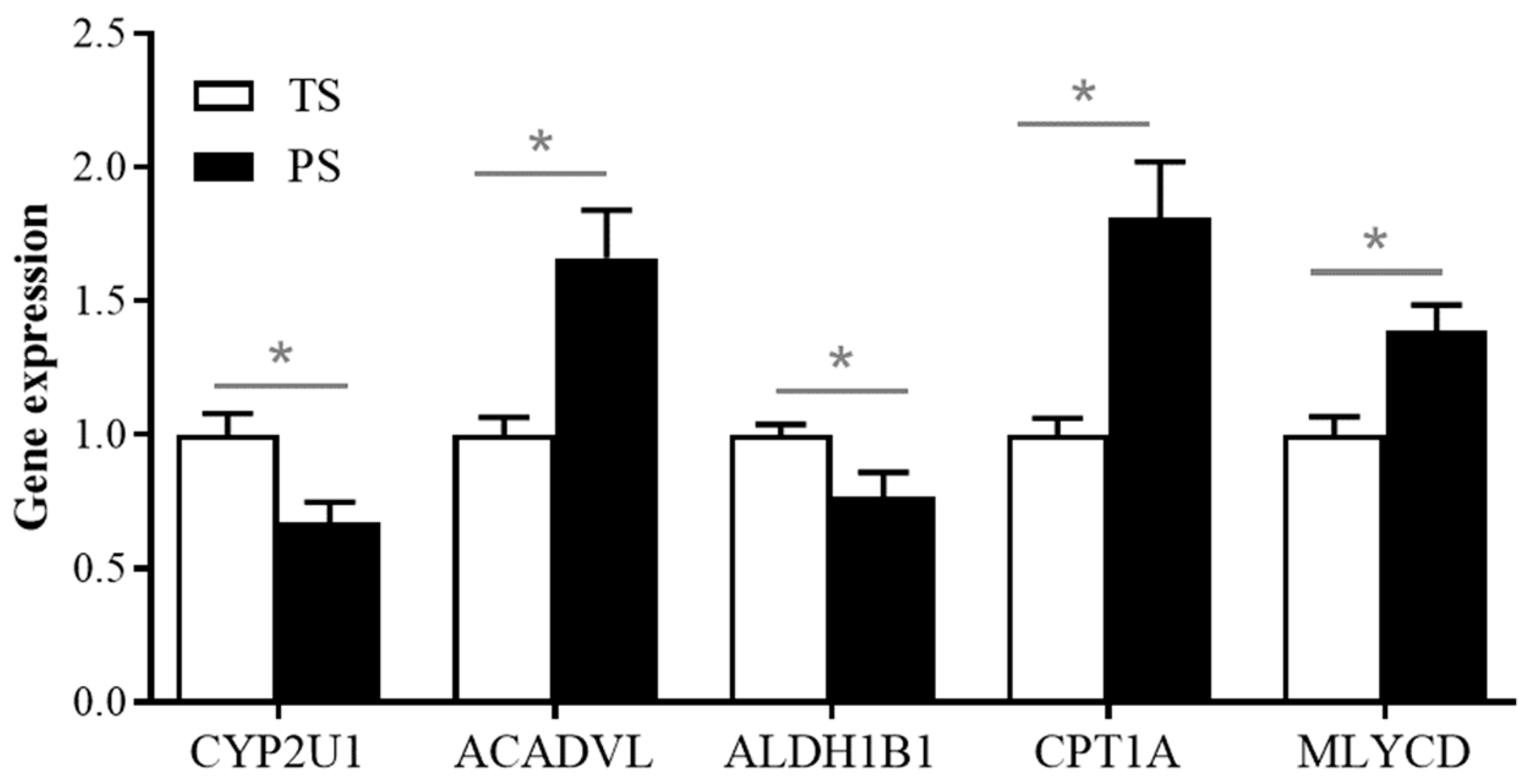
3.5. Validation of the Expressions of DEGs Involved in the Fatty Acid Degradation Using the Real-Time PCR Method
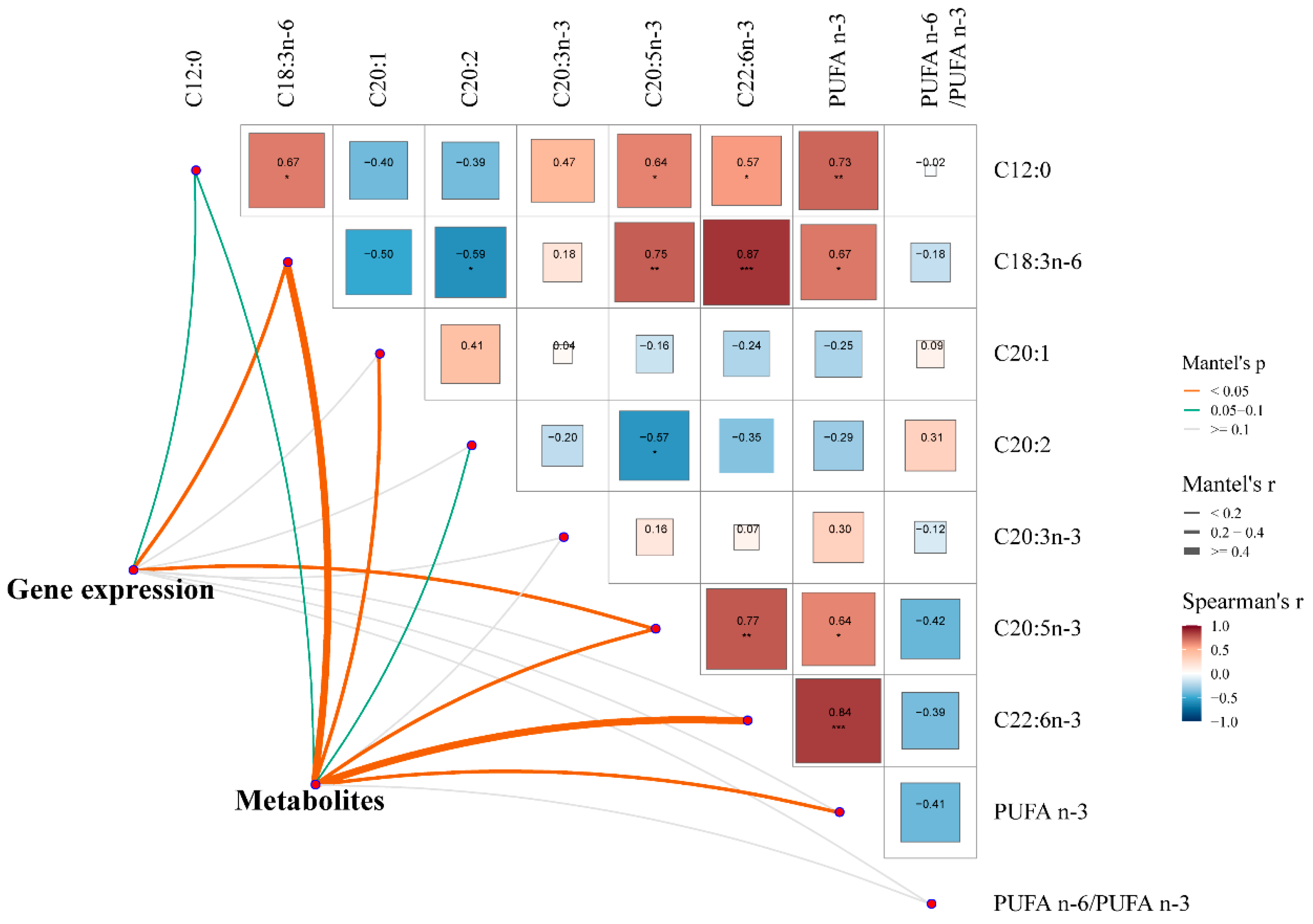
3.6. The Relationship Between Liver Status (Gene Expression and Metabolites) and Muscle Fatty Acids in Pigs
4. Discussion
4.1. Diets Containing Higher-Amylose-Content Starches Increased the Contents of Beneficial Unsaturated Fatty Acids and Amino Acids in the Liver of Pigs
4.2. Diets Containing Higher-Amylose-Content Starches Promoted the β-Oxidation of Fatty Acids and the Accumulation of Unsaturated Fatty Acids
4.3. Muscle Fatty Acids Were Significantly Closely Correlated with Liver Gene Expressions and Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Yu, B.; Yu, J.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Zheng, P.; Yan, H.; He, J. Effects of different starch structures on energy metabolism in pigs. J. Anim. Sci. Biotechnol. 2023, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Maier, T.V.; Lucio, M.; Lee, L.H.; VerBerkmoes, N.C.; Brislawn, C.J.; Bernhardt, J.; Lamendella, R.; McDermott, J.E.; Bergeron, N.; Heinzmann, S.S. Impact of dietary resistant starch on the human gut microbiome, metaproteome, and metabolome. MBio 2017, 8, e1343-17. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Zhang, Z.; Huang, J.; Yin, Y. Digestion rate of dietary starch affects systemic circulation of amino acids in weaned pigs. Br. J. Nutr. 2010, 103, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Doti, S.; Suárez-Belloch, J.; Latorre, M.A.; Guada, J.A.; Fondevila, M. Effect of dietary starch source on growth performances, digestibility and quality traits of growing pigs. Livest. Sci. 2014, 164, 119–127. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Zhang, L.; Gao, F.; Zhou, G. Effects of dietary starch types on growth performance, meat quality and myofibre type of finishing pigs. Meat Sci. 2017, 131, 60–67. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Chen, W.; Rong, T.; Wang, G.; Ma, X. Microbiome-metabolomics analysis investigating the impacts of dietary starch types on the composition and metabolism of colonic microbiota in finishing pigs. Front. Microbiol. 2019, 10, 1143. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Rong, T.; Wang, G.; Liu, Z.; Chen, W.; Li, J.; Li, J.; Ma, X. Different dietary starch sources alter the carcass traits, meat quality, and the profile of muscle amino acid and fatty acid in finishing pigs. J. Anim. Sci. Biotechno. 2020, 11, 78. [Google Scholar] [CrossRef]
- Bird, A.R.; Brown, I.L.; Topping, D.L. Starches, resistant starches, the gut microflora and human health. Curr. Issues Intest. Microbiol. 2000, 1, 25–37. [Google Scholar]
- Postic, C.; Dentin, R.; Girard, J. Role of the liver in the control of carbohydrate and lipid homeostasis. Diabetes Metab. 2004, 30, 398–408. [Google Scholar] [CrossRef]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef]
- Xie, C.; Li, Y.; Li, J.; Zhang, L.; Zhou, G.; Gao, F. Dietary starch types affect liver nutrient metabolism of finishing pigs. Br. J. Nutr. 2017, 118, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; He, J.; Yu, B.; Yu, J.; Mao, X.; Chen, D.; Yin, Y. The effect of dietary amylose/amylopectin ratio on serum and hepatic lipid content and its molecular mechanisms in growing-finishing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Yang, C.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Yu, J.; Zheng, P.; He, J. Effect of dietary amylose/amylopectin ratio on growth performance, carcass traits, and meat quality in finishing pigs. Meat Sci. 2015, 108, 55–60. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Liu, X.; Luo, R.; Liao, G.; Li, L.; Liu, J.; Cheng, J.; Lu, Y.; Chen, Y. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018, 203, 291–304. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar]
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The role of the novel lipokine palmitoleic acid in health and disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.O.; Valenzuela, C.A.; Baker, E.J.; Miles, E.A.; Rosa Neto, J.C.; Calder, P.C. Palmitoleic acid has stronger anti-inflammatory potential in human endothelial cells compared to oleic and palmitic acids. Mol. Nutr. Food Res. 2018, 62, 1800322. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-W.; Lu, C.-H.; Chang, R.C.-A.; Hsu, Y.-P.; Ho, L.-T.; Shih, K.-C. Palmitoleic acid ameliorates palmitic acid-induced proinflammation in J774A. 1 macrophages via TLR4-dependent and TNF-α-independent signallings. Prostaglandins Leukot. Essent. Fat. Acids 2021, 169, 102270. [Google Scholar] [CrossRef] [PubMed]
- Bolsoni-Lopes, A.; Festuccia, W.T.; Farias, T.S.; Chimin, P.; Torres-Leal, F.L.; Derogis, P.B.; de Andrade, P.B.; Miyamoto, S.; Lima, F.B.; Curi, R. Palmitoleic acid (n-7) increases white adipocyte lipolysis and lipase content in a PPARα-dependent manner. Am. J. Physiol-Endoc. M. 2013, 305, E1093–E1102. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.O.; Teixeira, A.A.; Biondo, L.A.; Silveira, L.S.; Calder, P.C.; Rosa Neto, J.C. Palmitoleic acid reduces the inflammation in LPS-stimulated macrophages by inhibition of NF κB, independently of PPAR s. Clin. Exp. Pharmacol. Physiol. 2017, 44, 566–575. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Attar-Bashi, N.M.; Li, D. What is the role of α-linolenic acid for mammals? Lipids 2002, 37, 1113–1123. [Google Scholar] [CrossRef]
- Burdge, G. Metabolism of α-linolenic acid in humans. Prosta. Leukotr. Ess. 2006, 75, 161–168. [Google Scholar] [CrossRef]
- Wang, C.; Li, L.; Guan, H.; Tong, S.; Liu, M.; Liu, C.; Zhang, Z.; Du, C.; Li, P. Effects of taurocholic acid on immunoregulation in mice. Int. Immunopharmacol. 2013, 15, 217–222. [Google Scholar] [CrossRef]
- Homayouni, A.; Amini, A.; Keshtiban, A.K.; Mortazavian, A.M.; Esazadeh, K.; Pourmoradian, S. Resistant starch in food industry: A changing outlook for consumer and producer. Starch-Stärke 2014, 66, 102–114. [Google Scholar] [CrossRef]
- Yu, M.; Li, Z.; Rong, T.; Tian, Z.; Deng, D.; Lu, H.; Zhang, R.; Ma, X. Integrated metagenomics-metabolomics analysis reveals the cecal microbial composition, function, and metabolites of pigs fed diets with different starch sources. Food Res. Int. 2022, 154, 110951. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, K.; Zhou, L.; Fang, L.; Su, Y.; Zhu, W. Metabolomic and transcriptomic responses induced in the livers of pigs by the long-term intake of resistant starch. J. Anim. Sci. 2016, 94, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Van Rensburg, C.J.; Gous, R. The response of weaned piglets to dietary valine and leucine. Animal 2017, 11, 1279–1286. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, X.; Liu, X.; Jia, H.; He, P.; Mao, X.; Qiao, S.; Zeng, X. Valine supplementation in a reduced protein diet regulates growth performance partially through modulation of plasma amino acids profile, metabolic responses, endocrine, and neural factors in piglets. J. Agric. Food Chem. 2018, 66, 3161–3168. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.-H.; Vo, T.S. An updated review on pharmaceutical properties of gamma-aminobutyric acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef] [PubMed]
- Guiotto, A.; Calderan, A.; Ruzza, P.; Borin, G. Carnosine and carnosine-related antioxidants: A Review. Curr. Med. Chem. 2005, 12, 2293–2315. [Google Scholar] [CrossRef]
- Cole, L.K.; Vance, J.E.; Vance, D.E. Phosphatidylcholine biosynthesis and lipoprotein metabolism. BBA-Mol. Cell Biol. L. 2012, 1821, 754–761. [Google Scholar] [CrossRef]
- Wong, J.T.; Chan, M.; Lee, D.; Jiang, J.Y.; Skrzypczak, M.; Choy, P.C. Phosphatidylcholine metabolism in human endothelial cells: Modulation by phosphocholine. Mol. Cell Biochem. 2000, 207, 95–100. [Google Scholar] [CrossRef]
- Lee, E.-S.Y.; Charlton, C.G. 1-Methyl-4-phenyl-pyridinium increases S-adenosyl-L-methionine dependent phospholipid methylation. Pharmacol. Biochem. Behav. 2001, 70, 105–114. [Google Scholar] [CrossRef]
- Obeid, R.; Herrmann, W. Homocysteine and lipids: S-adenosyl methionine as a key intermediate. FEBS Lett. 2009, 583, 1215–1225. [Google Scholar] [CrossRef]
- Deng, J.; Wu, X.; Bin, S.; Li, T.J.; Huang, R.; Liu, Z.; Liu, Y.; Ruan, Z.; Deng, Z.; Hou, Y. Dietary amylose and amylopectin ratio and resistant starch content affects plasma glucose, lactic acid, hormone levels and protein synthesis in splanchnic tissues. J. Anim. Physiol. Anim. Nutr. 2010, 94, 220–226. [Google Scholar] [CrossRef]
- Evans, R.M.; Barish, G.D.; Wang, Y.-X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.; Daiwen, C.; Bing, Y. Metabolic and transcriptomic responses of weaned pigs induced by different dietary amylose and amylopectin ratio. PLoS ONE 2010, 5, e15110. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef] [PubMed]
- Polakof, S.; Díaz-Rubio, M.E.; Dardevet, D.; Martin, J.-F.; Pujos-Guillot, E.; Scalbert, A.; Sebedio, J.-L.; Mazur, A.; Comte, B. Resistant starch intake partly restores metabolic and inflammatory alterations in the liver of high-fat-diet-fed rats. J. Nutr. Biochem. 2013, 24, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Ghisla, S.; Thorpe, C. Acyl-CoA dehydrogenases: A mechanistic overview. Eur. J. Biochem. 2004, 271, 494–508. [Google Scholar] [CrossRef]
- Wang, W.; Palmfeldt, J.; Mohsen, A.-W.; Gregersen, N.; Vockley, J. Fasting induces prominent proteomic changes in liver in very long chain Acyl-CoA dehydrogenase deficient mice. Biochem. Biophys. Rep. 2016, 8, 333–339. [Google Scholar] [CrossRef]
- Ewida, H.; Zoubi, S.; Kallem, R.R.; Patel, D.; Patra, S.; Szweda, L.; Chelikani, P.K.; Ahmed, M.S. Structure-based drug repurposing targeting malonyl-CoA decarboxylase for treatment of obesity. J. Pharmacol. Exp. Ther. 2024, 389, 282. [Google Scholar]
- Wekesa, B.M. Expression and Purification of Cytochrome 2U1 and Canine 1A2 and Metabolism of Caffeine by Cytochrome 1A2. Master’s Thesis, Missouri State University, Springfield, MO, USA, 2021. [Google Scholar]
- Arnold, C.; Konkel, A.; Fischer, R.; Schunck, W.-H. Cytochrome P450–dependent metabolism of ω-6 and ω-3 long-chain polyunsaturated fatty acids. Pharmacol. Rep. 2010, 62, 536–547. [Google Scholar] [CrossRef]
- Duan, X.; Hu, H.; Wang, L.; Chen, L. Aldehyde dehydrogenase 1 family: A potential molecule target for diseases. Cell Biol. Int. 2024, 48, 909–922. [Google Scholar] [CrossRef]
- Xing, K.; Zhao, X.; Ao, H.; Chen, S.; Yang, T.; Tan, Z.; Wang, Y.; Zhang, F.; Liu, Y.; Ni, H. Transcriptome analysis of miRNA and mRNA in the livers of pigs with highly diverged backfat thickness. Sci. Rep. 2019, 9, 16740. [Google Scholar] [CrossRef]
- Kalbe, C.; Priepke, A.; Nürnberg, G.; Dannenberger, D. Effects of long-term microalgae supplementation on muscle microstructure, meat quality and fatty acid composition in growing pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 574–582. [Google Scholar] [CrossRef] [PubMed]


| Diet 1 | ||
|---|---|---|
| Items | TS | PS |
| Ingredient, % | ||
| Tapioca starch | 59 | |
| Pea starch | 59 | |
| Soybean meal | 27 | 27 |
| Corn gluten meal | 3.4 | 3.4 |
| Wheat bran | 3.36 | 3.36 |
| Soybean oil | 3.25 | 3.25 |
| L-Lysine-HCl (98%) | 0.3 | 0.3 |
| DL-Methionine | 0.13 | 0.13 |
| L-Threonine | 0.06 | 0.06 |
| Dicalcium phosphate | 1.5 | 1.5 |
| Limestone | 0.3 | 0.3 |
| Choline chloride (50 %) | 0.4 | 0.4 |
| Salt | 0.3 | 0.3 |
| Vitamin and mineral Premix 2 | 1 | 1 |
| Total | 100 | 100 |
| Calculated content 3 | ||
| ME 4, MJ/kg | 13.81 | 13.81 |
| Standardized ileal digestible amino acids 3, % | ||
| Lysine | 0.88 | 0.88 |
| Methionine + Cysteine | 0.49 | 0.49 |
| Threonine | 0.5 | 0.5 |
| Tryptophan | 0.15 | 0.15 |
| Analyzed nutrient composition | ||
| 5 Dry matter, % | 88.48 | 88.45 |
| 5 Crude protein, % | 14.54 | 14.55 |
| 5 Crude fat, % | 1.26 | 1.25 |
| 5 Crude ash, % | 4.09 | 4.11 |
| Total starch, % DM | 52.25 | 52.25 |
| Amylose/amylopectin | 0.11 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Li, Z.; Cui, Y.; Rong, T.; Tian, Z.; Deng, D.; Liu, Z.; Zhang, R.; Ma, X. An Integrated Profiling of Liver Metabolome and Transcriptome of Pigs Fed Diets with Different Starch Sources. Animals 2024, 14, 3192. https://doi.org/10.3390/ani14223192
Yu M, Li Z, Cui Y, Rong T, Tian Z, Deng D, Liu Z, Zhang R, Ma X. An Integrated Profiling of Liver Metabolome and Transcriptome of Pigs Fed Diets with Different Starch Sources. Animals. 2024; 14(22):3192. https://doi.org/10.3390/ani14223192
Chicago/Turabian StyleYu, Miao, Zhenming Li, Yiyan Cui, Ting Rong, Zhimei Tian, Dun Deng, Zhichang Liu, Ruiyang Zhang, and Xianyong Ma. 2024. "An Integrated Profiling of Liver Metabolome and Transcriptome of Pigs Fed Diets with Different Starch Sources" Animals 14, no. 22: 3192. https://doi.org/10.3390/ani14223192
APA StyleYu, M., Li, Z., Cui, Y., Rong, T., Tian, Z., Deng, D., Liu, Z., Zhang, R., & Ma, X. (2024). An Integrated Profiling of Liver Metabolome and Transcriptome of Pigs Fed Diets with Different Starch Sources. Animals, 14(22), 3192. https://doi.org/10.3390/ani14223192






