Effects of Dietary Net Energy Concentration on Reproductive Performance, Immune Function, Milk Composition, and Gut Microbiota in Primiparous Lactating Sows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Diets and Feeding
2.3. Sample Collection
2.4. Performance Measurement of Sows and Piglets
2.5. Chemical Analyses
2.6. Microbiota Profiling
2.7. Statistical Analysis
3. Results
3.1. Performance of Sows and Piglets
3.2. Plasma Biochemical Indices and Hormone Levels
3.3. Plasma Antioxidant Capacity
3.4. Milk Composition
3.5. Immunoglobulins
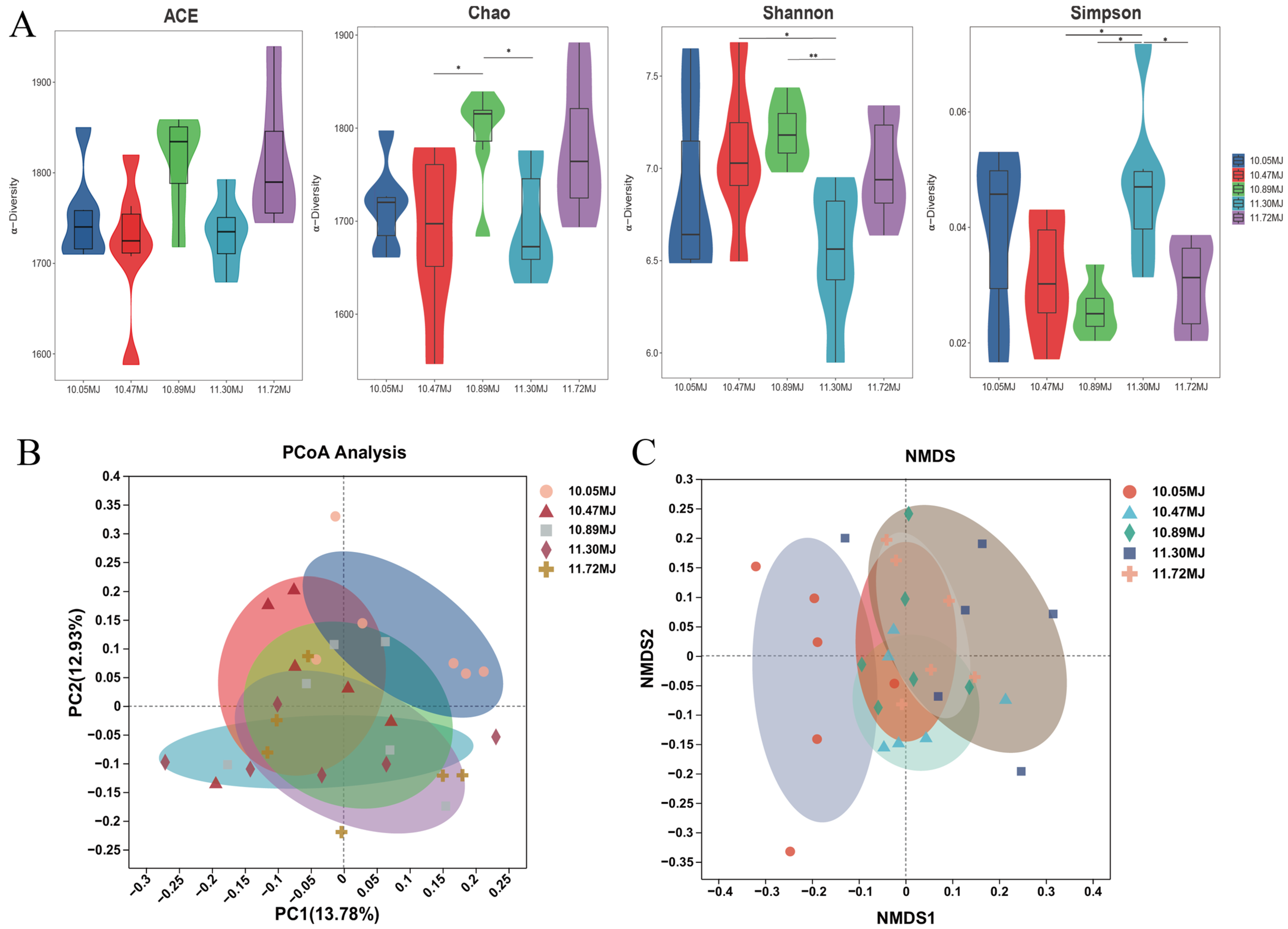
3.6. Gut Microbiota
3.7. Spearman’s Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tokach, M.D.; Menegat, M.B.; Gourley, K.M.; Goodband, R.D. Review: Nutrient requirements of the modern high-producing lactating sow, with an emphasis on amino acid requirements. Animal 2019, 13, 2967–2977. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Easter, R.A. Nutrient mobilization from body tissues as influenced by litter size in lactating sows. J. Anim. Sci. 2001, 79, 2179–2186. [Google Scholar] [CrossRef]
- Feyera, T.; Theil, P.K. Energy and lysine requirements and balances of sows during transition and lactation: A factorial approach. Livest. Sci. 2017, 201, 50–57. [Google Scholar] [CrossRef]
- Noblet, J.; Dourmad, J.Y.; Etienne, M. Energy utilization in pregnant and lactating sows: Modeling of energy requirements. J. Anim. Sci. 1990, 68, 562–572. [Google Scholar] [CrossRef]
- Prunier, A.; de Bragança, M.M.; Le Dividich, J. Influence of high ambient temperature on performance of reproductive sows. Livest. Prod. Sci. 1997, 52, 123–133. [Google Scholar] [CrossRef]
- Renaudeau, D.; Collin, A.; Yahav, S.; de Basilio, V.; Gourdine, J.L.; Collier, R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 2012, 6, 707–728. [Google Scholar] [CrossRef]
- Muns, R.; Malmkvist, J.; Larsen, M.L.; Sorensen, D.; Pedersen, L.J. High environmental temperature around farrowing induced heat stress in crated sows. J. Anim. Sci. 2016, 94, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Koketsu, Y. Sows having high lifetime efficiency and high longevity associated with herd productivity in commercial herds. Livest. Sci. 2008, 118, 140–146. [Google Scholar] [CrossRef]
- Thaker, M.Y.; Bilkei, G. Lactation weight loss influences subsequent reproductive performance of sows. Anim. Reprod. Sci. 2005, 88, 309–318. [Google Scholar] [CrossRef]
- Prunier, A.; Quesnel, H. Influence of the nutritional status on ovarian development in female pigs. Anim. Reprod. Sci. 2000, 60-61, 185–197. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Johnston, L.J.; Levesque, C.L.; Yin, J.; Dong, B. A systematic review and meta-analysis of dietary fat effects on reproductive performance of sows and growth performance of piglets. J. Anim. Sci. Biotechnol. 2022, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Yang, Y.X.; Choi, J.Y.; Yoon, S.Y.; Ahn, S.S.; Lee, S.H.; Yang, B.K.; Lee, J.K.; Chae, B.J. Effects of dietary fat inclusion at two energy levels on reproductive performance, milk compositions and blood profiles in lactating sows. Acta Agriculturae Scandinavica. Sect. A Anim. Sci. 2008, 58, 121–128. [Google Scholar] [CrossRef]
- Pedersen, T.F.; Bruun, T.S.; Feyera, T.; Larsen, U.K.; Theil, P.K. A two-diet feeding regime for lactating sows reduced nutrient deficiency in early lactation and improved milk yield. Livest. Sci. 2016, 191, 165–173. [Google Scholar] [CrossRef]
- Xue, L.; Piao, X.; Li, D.; Li, P.; Zhang, R.; Kim, S.W.; Dong, B. The effect of the ratio of standardized ileal digestible lysine to metabolizable energy on growth performance, blood metabolites and hormones of lactating sows. J. Anim. Sci. Biotechnol. 2012, 3, 11. [Google Scholar] [CrossRef]
- Rooney, H.B.; O’Driscoll, K.; O’Doherty, J.V.; Lawlor, P.G. Effect of increasing dietary energy density during late gestation and lactation on sow performance, piglet vitality, and lifetime growth of offspring. J. Anim. Sci. 2020, 98, skz379. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Choi, Y.H.; Hosseindoust, A.; Kim, M.J.; Moturi, J.; Kim, T.G.; Song, C.H.; Lee, J.H.; Chae, B.J. Effects of free feeding time system and energy level to improve the reproductive performance of lactating sows during summer. J. Anim. Sci. Technol. 2020, 62, 356–364. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; National Academic Press: Washington, DC, USA, 2012. [Google Scholar]
- Zhao, Y.; Huang, Y.; Gao, K.; Wen, X.; Hu, S.; Wang, L.; Jiang, Z.; Xiao, H. Maternal resveratrol regulates the growth performance, antioxidant capacity, and intestinal health of suckling piglets through intestinal microorganisms at high summer temperatures. Front. Nutr. 2022, 9, 971496. [Google Scholar] [CrossRef]
- Choi, Y.; Hosseindoust, A.; Shim, Y.; Kim, M.; Kumar, A.; Oh, S.; Kim, Y.; Chae, B.J. Evaluation of high nutrient diets on litter performance of heat-stressed lactating sows. Asian Australas. J. Anim. Sci. 2017, 30, 1598–1604. [Google Scholar] [CrossRef]
- Lu, N.; Vier, C.; Cast, W.; Orlando, U.; Gonçalves, M.; Zhou, Z.; Young, M. Effects of dietary net energy and neutral detergent fiber levels on growth performance and carcass characteristics of growing finishing pigs. J. Anim. Sci. 2020, 98, 55–56. [Google Scholar] [CrossRef]
- Kerr, J.C.; Cameron, N.D. Reproductive performance of pigs selected for components of efficient lean growth. Anim. Sci. 1995, 60, 281–290. [Google Scholar] [CrossRef]
- Pettigrew, J.E.; Moser, R.L. Chapter 8—Fat in Swine Nutrition. In Swine Nutrition; Miller, E.R., Ullrey, D.E., Lewis, A.J., Eds.; Butterworth-Heinemann: Oxford, UK, 1991; pp. 133–145. [Google Scholar]
- Black, J.L.; Mullan, B.P.; Lorschy, M.L.; Giles, L.R. Lactation in the sow during heat stress. Livest. Prod. Sci. 1993, 35, 153–170. [Google Scholar] [CrossRef]
- Lellis, W.A.; Speer, V.C. Nutrient balance of lactating sows fed supplemental tallow. J. Anim. Sci. 1983, 56, 1334–1339. [Google Scholar] [CrossRef]
- Hojgaard, C.K.; Bruun, T.S.; Theil, P.K. Impact of milk and nutrient intake of piglets and sow milk composition on piglet growth and body composition at weaning. J. Anim. Sci. 2020, 98, skaa060. [Google Scholar] [CrossRef]
- Barber, M.C.; Clegg, R.A.; Travers, M.T.; Vernon, R.G. Lipid Metabolism in the Lactating Mammary Gland; Elsevier B.V: Amsterdam, The Netherlands, 1997; Volume 1347, pp. 101–126. [Google Scholar]
- Emery, R.S. Biosynthesis of milk fat. J. Dairy Sci. 1973, 56, 1187–1195. [Google Scholar] [CrossRef]
- Lauridsen, C.; Danielsen, V. Lactational dietary fat levels and sources influence milk composition and performance of sows and their progeny. Livest. Prod. Sci. 2004, 91, 95–105. [Google Scholar] [CrossRef]
- Rooke, J.A.; Carranca, C.; Bland, I.M.; Sinclair, A.G.; Ewen, M.; Bland, V.C.; Edwards, S.A. Relationships between passive absorption of immunoglobulin G by the piglet and plasma concentrations of immunoglobulin G at weaning. Livest. Prod. Sci. 2003, 81, 223–234. [Google Scholar] [CrossRef]
- Bandrick, M.; Ariza-Nieto, C.; Baidoo, S.K.; Molitor, T.W. Colostral antibody-mediated and cell-mediated immunity contributes to innate and antigen-specific immunity in piglets. Dev. Comp. Immunol. 2014, 43, 114–120. [Google Scholar] [CrossRef]
- Lee, I.K.; Kye, Y.C.; Kim, G.; Kim, H.W.; Gu, M.J.; Umboh, J.; Maaruf, K.; Kim, S.W.; Yun, C.H. Stress, Nutrition, and Intestinal Immune Responses in Pigs—A Review. Asian Australas. J. Anim. Sci. 2016, 29, 1075–1082. [Google Scholar] [CrossRef]
- Yoon, J.H.; Ingale, S.L.; Kim, J.S.; Kim, K.H.; Lee, S.H.; Park, Y.K.; Kwon, I.K.; Chae, B.J. Effects of dietary supplementation of antimicrobial peptide-A3 on growth performance, nutrient digestibility, intestinal and fecal microflora and intestinal morphology in weanling pigs. Anim. Feed Sci. Technol. 2012, 177, 98–107. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Zhe, L.; Krogh, U.; Lauridsen, C.; Nielsen, M.O.; Fang, Z.; Theil, P.K. Impact of dietary fat levels and fatty acid composition on milk fat synthesis in sows at peak lactation. J. Anim. Sci. Biotechnol. 2023, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Declerck, I.; Dewulf, J.; Sarrazin, S.; Maes, D. Long-term effects of colostrum intake in piglet mortality and performance. J. Anim. Sci. 2016, 94, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Nelssen, J.L.; Lewis, A.J.; Peo, E.J.; Moser, B.D. Effect of source of dietary energy and energy restriction during lactation on sow and litter performance. J. Anim. Sci. 1985, 60, 171–178. [Google Scholar] [CrossRef][Green Version]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef]
- Kojta, I.; Chacinska, M.; Blachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Pere, M.C.; Etienne, M. Insulin sensitivity during pregnancy, lactation, and postweaning in primiparous gilts. J. Anim. Sci. 2007, 85, 101–110. [Google Scholar] [CrossRef]
- Parry, S.A.; Smith, J.R.; Corbett, T.R.; Woods, R.M.; Hulston, C.J. Short-term, high-fat overfeeding impairs glycaemic control but does not alter gut hormone responses to a mixed meal tolerance test in healthy, normal-weight individuals. Br. J. Nutr. 2017, 117, 48–55. [Google Scholar] [CrossRef]
- Numao, S.; Kawano, H.; Endo, N.; Yamada, Y.; Konishi, M.; Takahashi, M.; Sakamoto, S. Short-term low carbohydrate/high-fat diet intake increases postprandial plasma glucose and glucagon-like peptide-1 levels during an oral glucose tolerance test in healthy men. Eur. J. Clin. Nutr. 2012, 66, 926–931. [Google Scholar] [CrossRef]
- Berchieri-Ronchi, C.B.; Kim, S.W.; Zhao, Y.; Correa, C.R.; Yeum, K.J.; Ferreira, A.L. Oxidative stress status of highly prolific sows during gestation and lactation. Animal 2011, 5, 1774–1779. [Google Scholar] [CrossRef]
- Serdar, Z.; Gur, E.; Colakoethullary, M.; Develioethlu, O.; Sarandol, E. Lipid and protein oxidation and antioxidant function in women with mild and severe preeclampsia. Arch. Gynecol. Obstet. 2003, 268, 19–25. [Google Scholar] [CrossRef]
- Piironen, V.; Lindsay, D.G.; Miettinen, T.A.; Toivo, J.; Lampi, A.M. Plant sterols: Biosynthesis, biological function and their importance to human nutrition. J. Sci. Food. Agric. 2000, 80, 939–966. [Google Scholar] [CrossRef]
- Cui, L.; Decker, E.A. Phospholipids in foods: Prooxidants or antioxidants? J. Sci. Food. Agric. 2016, 96, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; de Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.A.; Park, S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014, 8, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Lu, D.; Tiezzi, F.; Schillebeeckx, C.; McNulty, N.P.; Schwab, C.; Shull, C.; Maltecca, C. Host contributes to longitudinal diversity of fecal microbiota in swine selected for lean growth. Microbiome 2018, 6, 4. [Google Scholar] [CrossRef]
- Wang, C.; Wei, S.; Xu, B.; Hao, L.; Su, W.; Jin, M.; Wang, Y. Bacillus subtilis and Enterococcus faecium co-fermented feed regulates lactating sow’s performance, immune status and gut microbiota. Microb. Biotechnol. 2021, 14, 614–627. [Google Scholar] [CrossRef]
- Tao, N.; Ochonicky, K.L.; German, J.B.; Donovan, S.M.; Lebrilla, C.B. Structural determination and daily variations of porcine milk oligosaccharides. J. Agric. Food. Chem. 2010, 58, 4653–4659. [Google Scholar] [CrossRef]
- Valeriano, V.D.; Balolong, M.P.; Kang, D.K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef]
- Bergamaschi, M.; Maltecca, C.; Schillebeeckx, C.; McNulty, N.P.; Schwab, C.; Shull, C.; Fix, J.; Tiezzi, F. Heritability and genome-wide association of swine gut microbiome features with growth and fatness parameters. Sci. Rep. 2020, 10, 10134. [Google Scholar] [CrossRef]
- Angelakis, E.; Bachar, D.; Yasir, M.; Musso, D.; Djossou, F.; Gaborit, B.; Brah, S.; Diallo, A.; Ndombe, G.M.; Mediannikov, O.; et al. Treponema species enrich the gut microbiota of traditional rural populations but are absent from urban individuals. New Microbes New Infect. 2019, 27, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Li, P.; Hao, S.; Zhang, Y.; Kim, S.W.; Li, H.; Ma, X.; Gao, S.; He, L.; Wu, W.; et al. Dynamic distribution of the gut microbiota and the relationship with apparent crude fiber digestibility and growth stages in pigs. Sci. Rep. 2015, 5, 9938. [Google Scholar] [CrossRef] [PubMed]
- Delmastro-Greenwood, M.M.; Piganelli, J.D. Changing the energy of an immune response. Am. J. Clin. Exp. Immunol. 2013, 2, 30–54. [Google Scholar] [PubMed]


| Item | Dietary Net Energy Concentration, MJ/kg | ||||
|---|---|---|---|---|---|
| 10.05 | 10.47 | 10.89 | 11.30 | 11.72 | |
| Ingredient, % | |||||
| Corn, 8% | 58.80 | 58.20 | 57.86 | 57.34 | 56.74 |
| Wheat meal | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Soybean meal, 44% | 21.50 | 22.18 | 22.60 | 23.19 | 23.85 |
| Fish meal, 63% | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Soybean hulls | 8.00 | 6.00 | 4.00 | 2.00 | 0 |
| Soybean oil | 0 | 1.90 | 3.80 | 5.70 | 7.60 |
| Choline chloride, 50% | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Salt | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| CaHPO4 | 1.10 | 1.10 | 1.10 | 1.10 | 1.10 |
| L-Lysine-HCl | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Limestone | 0.80 | 0.82 | 0.84 | 0.87 | 0.91 |
| Premix a | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated composition b | |||||
| ME, MJ/kg | 13.51 | 13.97 | 14.43 | 14.89 | 15.35 |
| NE, MJ/kg | 10.05 | 10.47 | 10.89 | 11.30 | 11.72 |
| CP, % | 17.8 | 17.8 | 17.8 | 17.8 | 17.8 |
| SID Lysine, % | 1.07 | 1.07 | 1.07 | 1.07 | 1.07 |
| SID Methionine + Cysteine, % | 0.63 | 0.63 | 0.63 | 0.63 | 0.63 |
| SID Threonine, % | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 |
| SID Tryptophan, % | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| Ca, % | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| P, % | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Item | Dietary Net Energy Concentration, MJ/kg | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10.05 | 10.47 | 10.89 | 11.30 | 11.72 | ANOVA | Linear | Quadratic | ||
| Daily feed intake, kg/d | |||||||||
| Lactation d1 to 21 | 5.18 | 5.15 | 4.98 | 4.30 | 4.93 | 0.11 | 0.082 | 0.079 | 0.358 |
| Energy intake, MJ/d | |||||||||
| Lactation d1 to 21 | 52.09 | 53.92 | 54.27 | 48.59 | 57.82 | 1.20 | 0.172 | 0.455 | 0.366 |
| Sow backfat thickness, mm | |||||||||
| Lactation d0 | 26.50 | 27.50 | 26.83 | 28.50 | 28.50 | 0.47 | 0.561 | 0.149 | 0.934 |
| Lactation d21 | 21.50 | 23.67 | 21.67 | 21.83 | 24.67 | 0.49 | 0.145 | 0.181 | 0.374 |
| Backfat loss d0–21 | −5.00 | −3.83 | −5.17 | −6.67 | −3.83 | 0.42 | 0.191 | 0.863 | 0.361 |
| Average weight of piglets, kg | |||||||||
| Lactation d0 | 1.71 | 1.70 | 1.58 | 1.53 | 1.54 | 0.05 | 0.657 | 0.158 | 0.789 |
| Lactation d21 | 6.65 | 6.46 | 6.45 | 6.13 | 6.56 | 0.15 | 0.878 | 0.661 | 0.500 |
| ADG of piglets | 0.24 | 0.23 | 0.23 | 0.22 | 0.24 | 0.05 | 0.866 | 0.984 | 0.472 |
| Item | Dietary Net Energy Concentration, MJ/kg | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10.05 | 10.47 | 10.89 | 11.30 | 11.72 | ANOVA | Linear | Quadratic | ||
| GLU, mmol/L | 4.48 | 4.79 | 4.27 | 5.35 | 5.18 | 0.16 | 0.149 | 0.074 | 0.606 |
| BUN, mmol/L | 12.34 ab | 12.93 a | 12.24 ab | 9.40 b | 11.87 ab | 0.38 | 0.020 | 0.063 | 0.561 |
| INS, mIU/L | 48.98 b | 52.76 b | 60.76 b | 67.98 ab | 85.20 a | 3.08 | <0.001 | <0.001 | 0.149 |
| PROG, pmol/L | 1698.06 | 1660.56 | 1699.44 | 1696.67 | 1706.39 | 29.33 | 0.991 | 0.814 | 0.843 |
| E2, pmol/L | 111.13 | 106.16 | 112.07 | 108.31 | 117.31 | 2.32 | 0.643 | 0.396 | 0.367 |
| Item | Dietary Net Energy Concentration, MJ/kg | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10.05 | 10.47 | 10.89 | 11.30 | 11.72 | ANOVA | Linear | Quadratic | ||
| T-AOC, mM | 0.42 | 0.40 | 0.39 | 0.40 | 0.39 | 0.03 | 0.998 | 0.823 | 0.889 |
| T-SOD, U/mL | 184.43 ab | 104.46 b | 118.13 b | 196.50 ab | 233.24 a | 13.76 | 0.005 | 0.024 | 0.004 |
| MDA, nmol/mL | 3.49 ab | 5.00 a | 4.09 ab | 3.81 ab | 1.63 b | 0.36 | 0.041 | 0.041 | 0.020 |
| Item | Dietary Net Energy Concentration, MJ/kg | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10.05 | 10.47 | 10.89 | 11.30 | 11.72 | ANOVA | Linear | Quadratic | ||
| Milk fat content, % | 6.78 ab | 6.12 b | 7.24 a | 7.35 a | 7.40 a | 0.14 | 0.007 | 0.005 | 0.667 |
| Nonfat solids content, % | 11.08 | 11.27 | 11.27 | 11.22 | 11.33 | 0.10 | 0.958 | 0.552 | 0.837 |
| Lactose content, % | 4.20 | 4.26 | 4.28 | 4.26 | 4.30 | 0.04 | 0.958 | 0.513 | 0.824 |
| Lactoprotein content, % | 6.04 | 6.13 | 6.13 | 6.10 | 6.15 | 0.05 | 0.976 | 0.641 | 0.794 |
| Item | Dietary Net Energy Concentration, MJ/kg | SEM | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10.05 | 10.47 | 10.89 | 11.30 | 11.72 | ANOVA | Linear | Quadratic | ||
| Plasma | |||||||||
| IgA, μg/mL | 32.49 | 33.83 | 35.17 | 30.73 | 32.74 | 0.79 | 0.508 | 0.651 | 0.515 |
| IgG, μg/mL | 190.00 | 190.44 | 197.46 | 180.13 | 184.74 | 3.75 | 0.688 | 0.454 | 0.625 |
| IgM, μg/mL | 36.81 | 34.37 | 39.85 | 40.05 | 45.15 | 1.25 | 0.066 | 0.010 | 0.314 |
| Milk | |||||||||
| SIgA, μg/mL | 25.05 b | 27.21 b | 27.00 b | 27.97 b | 34.53 a | 0.69 | <0.001 | <0.001 | 0.003 |
| IgA, μg/mL | 31.05 b | 31.12 b | 31.53 ab | 33.70 ab | 37.27 a | 0.74 | 0.021 | 0.003 | 0.111 |
| IgG, μg/mL | 365.32 b | 396.09 ab | 381.03 ab | 391.44 ab | 440.96 a | 7.81 | 0.020 | 0.005 | 0.273 |
| IgM, μg/mL | 34.99 b | 38.65 b | 39.40 b | 43.30 b | 47.74 a | 0.91 | <0.001 | <0.001 | 0.219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, F.; Hou, L.; Gao, K.; Wen, X.; Mi, S.; Qin, G.; Huang, L.; Wu, Q.; Yang, X.; Wang, L.; et al. Effects of Dietary Net Energy Concentration on Reproductive Performance, Immune Function, Milk Composition, and Gut Microbiota in Primiparous Lactating Sows. Animals 2024, 14, 3044. https://doi.org/10.3390/ani14203044
Gu F, Hou L, Gao K, Wen X, Mi S, Qin G, Huang L, Wu Q, Yang X, Wang L, et al. Effects of Dietary Net Energy Concentration on Reproductive Performance, Immune Function, Milk Composition, and Gut Microbiota in Primiparous Lactating Sows. Animals. 2024; 14(20):3044. https://doi.org/10.3390/ani14203044
Chicago/Turabian StyleGu, Fang, Lei Hou, Kaiguo Gao, Xiaolu Wen, Shuyun Mi, Guoxi Qin, Lijun Huang, Qiwen Wu, Xuefen Yang, Li Wang, and et al. 2024. "Effects of Dietary Net Energy Concentration on Reproductive Performance, Immune Function, Milk Composition, and Gut Microbiota in Primiparous Lactating Sows" Animals 14, no. 20: 3044. https://doi.org/10.3390/ani14203044
APA StyleGu, F., Hou, L., Gao, K., Wen, X., Mi, S., Qin, G., Huang, L., Wu, Q., Yang, X., Wang, L., Jiang, Z., & Xiao, H. (2024). Effects of Dietary Net Energy Concentration on Reproductive Performance, Immune Function, Milk Composition, and Gut Microbiota in Primiparous Lactating Sows. Animals, 14(20), 3044. https://doi.org/10.3390/ani14203044






