Avian and Mammalian Diversity and Abundance in Jhalana Reserve Forest, Jaipur, India
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
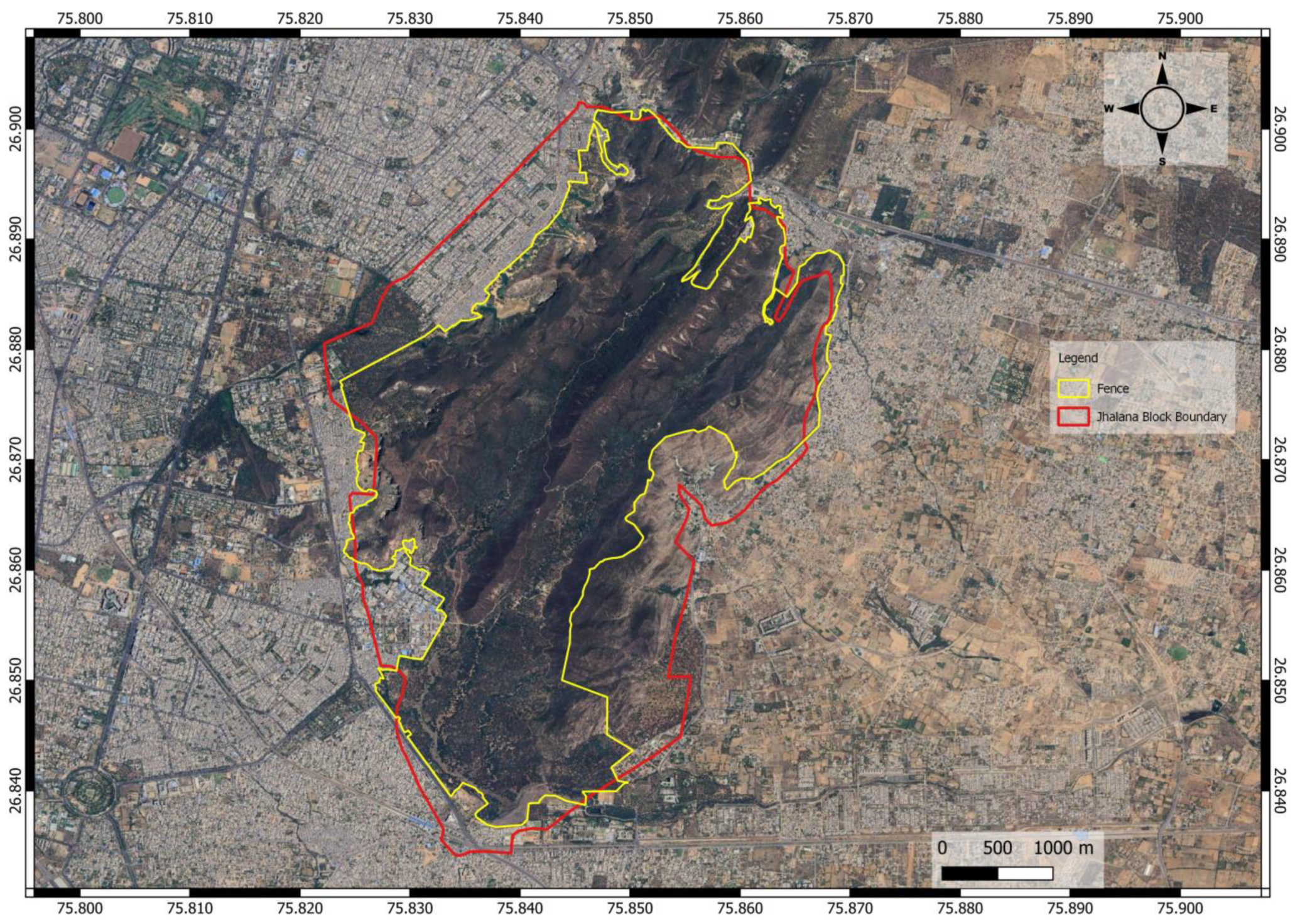
2.1. Study Area
2.2. Field Methods
2.3. Statistical Analysis
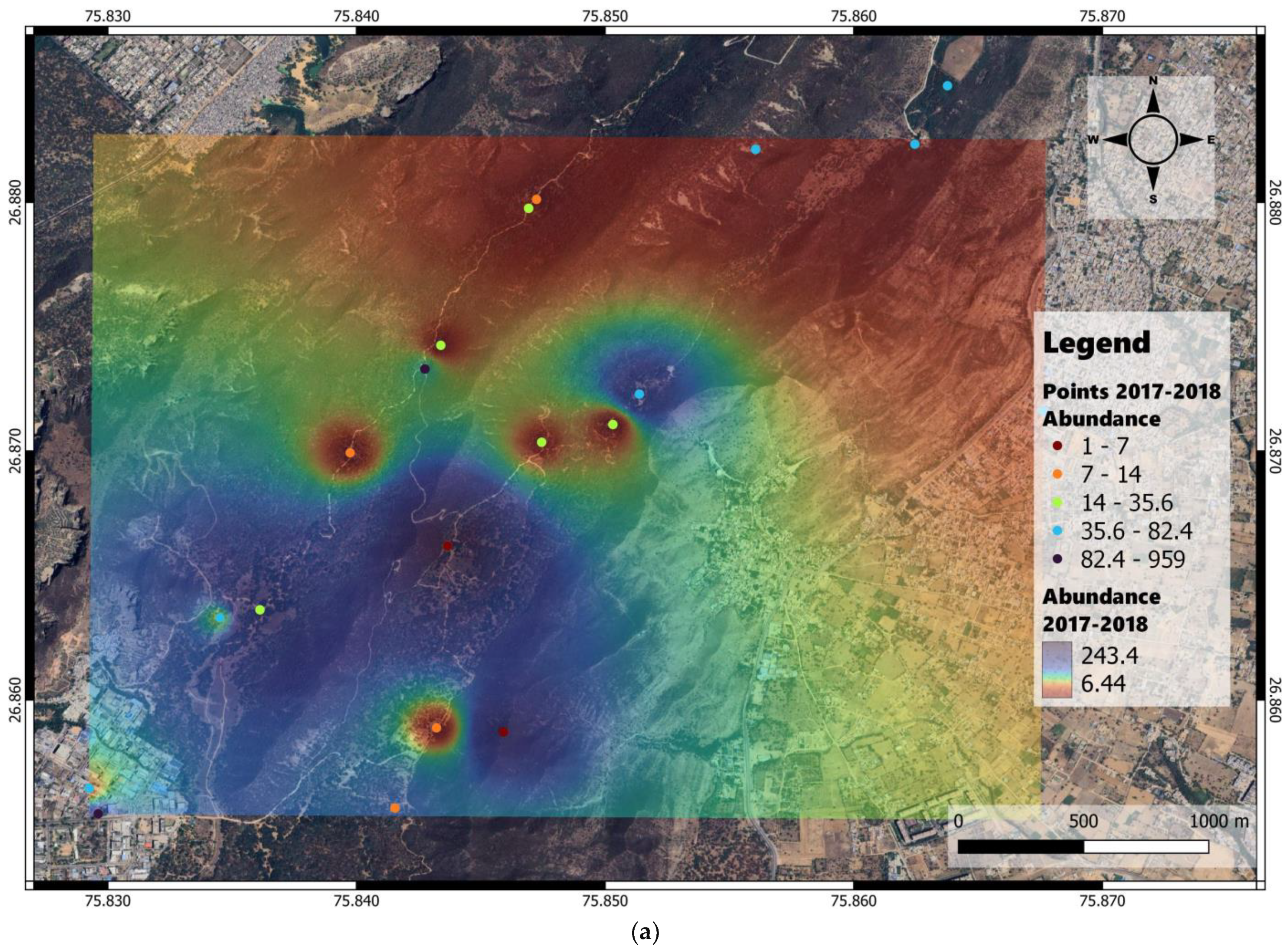
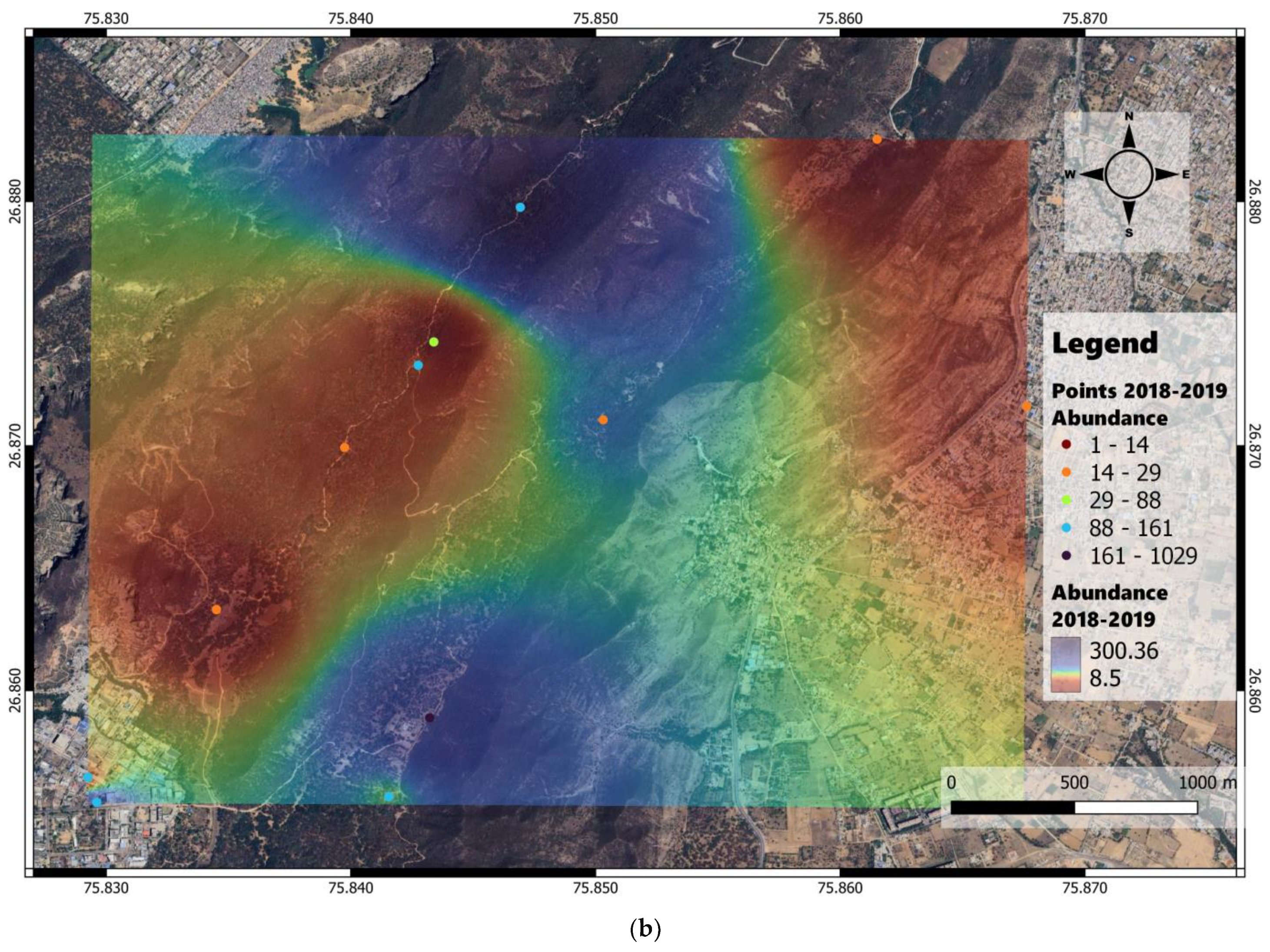
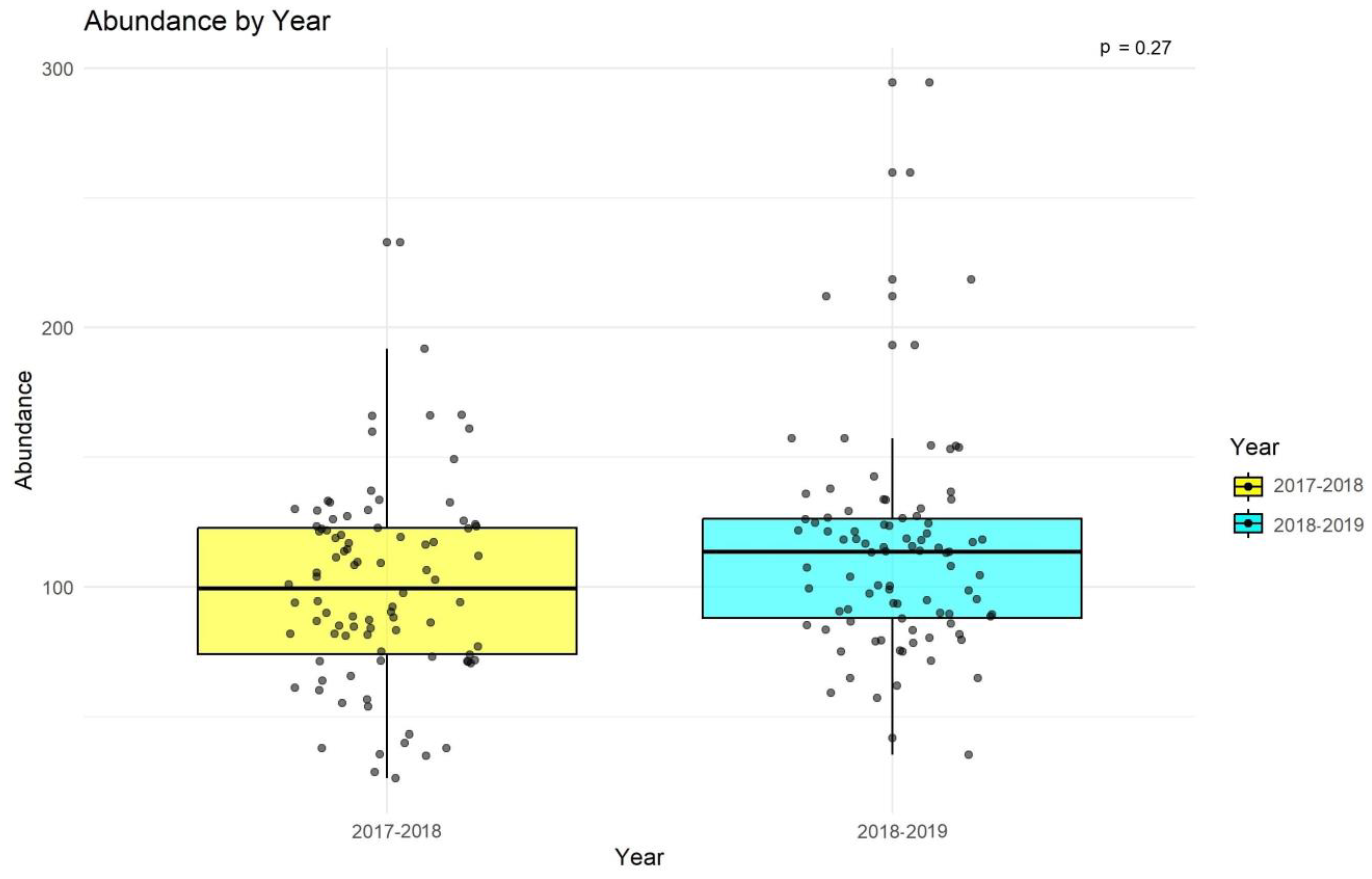
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacKenzie, D.I.; Bailey, L.L.; Hines, J.E.; Nichols, J.D. An integrated model of habitat and species occurrence dynamics. Methods Ecol. Evol. 2011, 2, 612–622. [Google Scholar] [CrossRef]
- Findlay, C.S.; Houlahan, J. Anthropogenic Correlates of Species Richness in Southeastern Ontario Wetlands: Correlativos Antropogénicos de la Riqueza de Especies en Humedales del Sureste de Ontario. Conserv. Biol. 1997, 11, 1000–1009. [Google Scholar] [CrossRef]
- Gurd, D.B.; Nudds, T.D.; Rivard, D.H. Conservation of mammals in eastern North American wildlife reserves: How small is too small? Conserv. Biol. 2001, 15, 1355–1363. [Google Scholar] [CrossRef]
- Caughley, G. Analysis of Vertebrate Populations; John Wiley: Chichester, UK, 1977. [Google Scholar]
- O’Brien, T.G.; Kinnaird, M.F. A picture is worth a thousand words: The application of camera trapping to the study of birds. Bird Conserv. Int. 2008, 18, S144–S162. [Google Scholar] [CrossRef]
- Balme, G.A.; Hunter, L.T.B.; Slotow, R. Evaluating methods for counting cryptic carnivores. J. Wildl. Manag. 2009, 73, 433–441. [Google Scholar] [CrossRef]
- Harihar, A.; Pandav, B.; Goyal, S.P. Density of leopards (Panthera pardus) in the Chilla Range of Rajaji National Park, Uttarakhand, India. Mammalia 2009, 73, 68–71. [Google Scholar] [CrossRef]
- Harihar, A.; Pandav, B.; Goyal, S.P. Responses of leopard Panthera pardus to the recovery of a tiger Panthera tigris population. J. Appl. Ecol. 2011, 48, 806–814. [Google Scholar] [CrossRef]
- Harihar, A.; Ghosh, M.; Fernandes, M.; Pandav, B.; Goyal, S.P. Use of photographic capture-recapture sampling to estimate density of Striped Hyena (Hyaena hyaena): Implications for conservation. Mammalia 2010, 74, 83–87. [Google Scholar] [CrossRef]
- Gupta, S.; Mondal, K.; Sankar, K.; Qureshi, Q. Estimation of striped hyena Hyaena hyaena population using camera traps in Sariska Tiger Reserve, Rajasthan, India. J. Bombay Nat. Hist. Soc. 2009, 106, 284. [Google Scholar]
- Singh, P.; Gopalaswamy, A.M.; Karanth, K.U. Factors influencing densities of striped hyenas (Hyaena hyaena) in arid regions of India. J. Mammal 2010, 91, 1152–1159. [Google Scholar] [CrossRef]
- Hadad, E.; Balaban, A.; Yosef, R. Alloparenting by helpers in striped hyena (Hyaena hyaena). Animals 2023, 13, 1914. [Google Scholar] [CrossRef] [PubMed]
- Karanth, K.U.; Nichols, J.D. Estimation of tiger densities in India using photographic captures and recaptures. Ecololgy 1998, 79, 2852–2862. [Google Scholar] [CrossRef]
- Karanth, K.U.; Nichols, J.D.; Kumar, N.S.; Hines, J.E. Assessing tiger population dynamics using photographic capture–recapture sampling. Ecology 2006, 87, 2925–2937. [Google Scholar] [CrossRef]
- Janečka, J.E.; Munkhtsog, B.; Jackson, R.M.; Naranbaatar, G.; Mallon, D.P.; Murphy, W.J. Comparison of noninvasive genetic and camera-trapping techniques for surveying snow leopards. J. Mammal. 2011, 92, 771–783. [Google Scholar] [CrossRef]
- Soisalo, M.K.; Cavalcanti, S.M. Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture–recapture sampling in combination with GPS radio-telemetry. Biol. Conserv. 2006, 129, 487–496. [Google Scholar] [CrossRef]
- Martyr, D. Important findings by FFI team in Kerinci Seblat, Sumatra, Indonesia. Oryx 1997, 31, 80–82. [Google Scholar]
- Zetra, B.; Rafiastanto, A.; Rombang, W.M.; Trainor, C.R. Rediscovery of the critically endangered Sumatran Ground Cuckoo Carpococcyx viridis. Forktail 2002, 18, 63–66. [Google Scholar]
- Dinata, Y.; Nugroho, A.; Haidir, I.A.; Linkie, M. Camera trapping rare and threatened avifauna in west-central Sumatra. Bird Conserv. Int. 2008, 18, 30–37. [Google Scholar] [CrossRef]
- Ontiveros, Y.; Cappa, F.M.; Andino, N.; Campos, C.M.; Borghi, C.E.; Giannoni, S.M. Mammal and bird diversity in a system of protected areas in Argentina. Biodiversity 2022, 23, 61–71. [Google Scholar] [CrossRef]
- Trofino-Falasco, C.; Simoy, M.V.; Aranguren, M.F.; Pizzarello, M.G.; Cortelezzi, A.; Vera, D.G.; Simoy, M.I.; Marinelli, C.B.; Cepeda, R.E.; Di Giacomo, A.S.; et al. How effective is camera trapping in monitoring grassland species in the southern Pampas ecoregion? Rev. Mex. Biodivers. 2023, 94, e945243. [Google Scholar] [CrossRef]
- Kumbhojkar, S.; Yosef, R.; Mehta, A.; Rakholia, S. A camera-trap home-range analysis of the Indian leopard (Panthera pardus fusca) in Jaipur, India. Animals 2020, 10, 1600. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Sihag, V.; Singh, N.P. Distribution and biocontrol potential of chosen spiders. J. Biopestic. 2009, 2, 151–155. [Google Scholar] [CrossRef]
- Reena, M.; Ram, M.B. Rate of takeovers in groups of Hanuman langurs (Presbytis entellus) at Jaipur. Folia Primatol. 1992, 58, 61–71. [Google Scholar] [CrossRef]
- Sultana, A.; Hussain, M.S.; Rathore, D.K. Diversity of tree vegetation of Rajasthan, India. Trop. Ecol. 2014, 55, 403–410. [Google Scholar]
- Yosef, R.; Rakholia, S.; Mehta, A.; Bhatt, A.; Kumbhojkar, S. Land Surface Temperature Regulation Ecosystem Service: A Case Study of Jaipur, India, and the Urban Island of Jhalana Reserve Forest. Forests 2022, 13, 1101. [Google Scholar] [CrossRef]
- Kumbhojkar, S.; Yosef, R.; Benedetti, Y.; Morelli, F. Human-leopard (Panthera pardus fusca) co-existence in Jhalana Forest Reserve, India. Sustainability 2019, 11, 3912. [Google Scholar] [CrossRef]
- Kumbhojkar, S.; Yosef, R.; Kosicki, J.Z.; Kwiatkowska, P.K.; Tryjanowski, P. Dependence of the leopard Panthera pardus fusca in Jaipur, India, on domestic animals. Oryx 2021, 55, 692–698. [Google Scholar] [CrossRef]
- Kumbhojkar, S.; Yosef, R.; Mehta, A.; Rakholia, S. Ecosystem services of Leopards (Panthera pardus fusca) to the conurbation of Jaipur, India. In Ecology of Tropical Cities: Nature and Social Sciences Applied to the Conservation of Urban Biodiversity; Angeoletto, F., Tryjanowski, P., Fellowes, K., Eds.; Springer Nature: Berlin, Germany, 2024; Chapter 33. [Google Scholar]
- Yosef, R.; Dabi, H.; Kumbhojkar, S. Thanatological behavior of a female Leopard (Panthera pardus fusca). Acta Ethologica 2021, 24, 137–140. [Google Scholar] [CrossRef]
- Yosef, R.; Kumbhojkar, S.; Sharma, S.; Morelli, F. Electric vehicles minimize disturbance to mammals. Eur. J. Wildl. Res. 2021, 67, 74. [Google Scholar] [CrossRef]
- Yosef, R.; Kumbhojkar, S.; Gurjar, B.; Kosicki, J.Z. Magnetic alignment in free-ranging Indian Leopard (Panthera pardus fusca). PLoS ONE 2022, 17, e0266129. [Google Scholar] [CrossRef]
- Larrucea, E.S.; Brussard, P.F.; Jaeger, M.M.; Barrett, R.H. Cameras, coyotes, and the assumption of equal detectability. J. Wildl. Manag. 2007, 71, 1682–1689. [Google Scholar] [CrossRef]
- Tarugara, A.; Clegg, B.W.; Gandiwa, E.; Muposhi, V.K. Cost-benefit analysis of increasing sampling effort in a baited-camera trap survey of an African leopard (Panthera pardus) population. Glob. Ecol. Conserv. 2019, 18, e00627. [Google Scholar] [CrossRef]
- Treves, A.; Mwima, P.; Plumptre, A.J.; Isoke, S. Camera-trapping forest–woodland wildlife of western Uganda reveals how gregariousness biases estimates of relative abundance and distribution. Biol. Conserv. 2010, 143, 521–528. [Google Scholar] [CrossRef]
- Stein, A.B.; Gerngross, P.; Al Hikmani, H.; Balme, G.; Bertola, L.; Drouilly, M.; Farhadinia, M.S.; Feng, L.; Ghoddousi, A.; Henschel, P.; et al. Panthera pardus. The IUCN Red List of Threatened Species 2024:E.T15954A254576956. Available online: https://www.iucnredlist.org/species/15954/254576956 (accessed on 17 July 2024).
- Athreya, V.; Odden, M.; Linnell, J.D.C.; Krishnaswamy, J.; Karanth, U. Big cats in our backyards: Persistence of large carnivores in a human dominated landscape in India. PLoS ONE 2013, 8, e57872. [Google Scholar] [CrossRef]
- Karanth, K.U.; Sunquist, M.E. Prey selection by tiger, leopard and dhole in tropical forests. J. Anim. Ecol. 1995, 64, 439–450. [Google Scholar] [CrossRef]
- Reddy, C.S.; Yosef, R.; Calvi, G.; Fornasari, L. Inter-specific competition influences apex predator–prey populations. Wildl. Res. 2019, 46, 628–638. [Google Scholar] [CrossRef]
- Kalle, R.; Ramesh, T.; Qureshi, Q.; Sankar, K. Density of tiger and leopard in a tropical deciduous forest of Mudumalai Tiger Reserve, southern India, as estimated using photographic capture–recapture sampling. Acta Theriol. 2011, 56, 335–342. [Google Scholar] [CrossRef]
- Hiby, L.R.; Reece, J.F.; Wright, R.; Jaisinghani, R.; Singh, B.; Hiby, E.F. A mark-resight survey method to estimate the roaming dog population in three cities in Rajasthan, India. BMC Vet. Res. 2011, 7, 46. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Goyal, S.P. A Study on Distribution, Relative Abundance and Food Habits of Leopard (Panthera pardus) in Garhwal Himalayas; Wildlife Institute of India: Dehradun, India, 2000. [Google Scholar]
- Vijayan, S.; Pati, B.P. Impact of changing cropping patterns on man-animal conflicts around Gir Protected Area with specific reference to Talala Sub-District, Gujarat, India. Popul. Environ. 2002, 23, 541–559. [Google Scholar] [CrossRef]
- Breitenmoser, U.; Angst, C.; Landry, J.M.; Breitenmoser-Wursten, C.; Linnell, J.D.C. Non-lethal techniques for reducing depredation. In People and Wildlife. Conflict or Co-Existence? Woodroffe, R., Thirgood, S., Rabinowitz, A., Eds.; Cambridge University Press: New York, NY, USA, 2005; pp. 49–71. [Google Scholar]
- Lott, D.F.; McCoy, M. Asian rhinos Rhinoceros unicornis on the run? Impact of tourist visits on one population. Biol. Conserv. 1995, 73, 23–26. [Google Scholar] [CrossRef]
- Fowler, G.S. Behavioral and hormonal responses of Magellanic penguins (Spheniscus magellanicus) to tourism and nest site visitation. Biol. Conserv. 1999, 90, 143–149. [Google Scholar] [CrossRef]
- Romero, L.M.; Wikelski, M. Exposure to tourism reduces stress-induced corticosterone levels in Galapagos marine iguanas. Biol. Conserv. 2002, 108, 371–374. [Google Scholar] [CrossRef]
- Lacy, K.E.; Martins, E.P. The effect of anthropogenic habitat usage on the social behaviour of a vulnerable species, Cyclura nubila. Anim. Conserv. 2003, 6, 3–9. [Google Scholar] [CrossRef]
- Jones, J.P.G. Monitoring species abundance and distribution at the landscape scale. J. Appl. Ecol. 2011, 48, 9–13. [Google Scholar] [CrossRef]
- Gray, T.N.; Phan, C.; Pin, C.; Prum, S. Establishing a monitoring baseline for threatened large ungulates in eastern Cambodia. Wildl. Biol. 2012, 18, 406–413. [Google Scholar] [CrossRef]




| Common Name | Scientific Name | Family | N 2017_2018 | N 2018_2019 | Relative Abundance 2017_2018 | Relative Abundance 2018_2019 |
|---|---|---|---|---|---|---|
| Birds | ||||||
| Indian Peafowl * | Pavo cristatus | Phasianidae | 3047 | 1579 | 24.62 | 39.94 |
| Jungle Babblers * | Turdoides striata | Leothricidae | 9 | 334 | 0.07 | 8.45 |
| Rufous Treepie * | Dendrocitta vagabunda | Corvidae | 3 | 310 | 0.02 | 7.84 |
| Red-vented Bulbul * | Pycnonotus cafer | Pycnonotidae | 3 | 308 | 0.02 | 7.79 |
| Red-wattled Lapwing | Vanellus indicus | Charadriiidae | 1 | 62 | 0.01 | 1.57 |
| Blue Rock Pigeon * | Columba livia | Columbidae | 3 | 64 | 0.02 | 1.62 |
| Grey Francolin | Francolinus pondicerianus | Phasianidae | 8 | 10 | 0.06 | 0.25 |
| Cattle Egret | Bubulcus ibis | Ardeidae | 7 | 10 | 0.06 | 0.25 |
| Eurasian Collared Dove | Streptopelia decaocto | Columbidae | 0 | 7 | 0.00 | 0.18 |
| Greater Coucal | Centropus sinensis | Cuculidae | 4 | 5 | 0.03 | 0.13 |
| Shikra | Accipiter badius | Accipitridae | 2 | 5 | 0.02 | 0.13 |
| White-throated Kingfisher | Halcyon smymensis | Alcedinidae | 19 | 2 | 0.15 | 0.05 |
| Brahminy Starling * | Sturnia pagodarum | Sturnidae | 0 | 1 | 0.00 | 0.03 |
| Cinerous Tit | Parus cinereus | Paridae | 0 | 1 | 0.00 | 0.03 |
| Black Drongo | Dicrurus macrocercus | Dicruridae | 0 | 1 | 0.00 | 0.03 |
| Jacobin Cuckoo | Clamator jacobinus | Cuculidae | 0 | 1 | 0.00 | 0.03 |
| Indian Eagle Owl | Bubo bengalensis | Strigidae | 2 | 1 | 0.02 | 0.03 |
| Long-legged Buzzard | Buteo rufinus | Accipitridae | 4 | 0 | 0.03 | 0.00 |
| Mammals | ||||||
| Nilgai * | Boselaphus tragocamelus | Bovidae | 3377 | 622 | 27.29 | 15.73 |
| Indian Leopard | Panthera pardus fusca | Felidae | 473 | 188 | 3.82 | 4.76 |
| Small Indian Civet | Viverricula indica | Viverridae | 10 | 100 | 0.08 | 2.53 |
| Indian Crested Porcupine | Hystrix indica | Hystricidae | 178 | 60 | 1.44 | 1.52 |
| Bengal Fox | Vulpes bengalensis | Canidae | 295 | 48 | 2.38 | 1.21 |
| Striped Hyena | Hyaena hyaena | Hyaenidae | 328 | 30 | 2.65 | 0.76 |
| Black-naped Hare | Lepus nigricollis | Leporidae | 427 | 30 | 3.45 | 0.76 |
| Golden Jackal | Canius aureus | Canidae | 0 | 7 | 0.00 | 0.18 |
| Sambar Deer * | Rusa unicolor | Cervidae | 7 | 6 | 0.06 | 0.15 |
| Indian Mongoose | Herepestes edwardsii | Herpestidae | 8 | 5 | 0.06 | 0.13 |
| Indian Hedgehog | Paraechinus micropus | Erinaceidae | 1 | 1 | 0.01 | 0.03 |
| Spotted Deer * | Axis axis | Cervidae | 157 | 0 | 1.27 | 0.00 |
| Gray Langur | Semnopithecus | Pteropodidae | 1 | 0 | 0.01 | 0.00 |
| Fruit Bat | Pteropus giganteus | Pteropodidae | 1 | 0 | 0.01 | 0.00 |
| Domestic Animals | ||||||
| Cat | Felis catus | Felidae | 2 | 0 | 0.02 | 0.00 |
| Cattle | Bos taurus | Bovidae | 449 | 0 | 3.63 | 0.00 |
| Goats | Capra aegagrus hircus | Bovidae | 48 | 0 | 0.39 | 0.00 |
| Horse | Equus caballus | Equidae | 18 | 0 | 0.15 | 0.00 |
| Feral Dog | Canis lupus familiaris | Canidae | 10 | 0 | 0.08 | 0.00 |
| Domestic Pig | Sus scrofa domesticus | Suidae | 2 | 0 | 0.02 | 0.00 |
| Human | ||||||
| Humans * | Homo sapiens | Hominoidea | 3470 | 155 | 28.04 | 3.92 |
| Total | 12,374 | 3953 | 100 | 100 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumbhojkar, S.; Mahabal, A.; Rakholia, S.; Yosef, R. Avian and Mammalian Diversity and Abundance in Jhalana Reserve Forest, Jaipur, India. Animals 2024, 14, 2939. https://doi.org/10.3390/ani14202939
Kumbhojkar S, Mahabal A, Rakholia S, Yosef R. Avian and Mammalian Diversity and Abundance in Jhalana Reserve Forest, Jaipur, India. Animals. 2024; 14(20):2939. https://doi.org/10.3390/ani14202939
Chicago/Turabian StyleKumbhojkar, Swapnil, Anil Mahabal, Shrey Rakholia, and Reuven Yosef. 2024. "Avian and Mammalian Diversity and Abundance in Jhalana Reserve Forest, Jaipur, India" Animals 14, no. 20: 2939. https://doi.org/10.3390/ani14202939
APA StyleKumbhojkar, S., Mahabal, A., Rakholia, S., & Yosef, R. (2024). Avian and Mammalian Diversity and Abundance in Jhalana Reserve Forest, Jaipur, India. Animals, 14(20), 2939. https://doi.org/10.3390/ani14202939








