Immune Cells in the Spleen of Mice Mediate the Inflammatory Response Induced by Mannheimia haemolytica A2 Serotype
Abstract
Simple Summary
Abstract
1. Introduction
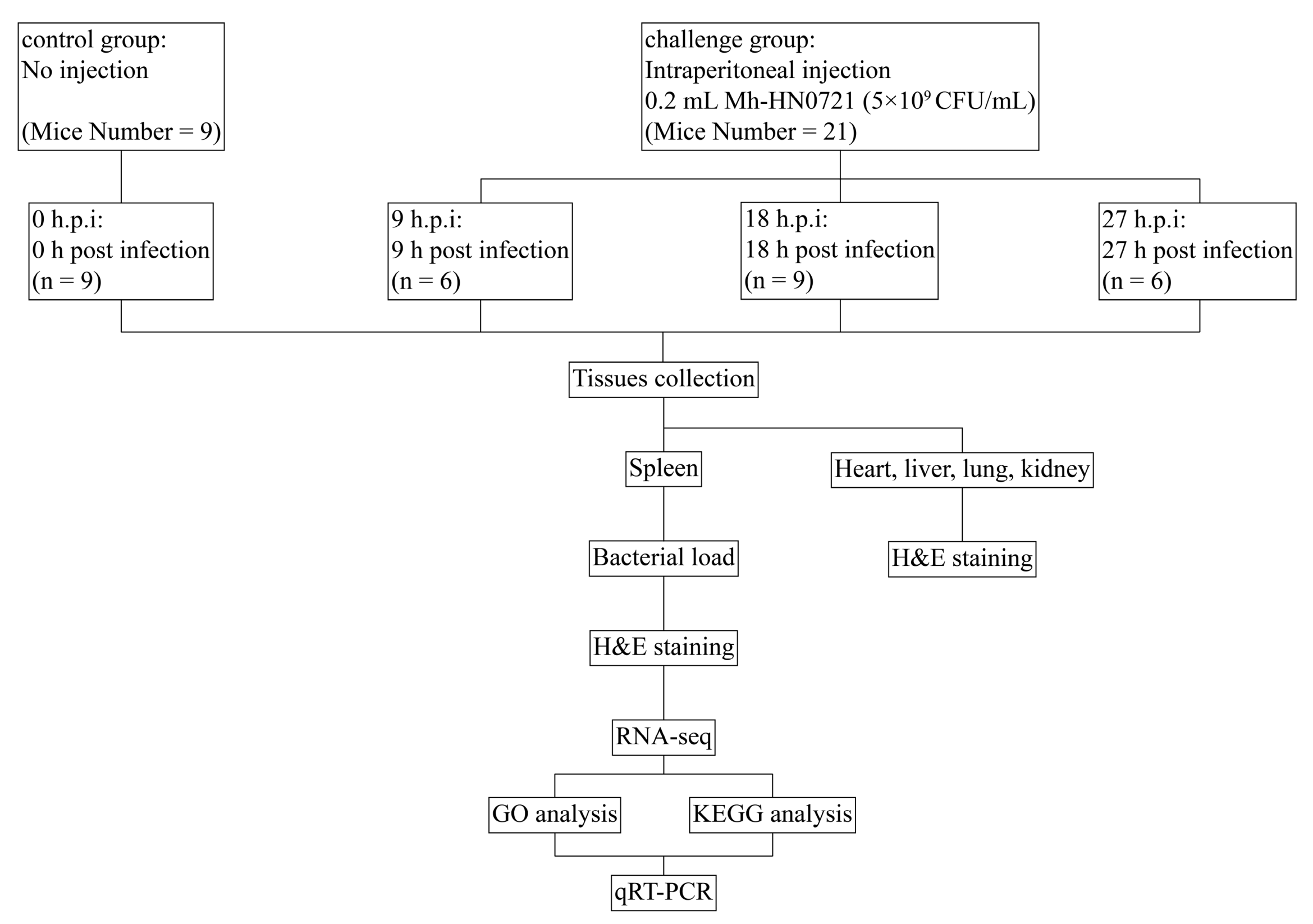
2. Materials and Methods
2.1. Ethical Review
2.2. Bacteria Source and Serotypes Identification
2.3. Experimental Animal
2.4. Culture and Pathogenicity of Mh-HN0721
2.5. Bacterial Load of Mh-HN0721 in Mice Spleen
2.6. Histopathological Examination of the Spleen
2.7. RNA Preparation, Library Construction, and Sequencing
2.8. Identification and Analysis of DEGs
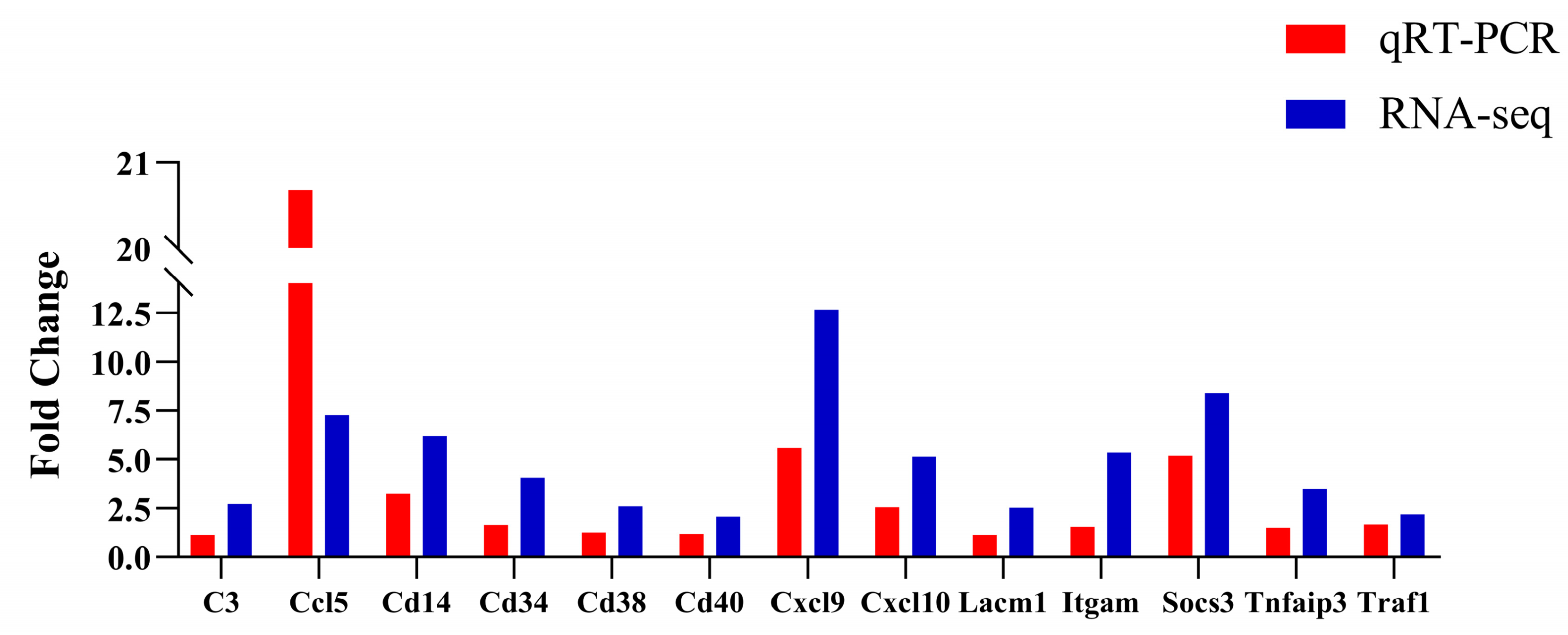
2.9. Verification of DEGs by qRT-PCR
3. Results
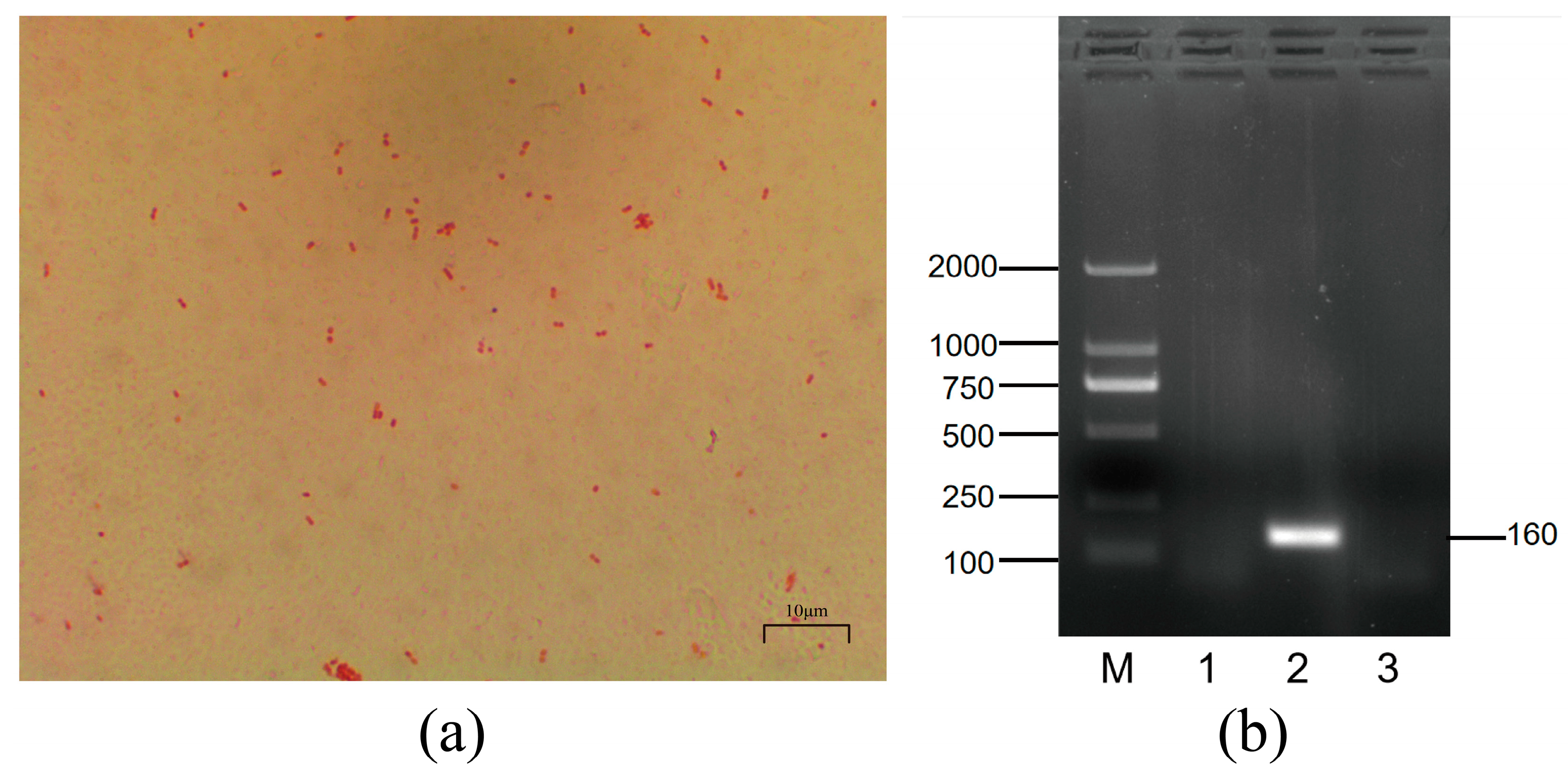
3.1. Morphological Characterization and Serotyping of Mh-HN0721
3.2. Growth Trends of Mh-HN0721
3.3. Pathogenicity and Bacterial Load in Mice Spleen
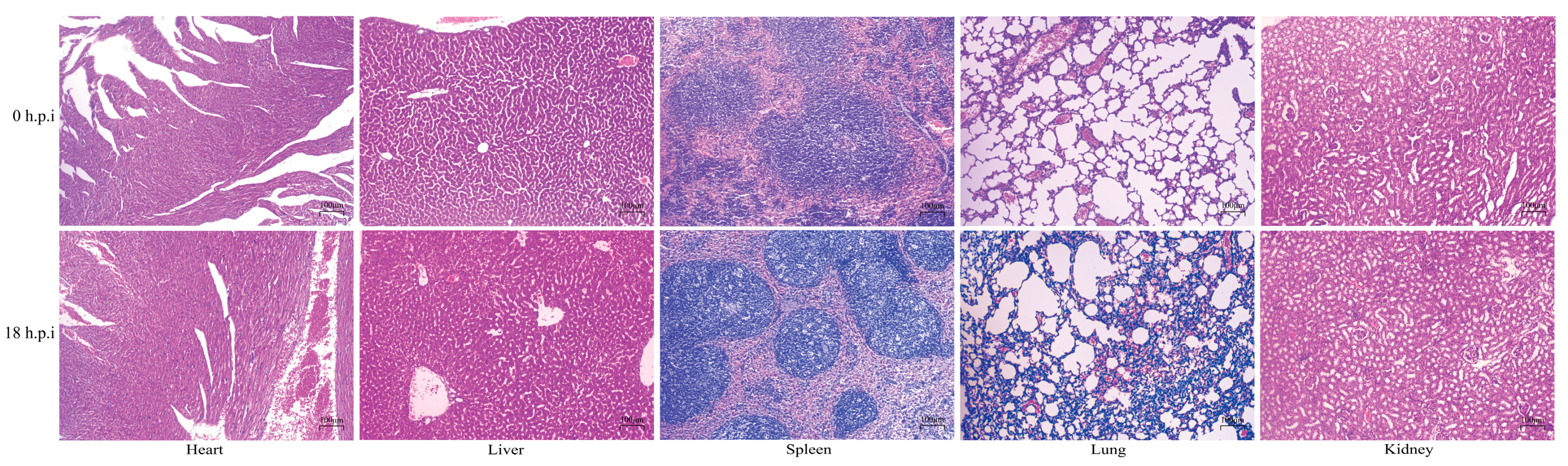
3.4. Histopathological Observations in Mice Organs
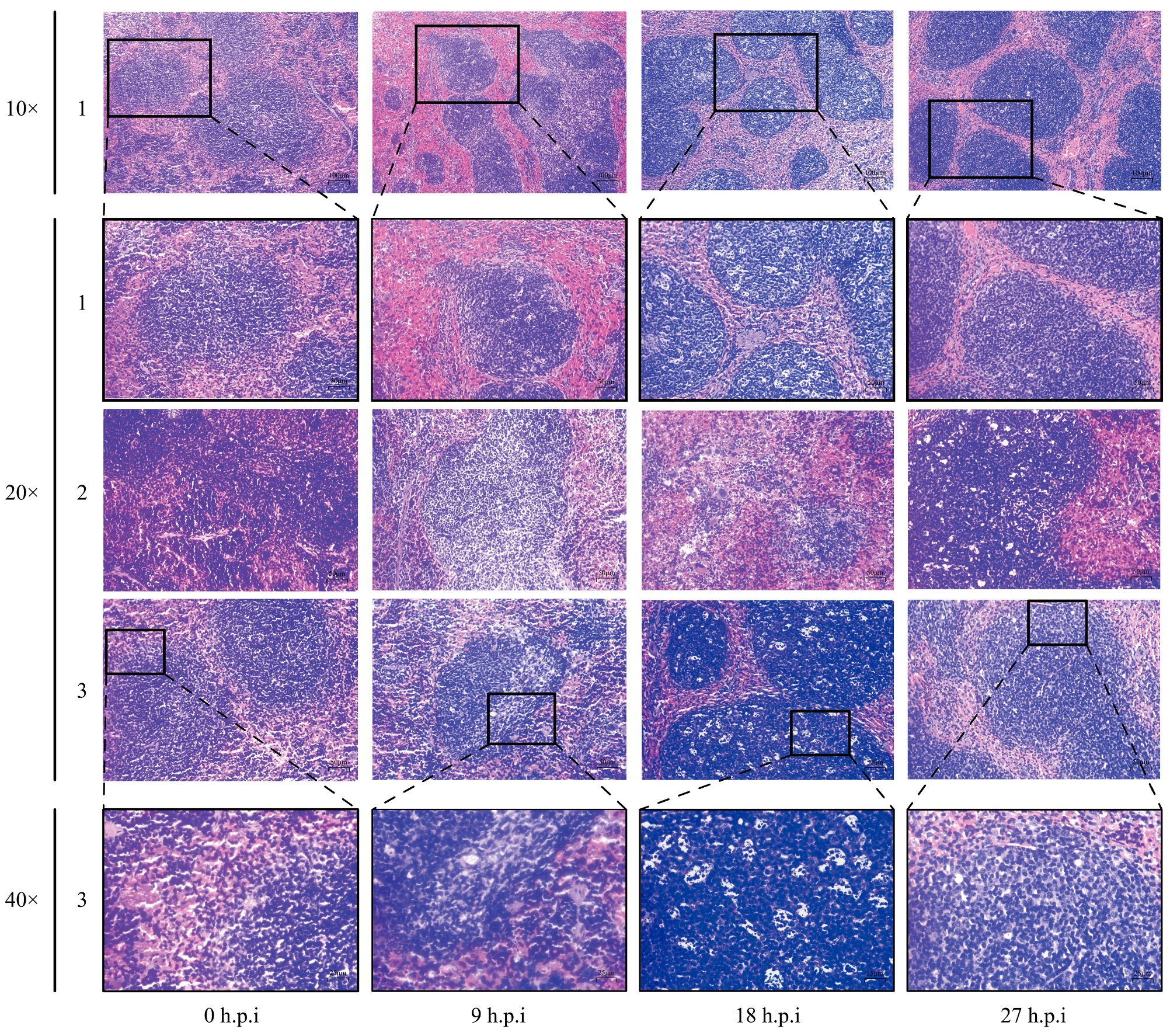
3.5. Histopathological Changes in Mice Spleen
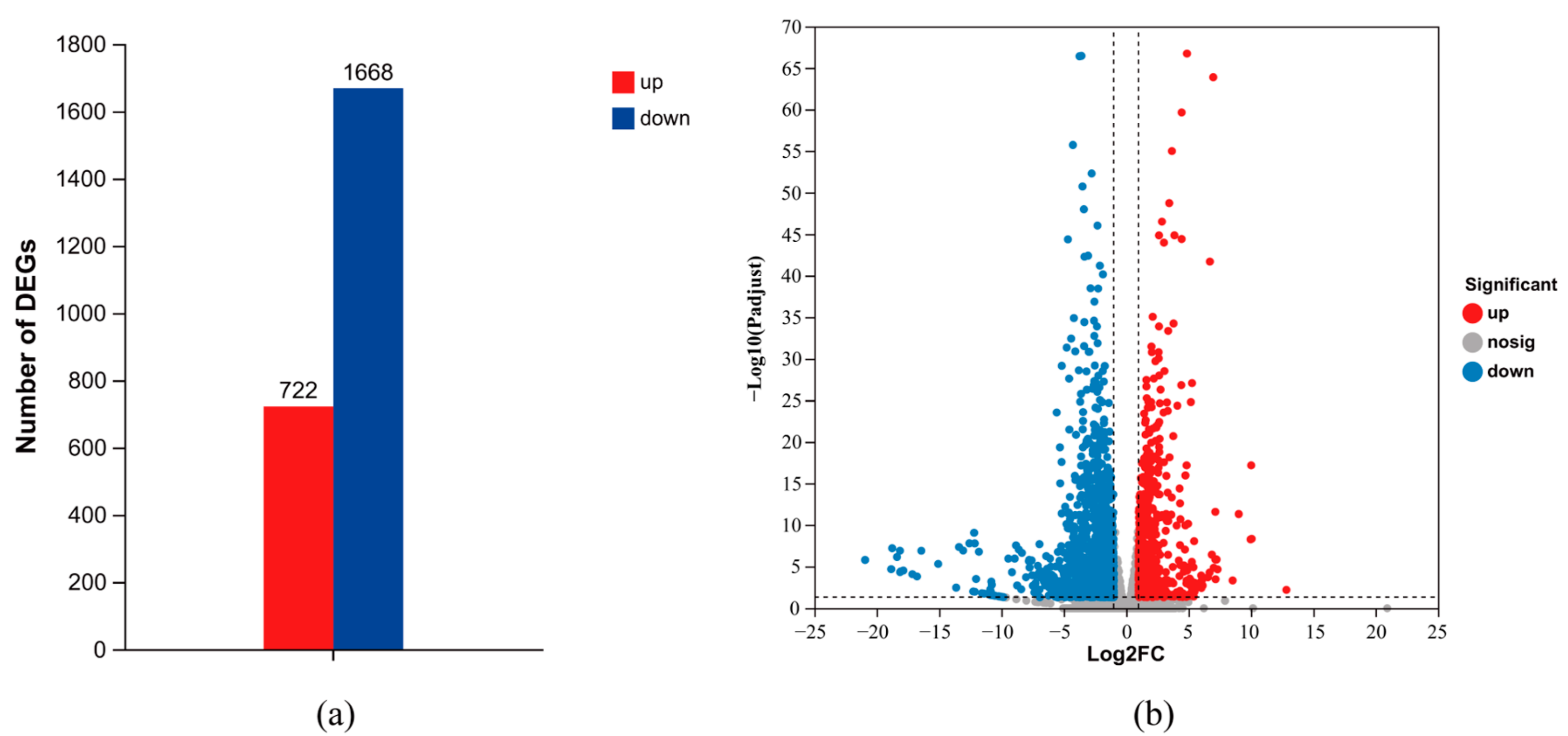
3.6. M. haemolytica Identification and RNA-Seq Analysis
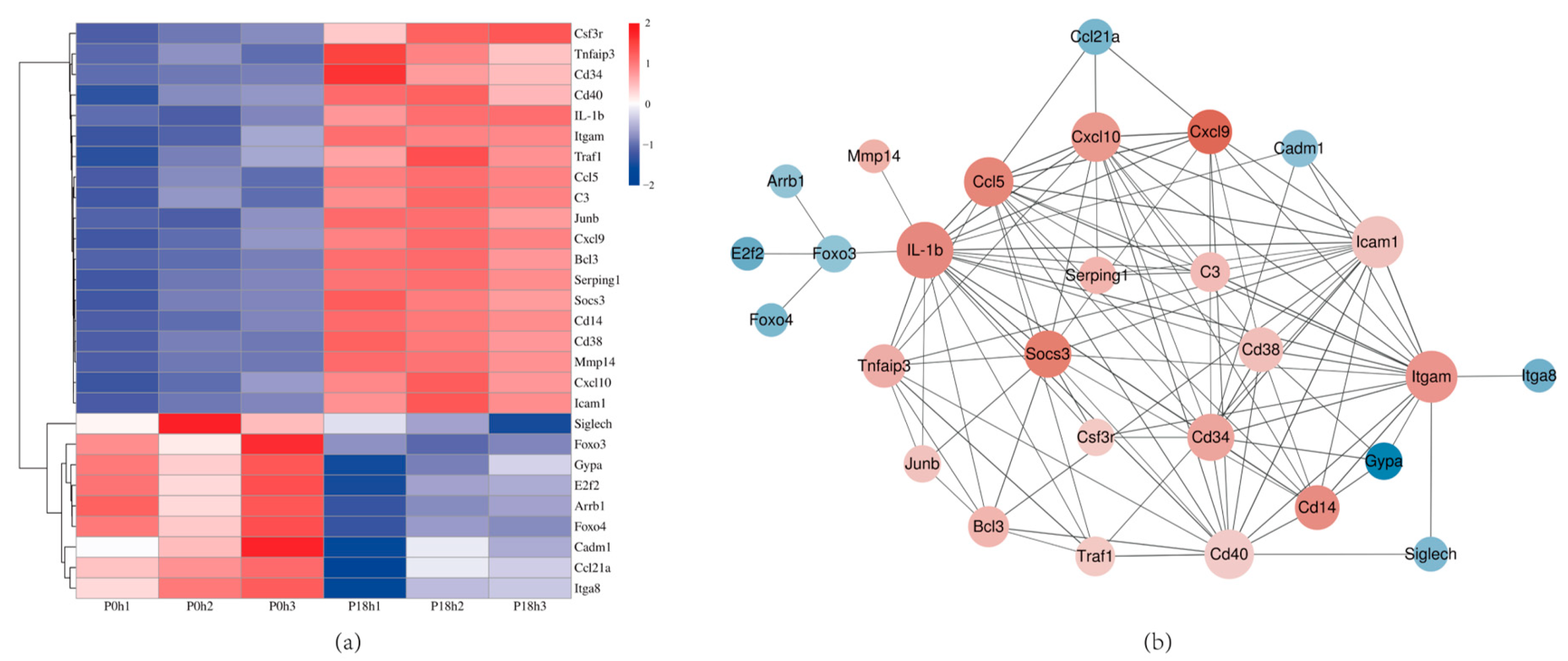
3.7. GO and KEGG Enrichment Analysis of DEGs
3.8. Validation of DEGs by qRT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mombeni, E.G.; Gharibi, D.; Ghorbanpoor, M.; Jabbari, A.R.; Cid, D. Molecular characterization of Mannheimia haemolytica associated with ovine and caprine pneumonic lung lesions. Microb. Pathog. 2021, 153, 104791. [Google Scholar] [CrossRef] [PubMed]
- Blackall, P.J.; Bojesen, A.M.; Christensen, H.; Bisgaard, M. Reclassification of Pasteurella trehalosi as Bibersteinia trehalosi gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 2007, 57, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, R.P.; Clawson, M.L.; Tatum, F.M.; Briggs, R.E.; Kaplan, B.S.; Casas, E. Differential identification of Mannheimia haemolytica genotypes 1 and 2 using colorimetric loop-mediated isothermal amplification. BMC Res. Notes 2023, 16, 4. [Google Scholar] [CrossRef] [PubMed]
- Santos-Rivera, M.; Woolums, A.; Thoresen, M.; Blair, E.; Jefferson, V.; Meyer, F.; Vance, C.K. Profiling Mannheimia haemolytica infection in dairy calves using near infrared spectroscopy (NIRS) and multivariate analysis (MVA). Sci. Rep. 2021, 11, 1392. [Google Scholar] [CrossRef]
- Dao, X.F.; Hung, C.C.; Yang, Y.W.; Wang, J.; Yang, F.L. Development and validation of an insulated isothermal PCR assay for the rapid detection of Mannheimia haemolytica. J. Vet. Diagn. Investig. 2022, 34, 302–305. [Google Scholar] [CrossRef]
- Prysliak, T.; Vulikh, K.; Caswell, J.L.; Perez-Casal, J. Mannheimia haemolytica increases Mycoplasma bovis disease in a bovine experimental model of BRD. Vet. Microbiol. 2023, 283, 109793. [Google Scholar] [CrossRef]
- Griffin, D.; Chengappa, M.M.; Kuszak, J.; McVey, D.S. Bacterial Pathogens of the Bovine Respiratory Disease Complex. Vet. Clin. North Am. Food Anim. Pract. 2010, 26, 381–394. [Google Scholar] [CrossRef]
- Angen, O.; Mutters, R.; Caugant, D.A.; Olsen, J.E.; Bisgaard, M. Taxonomic relationships of the Pasteurella haemolytica complex as evaluated by DNA-DNA hybridizations and 16S rRNA sequencing with proposal of Mannheimia haemolytica gen. nov., comb. nov., Mannheimia granulomatis comb. nov., Mannheimia glucosida sp. nov., Mannheimia ruminalis sp. nov. and Mannheimia varigena sp. nov. Int. J. Syst. Bacteriol. 1999, 49 Pt 1, 67–86. [Google Scholar] [CrossRef]
- Mason, C.; Errington, J.; Foster, G.; Thacker, J.; Grace, O.; Baxter-Smith, K. Mannheimia haemolytica serovars associated with respiratory disease in cattle in Great Britain. BMC Vet. Res. 2022, 18, 5. [Google Scholar] [CrossRef]
- Boorei, M.A.; Paul, B.T.; Jesse, F.F.A.; Chung, E.L.T.; Lila, M.A.M. Responses of selected biomarkers, female reproductive hormones and tissue changes in non-pregnant does challenged with Mannheimia haemolytica serotype A2 and its outer membrane protein (OMP) immunogen. Microb. Pathog. 2022, 169, 105674. [Google Scholar] [CrossRef]
- Avalos-Gomez, C.; Reyes-Lopez, M.; Ramirez-Rico, G.; Diaz-Aparicio, E.; Zenteno, E.; Gonzalez-Ruiz, C.; de la Garza, M. Effect of apo-lactoferrin on leukotoxin and outer membrane vesicles of Mannheimia haemolytica A2. Vet. Res. 2020, 51, 36. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.R.; Brogden, K.A. Response of the ruminant respiratory tract to Mannheimia (Pasteurella) haemolytica. Microbes Infect. 2000, 2, 1079–1088. [Google Scholar] [CrossRef]
- Christensen, H.; Bisgaard, M.; Menke, T.; Liman, M.; Timsit, E.; Foster, G.; Olsen, J.E. Prediction of Mannheimia haemolytica serotypes based on whole genomic sequences. Vet. Microbiol. 2021, 262, 109232. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, A.; Francisco Fernandez-Garayzabal, J.; Chaves, F.; Pinto, C.; Cid, D. Ovine Mannheimia haemolytica isolates from lungs with and without pneumonic lesions belong to similar genotypes. Vet. Microbiol. 2018, 219, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Ritchey, J.W.; Confer, A.W. Mannheimia haemolytica: Bacterial-Host Interactions in Bovine Pneumonia. Vet. Pathol. 2011, 48, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, G.; Tariku, S.; Ayelet, G.; Abebe, Z. Contagious caprine pleuropneumonia and Mannheimia haemolytica-associated acute respiratory disease of goats and sheep in Afar Region, Ethiopia. Rev. Sci. Tech. (Int. Off. Epizoot.) 2006, 25, 1153–1163. [Google Scholar] [CrossRef]
- Katsafadou, A.I.; Tsangaris, G.T.; Anagnostopoulos, A.K.; Billinis, C.; Barbagianni, M.S.; Vasileiou, N.G.C.; Spanos, S.A.; Mavrogianni, V.S.; Fthenakis, G.C. Differential quantitative proteomics study of experimental Mannheimia haemolytica mastitis in sheep. J. Proteom. 2019, 205, 103393. [Google Scholar] [CrossRef]
- Azhar, N.A.; Paul, B.T.; Jesse, F.F.A.; Chung, E.L.T.; Isa, K.M.; Lila, M.A.M.; Haron, A.W. Responses of testosterone hormone and important inflammatory cytokines in bucks after challenge with Mannheimia haemolytica A2 and its LPS endotoxin. Trop. Anim. Health Prod. 2021, 53, 242. [Google Scholar] [CrossRef]
- Kamarulrizal, M.I.; Chung, E.L.T.; Jesse, F.F.A.; Paul, B.T.; Azhar, A.N.; Lila, M.A.M.; Salleh, A.; Abba, Y.; Shamsuddin, M.S. Changes in selected cytokines, acute-phase proteins, gonadal hormones and reproductive organs of non-pregnant does challenged with Mannheimia haemolytica serotype A2 and its LPS endotoxin. Trop. Anim. Health Prod. 2022, 54, 161. [Google Scholar] [CrossRef]
- Lo, R.Y.; McKerral, L.J.; Hills, T.L.; Kostrzynska, M. Analysis of the capsule biosynthetic locus of Mannheimia (Pasteurella) haemolytica A1 and proposal of a nomenclature system. Infect. Immun. 2001, 69, 4458–4464. [Google Scholar] [CrossRef]
- Cai, Y.; Varasteh, S.; van Putten, J.P.M.; Folkerts, G.; Braber, S. Mannheimia haemolytica and lipopolysaccharide induce airway epithelial inflammatory responses in an extensively developed ex vivo calf model. Sci. Rep. 2020, 10, 13042. [Google Scholar] [CrossRef] [PubMed]
- Aulik, N.A.; Hellenbrand, K.M.; Czuprynski, C.J. Mannheimia haemolytica and Its Leukotoxin Cause Macrophage Extracellular Trap Formation by Bovine Macrophages. Infect. Immun. 2012, 80, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Kisiela, D.I.; Czuprynski, C.J. Identification of Mannheimia haemolytica Adhesins Involved in Binding to Bovine Bronchial Epithelial Cells. Infect. Immun. 2009, 77, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Clawson, M.L.; Schuller, G.; Dickey, A.M.; Bono, J.L.; Murray, R.W.; Sweeney, M.T.; Apley, M.D.; DeDonder, K.D.; Capik, S.F.; Larson, R.L.; et al. Differences between predicted outer membrane proteins of genotype 1 and 2 Mannheimia haemolytica. BMC Microbiol. 2020, 20, 250. [Google Scholar] [CrossRef] [PubMed]
- Ramirez Rico, G.; Martinez-Castillo, M.; Gonzalez-Ruiz, C.; Luna-Castro, S.; de la Garza, M. Mannheimia haemolytica A2 secretes different proteases into the culture medium and in outer membrane vesicles. Microb. Pathog. 2017, 113, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, S.; Galapero, J.; Gomez, L.; Perez, C.J.; Cid, D.; Carmen Martin, M.; Manuel Alonso, J.; Rey, J. Mannheimia haemolytica and Bibersteinia trehalosi Serotypes Isolated from Merino Breed Lambs in Extremadura (Southwestern Spain). Indian J. Microbiol. 2016, 56, 513–515. [Google Scholar] [CrossRef]
- Zecchinon, L.; Fett, T.; Desmecht, D. How Mannheimia haemolytica defeats host defence through a kiss of death mechanism. Vet. Res. 2005, 36, 133–156. [Google Scholar] [CrossRef]
- Klima, C.L.; Zaheer, R.; Briggs, R.E.; McAllister, T.A. A multiplex PCR assay for molecular capsular serotyping of Mannheimia haemolytica serotypes 1, 2, and 6. J. Microbiol. Methods 2017, 139, 155–160. [Google Scholar] [CrossRef]
- Molaee, H.; Tahamtan, Y.; Saeednezhad, E.; Hayati, M. Isolation of the various serotypes of Mannheimia haemolytica and preparation of the first vaccine candidate in Iran. Mol. Biol. Rep. 2022, 49, 10367–10375. [Google Scholar] [CrossRef]
- Bonnet, A.; Bevilacqua, C.; Benne, F.; Bodin, L.; Cotinot, C.; Liaubet, L.; Sancristobal, M.; Sarry, J.; Terenina, E.; Martin, P.; et al. Transcriptome profiling of sheep granulosa cells and oocytes during early follicular development obtained by Laser Capture Microdissection. BMC Genom. 2011, 12, 417. [Google Scholar] [CrossRef]
- Kimura, I.; Yamasoba, D.; Tamura, T.; Nao, N.; Suzuki, T.; Oda, Y.; Mitoma, S.; Ito, J.; Nasser, H.; Zahradnik, J.; et al. Virological characteristics of the SARS-CoV-2 Omicron BA.2 subvariants, including BA.4 and BA.5. Cell 2022, 185, 3992–4007. [Google Scholar] [CrossRef]
- Alexander, T.W.; Cook, S.R.; Yanke, L.J.; Booker, C.W.; Morley, P.S.; Read, R.R.; Gow, S.P.; McAllister, T.A. A multiplex polymerase chain reaction assay for the identification of Mannheimia haemolytica, Mannheimia glucosida and Mannheimia ruminalis. Vet. Microbiol. 2008, 130, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.B.; Poston, M.D.; Brown, S.; Lawson, S.E.; Bond, C.E.; Downs, A.M.; Williams, D.L.; Ozment, T.R. Cholinergic leukocytes in sepsis and at the neuroimmune junction in the spleen. Int. Immunopharmacol. 2020, 81, 106359. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, R.L.; Liang, H.-E.; Bao, K.; Price, A.E.; Mohrs, M.; Kelly, B.L.; Locksley, R.M. A Novel Model for IFN-gamma-Mediated Autoinflammatory Syndromes. J. Immunol. 2015, 194, 2358–2368. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-kappa B pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Speiser, D.E.; Lee, S.Y.; Wong, B.; Arron, J.; Santana, A.; Kong, Y.Y.; Ohashi, P.S.; Choi, Y. A regulatory role for TRAF1 in antigen-induced apoptosis of T cells. J. Exp. Med. 1997, 185, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Wertz, I.E.; O’Rourke, K.M.; Zhou, H.; Eby, M.; Aravind, L.; Seshagiri, S.; Wu, P.; Wiesmann, C.; Baker, R.; Boone, D.L.; et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 2004, 430, 694–699. [Google Scholar] [CrossRef]
- Yoshimura, A.; Naka, T.; Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007, 7, 454–465. [Google Scholar] [CrossRef]
- Hoegl, S.; Bachmann, M.; Scheiermann, P.; Goren, I.; Hofstetter, C.; Pfeilschifter, J.; Zwissler, B.; Muhl, H. Protective Properties of Inhaled IL-22 in a Model of Ventilator-Induced Lung Injury. Am. J. Respir. Cell Mol. Biol. 2011, 44, 369–376. [Google Scholar] [CrossRef]
- Kuna, P.; Alam, R.; Ruta, U.; Gorski, P. RANTES induces nasal mucosal inflammation rich in eosinophils, basophils, and lymphocytes in vivo. Am. J. Respir. Crit. Care Med. 1998, 157, 873–879. [Google Scholar] [CrossRef]
- Palaniappan, R.; Singh, S.; Singh, U.P.; Singh, R.; Ades, E.W.; Briles, D.E.; Hollingshead, S.K.; Royal, W., 3rd; Sampson, J.S.; Stiles, J.K.; et al. CCL5 modulates pneumococcal immunity and carriage. J. Immunol. 2006, 176, 2346–2356. [Google Scholar] [CrossRef] [PubMed]
- Ozga, A.J.; Chow, M.T.; Lopes, M.E.; Servis, R.L.; Di Pilato, M.; Dehio, P.; Lian, J.; Mempel, T.R.; Luster, A.D. CXCL10 chemokine regulates heterogeneity of the CD8(+) T cell response and viral set point during chronic infection. Immunity 2022, 55, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Xu, D.; Li, F.; Huang, C.; Lai, K.; Deng, Z. Intrapulmonary IFN-& gamma; instillation causes chronic lymphocytic inflammation in the spleen and lung through the CXCR3 pathway. Int. Immunopharmacol. 2023, 122, 110675. [Google Scholar] [CrossRef] [PubMed]
- Amat, S.; Timsit, E.; Baines, D.; Yanke, J.; Alexander, T.W. Development of Bacterial Therapeutics against the Bovine Respiratory Pathogen Mannheimia haemolytica. Appl. Environ. Microbiol. 2019, 85, e01359-19. [Google Scholar] [CrossRef] [PubMed]
- Galkina, E.; Ley, K. Immune and Inflammatory Mechanisms of Atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Strukova, S.M. Thrombin as a regulator of inflammation and reparative processes in tissues. Biochemistry. Biokhimiia 2001, 66, 8–18. [Google Scholar] [CrossRef]
- N’Jai, A.U.; Rivera, J.; Atapattu, D.N.; Owusu-Ofori, K.; Czuprynski, C.J. Gene expression profiling of bovine bronchial epithelial cells exposed in vitro to bovine herpesvirus 1 and Mannheimia haemolytica. Vet. Immunol. Immunopathol. 2013, 155, 182–189. [Google Scholar] [CrossRef]
- Bronte, V.; Pittet, M.J. The Spleen in Local and Systemic Regulation of Immunity. Immunity 2013, 39, 806–818. [Google Scholar] [CrossRef]
- AbuSamra, D.B.; Aleisa, F.A.; Al-Amoodi, A.S.; Ahmed, H.M.J.; Chin, C.J.; Abuelela, A.F.; Bergam, P.; Sougrat, R.; Merzaban, J.S. Not just a marker: CD34 on human hematopoietic stem/progenitor cells dominates vascular selectin binding along with CD44. Blood Adv. 2017, 1, 2799–2816. [Google Scholar] [CrossRef]
- Szlasa, W.; Czarny, J.; Sauer, N.; Rakoczy, K.; Szymanska, N.; Stecko, J.; Kolodziej, M.; Kazmierczak, M.; Barg, E. Targeting CD38 in Neoplasms and Non-Cancer Diseases. Cancers 2022, 14, 4169. [Google Scholar] [CrossRef]
- Jimenez-Duran, G.; Luque-Martin, R.; Patel, M.; Koppe, E.; Bernard, S.; Sharp, C.; Buchan, N.; Rea, C.; de Winther, M.P.J.; Turan, N.; et al. Pharmacological validation of targets regulating CD14 during macrophage differentiation. Ebiomedicine 2020, 61, 103039. [Google Scholar] [CrossRef] [PubMed]
- Batista, C.F.; Souza, F.N.; Santos, K.R.; Sanchez, E.M.R.; Reis, L.C.; Bertagnon, H.G.; Blagitz, M.G.; Gomes, R.C.; Lage, A.P.; Heinemann, M.B.; et al. R-Phycoerythrin-labeled Mannheimia haemolytica for the simultaneous measurement of phagocytosis and intracellular reactive oxygen species production in bovine blood and bronchoalveolar lavage cells. Vet. Immunol. Immunopathol. 2018, 196, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, V.; Li, X.B.; Faridi, J.H.M.; Gupta, V. CD11b agonists offer a novel approach for treating lupus nephritis. Transl. Res. 2022, 245, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Retamozo, V.; Etzrodt, M.; Newton, A.; Rauch, P.J.; Chudnovskiy, A.; Berger, C.; Ryan, R.J.H.; Iwamoto, Y.; Marinelli, B.; Gorbatov, R.; et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl. Acad. Sci. USA 2012, 109, 2491–2496. [Google Scholar] [CrossRef]


| Serotype | Primer Sequence (5′–3′) | Product Length (bp) |
|---|---|---|
| A1 | F: CAT TTC CTT AGG TTC AGC R: CAA GTC ATC GTA ATG CCT | 306 |
| A2 | F: GGC ATA TCC TAA AGC CGT R: AGA ATC CAC TAT TGG GCA CC | 160 |
| A6 | F: TGA GAA TTT CGA CAG CAC T R: ACC TTG GCA TAT CGT ACC | 78 |
| Name of Genes | Primer Sequence (5′–3′) | Product Length (bp) |
|---|---|---|
| β-actin | F: GTA CCA CCA TGT ACC CAG GC R: AAC GCA GCT CAG TAA CAG TCC | 247 |
| Cd14 | F: CAC AAT TCA CTG CGG GAT GC R: AGC GAG TTT AGC TGA CTG GG | 65 |
| Itgam | F: CAG GGC AGG AGT CGT ATG TG R: GTC CAT CAG CTT CGG TGT TG | 289 |
| Cxcl10 | F: TCT CTC CAT CAC TCC CCT TTA R: GCT TCG GCA GTT ACT TTT GTC | 151 |
| Traf1 | F: AGC AGA GGG TGG TGG AAT TAC R: ATC ACG ATG AAG AGG GAC AGG | 137 |
| Tnfaip3 | F: TGG TGT CGT GAA GTC AGG AAG R: CAT TCG TCA TTC CAG TTC CG | 251 |
| Icam1 | F: CAC CGT GTA TTC GTT TCC G R: TGA TCT CCT TGG GGT CCT T | 197 |
| Cd34 | F: CCT TCAG GCT CTG GAA CTC R: TAT AGA TGG CAG GCT GGA CTT | 126 |
| Cd38 | F: GTG GTC CAA GTG ATG CTC R: GTC TAC ACG ATG GGT GCT | 286 |
| C3 | F: CAG CAA CGC AAG TTC ATC R: TTC GCA CTG TTT CTG GTA C | 204 |
| Cd40 | F: CCA ATC AAG GGC TTC GGG TTA R: CTG AGC ACA TGC CTC GCA AT | 110 |
| Cxcl9 | F: CGA GGC ACG ATC CAC TAC AA R: AGG CAG GTT TGA TCT CCG TT | 113 |
| Ccl5 | F: AGG AAC CGC CAA GTG TGT G R: CCG AGT GGG AGT AGG GGA TTA | 181 |
| Socs3 | F: AGA GCG GAT TCT ACT GGA GCG R: CTG GAT GCG TAG GTT CTT GGT C | 161 |
| Time (h) | OD600 Average Value | Dilution Factor | Number of Colonies | Estimated Concentration of Stock Solution (CFU/mL) |
|---|---|---|---|---|
| 4 | 0.829 | 1 × 10−6 | 110 | 1.12 × 109 |
| 107 | ||||
| 120 | ||||
| 5.5 | 1.167 | 1 × 10−7 | 28 | 3.20 × 109 |
| 37 | ||||
| 32 | ||||
| 7 | 1.235 | 1 × 10−6 | 35 | 3.90 × 108 |
| 37 | ||||
| 45 | ||||
| 8.5 | 1.221 | 1 × 10−5 | 135 | 1.24 × 108 |
| 111 | ||||
| 125 | ||||
| 10 | 1.211 | 1 × 10−5 | 24 | 2.60 × 107 |
| 22 | ||||
| 32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Z.; Jiang, J.; Meng, Y.; Wu, G.; Tang, J.; Chen, T.; Fu, Y.; Chen, Y.; Zhang, Z.; Gao, H.; et al. Immune Cells in the Spleen of Mice Mediate the Inflammatory Response Induced by Mannheimia haemolytica A2 Serotype. Animals 2024, 14, 317. https://doi.org/10.3390/ani14020317
Jiao Z, Jiang J, Meng Y, Wu G, Tang J, Chen T, Fu Y, Chen Y, Zhang Z, Gao H, et al. Immune Cells in the Spleen of Mice Mediate the Inflammatory Response Induced by Mannheimia haemolytica A2 Serotype. Animals. 2024; 14(2):317. https://doi.org/10.3390/ani14020317
Chicago/Turabian StyleJiao, Zizhuo, Junming Jiang, Yong Meng, Guansheng Wu, Jiayang Tang, Taoyu Chen, Yujing Fu, Yuanyuan Chen, Zhenxing Zhang, Hongyan Gao, and et al. 2024. "Immune Cells in the Spleen of Mice Mediate the Inflammatory Response Induced by Mannheimia haemolytica A2 Serotype" Animals 14, no. 2: 317. https://doi.org/10.3390/ani14020317
APA StyleJiao, Z., Jiang, J., Meng, Y., Wu, G., Tang, J., Chen, T., Fu, Y., Chen, Y., Zhang, Z., Gao, H., Man, C., Chen, Q., Du, L., Wang, F., & Chen, S. (2024). Immune Cells in the Spleen of Mice Mediate the Inflammatory Response Induced by Mannheimia haemolytica A2 Serotype. Animals, 14(2), 317. https://doi.org/10.3390/ani14020317





