Integrated Microbiome and Metabolomics Analysis of the Effects of Dietary Supplementation with Corn-Steep-Liquor-Derived Candida utilis Feed on Black Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Medium
2.2. Methods for the Determination of Strain Indicators
2.3. Single-Factor Experiment Design of Culture Medium Components
2.4. Response Surface Methodology
2.5. Animals, Diets, and Experimental Design
2.6. Sample Collection
2.7. Serum Biochemical Indicators Analysis
2.8. DNA Extraction and Purification and 16S rDNA Amplification Data Analysis
2.9. Serum-Related Metabolites Extraction and Non-Targeted Metabolomics Analysis
2.10. Integrated Microbiome–Metabolome Analysis
2.11. Statistical Analysis
3. Results
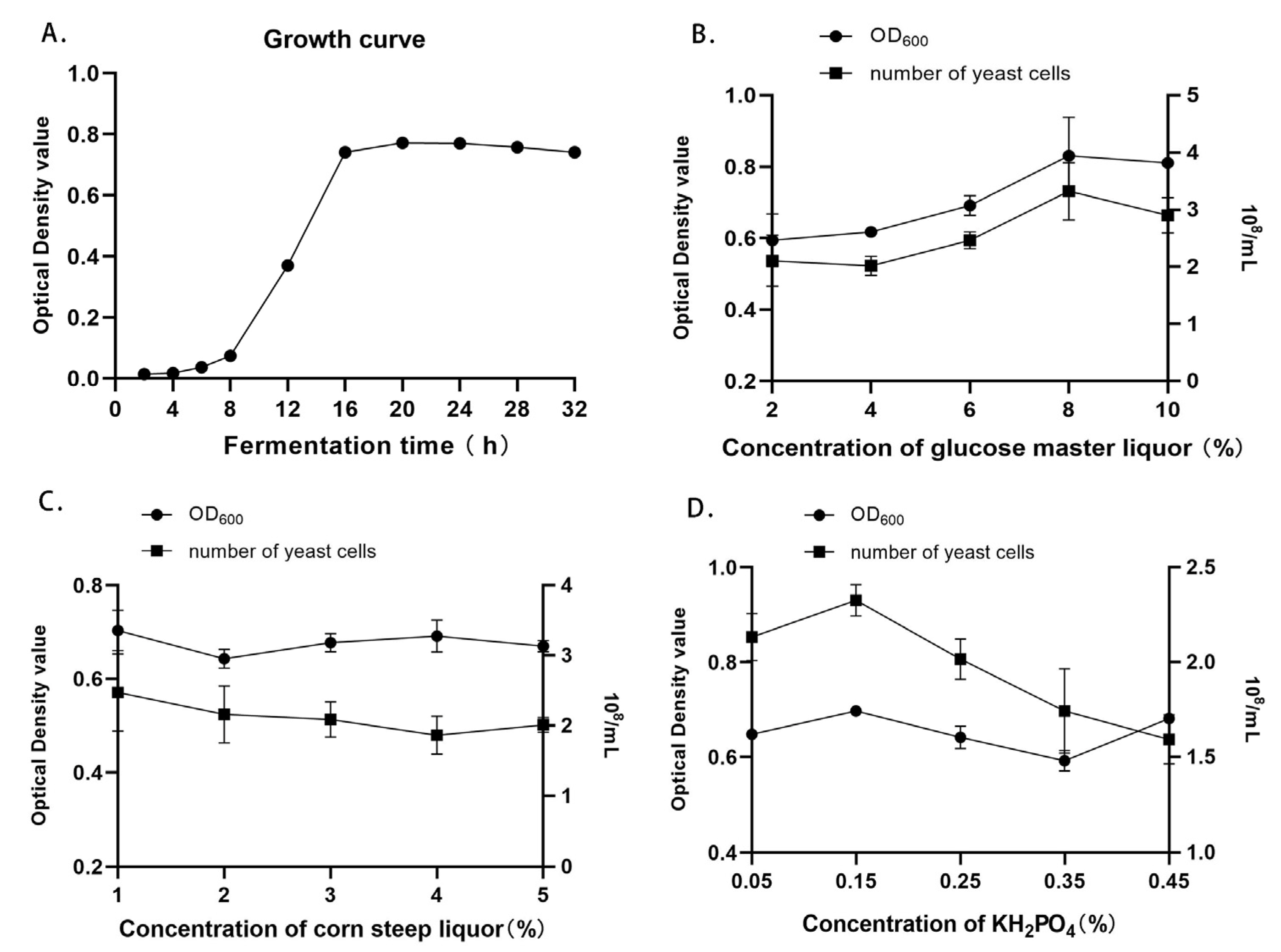
3.1. Determination of the Growth Curve of Candida utilis Feed
3.2. Single-Factor Experiment Results of Fermentation Medium Components for Candida utilis Feed
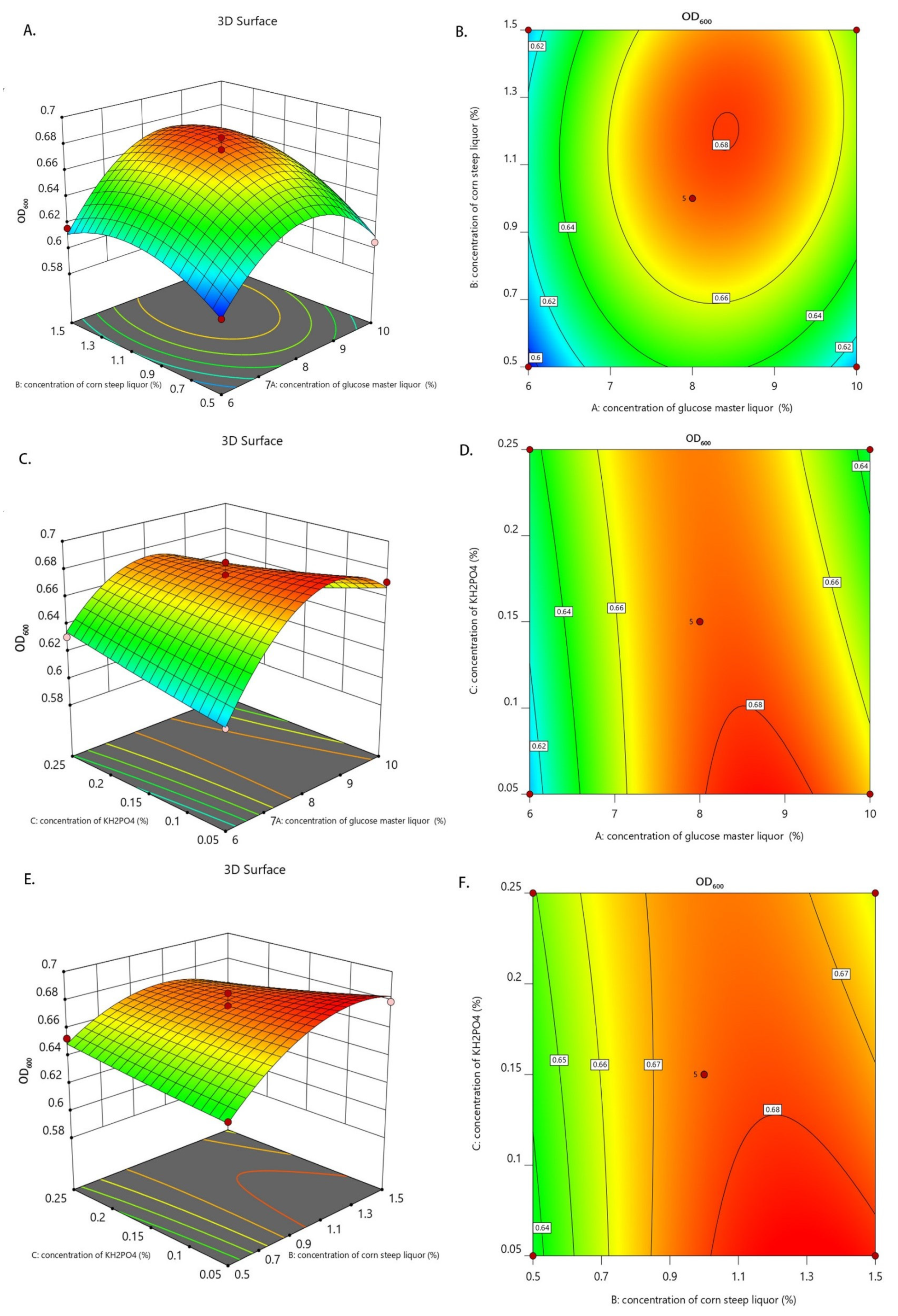
3.3. RSM Results of Culture Medium Components of Candida utilis Feed
3.3.1. Optimization of Medium Components
3.3.2. Interaction Analysis of Factors
3.4. Growth Performance
3.5. Serum Biochemical Variables
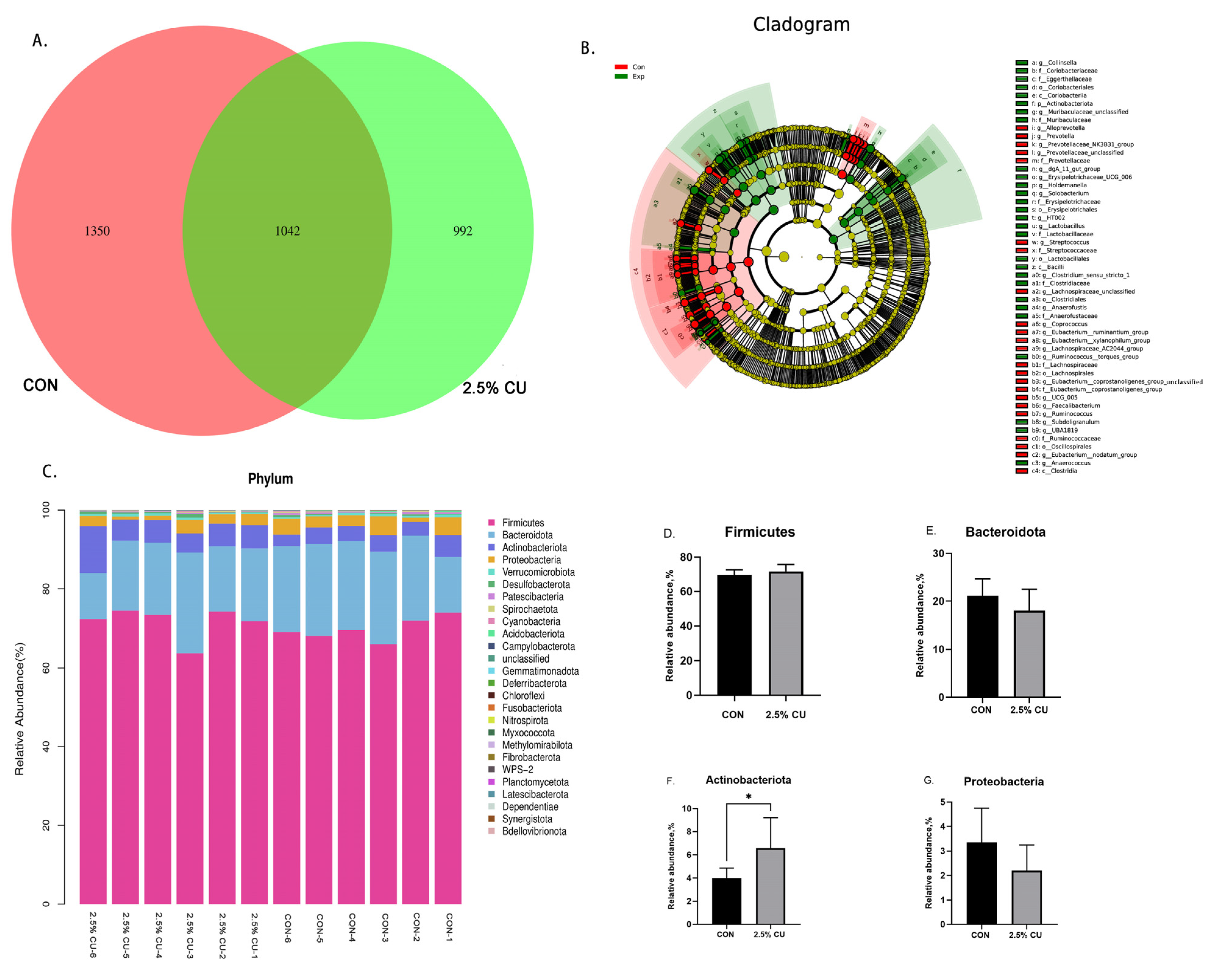
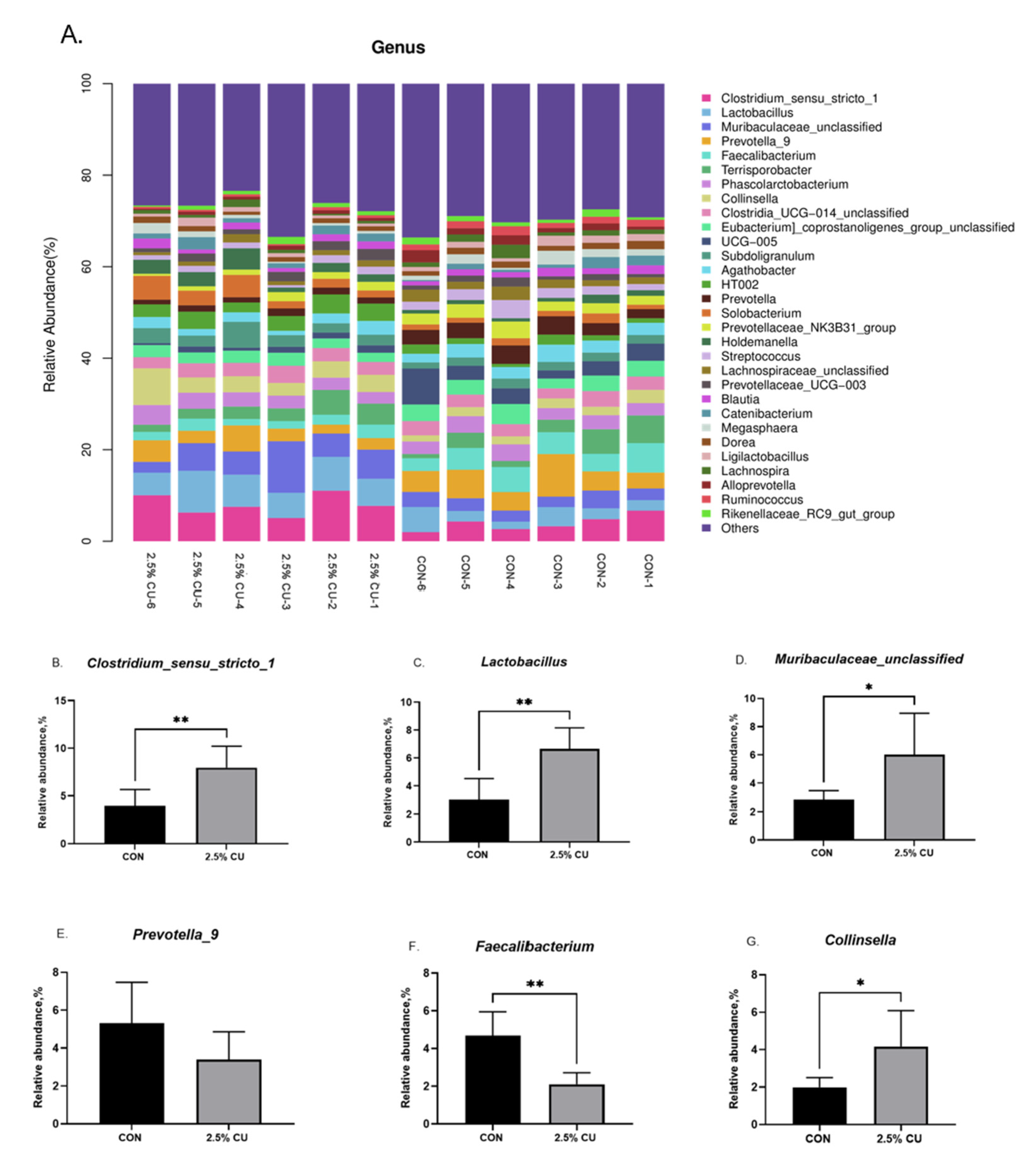
3.6. Effect of Candida utilis onFecal Microflora
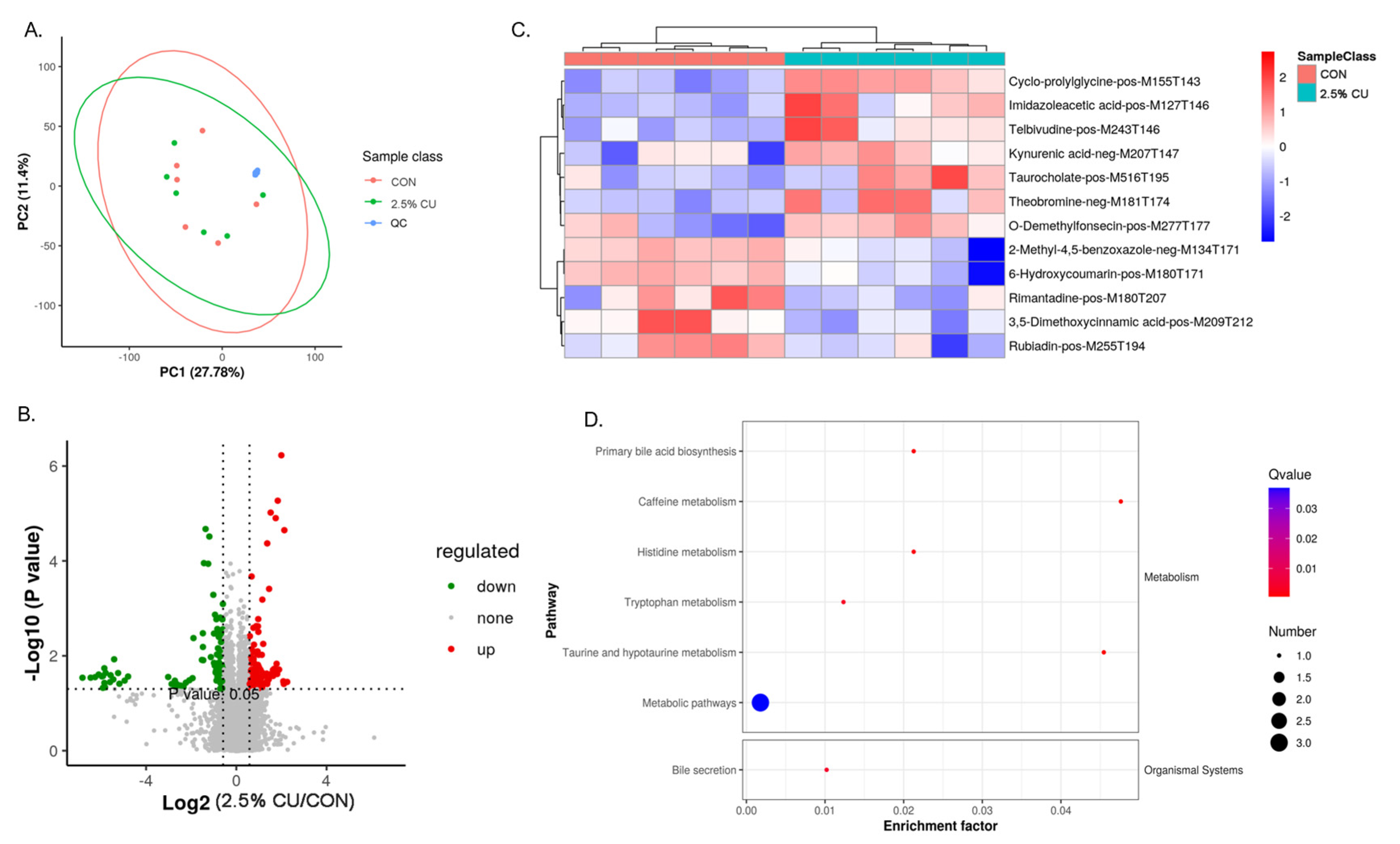
3.7. Supplementation with Candida utilis in a Basic Diet Is a Discriminating Factor of the Plasma Metabolome
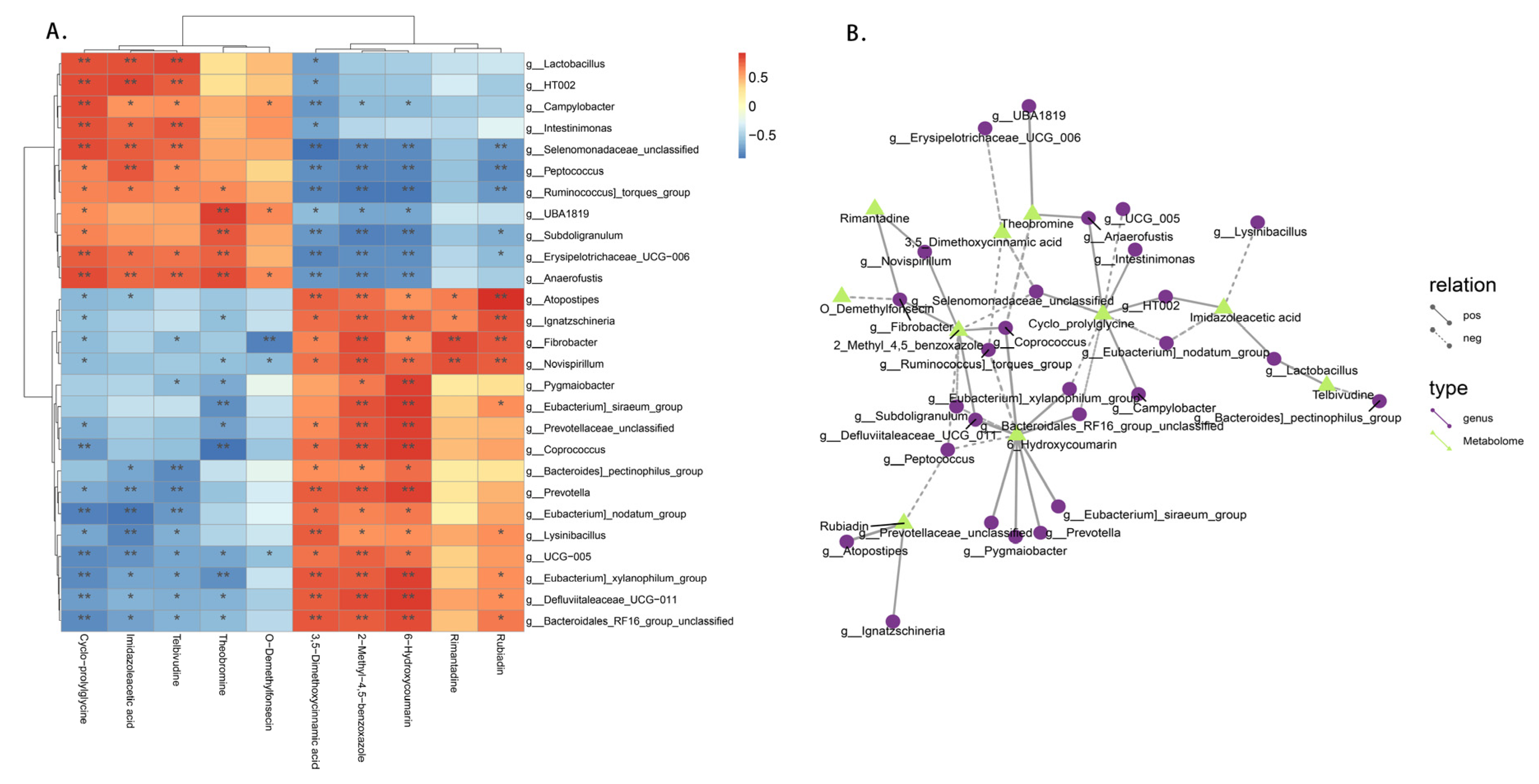
3.8. Fecal Microbiota Correlated with the Plasma Metabolome in Candida utilis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Delgado, J.; Fernandez-Diaz, B.; Nieto-Lopez, M.; Cruz-Suarez, L.E. Nutritional contribution of torula yeast and fish meal to the growth of shrimp Litopenaeus vannamei as indicated by natural nitrogen stable isotopes. Aquaculture 2016, 453, 116–121. [Google Scholar] [CrossRef]
- Yalcin, S.; Ozsoy, B.; Erol, H.; Yalcin, S. Yeast Culture Supplementation to Laying Hen Diets Containing Soybean Meal or Sunflower Seed Meal and Its Effect on Performance, Egg Quality Traits, and Blood Chemistry. J. Appl. Poult. Res. 2008, 17, 229–236. [Google Scholar] [CrossRef]
- Van der Peet-Schwering, C.M.C.; Jansman, A.J.M.; Smidt, H.; Yoon, I. Effects of yeast culture on performance, gut integrity, and blood cell composition of weanling pigs. J. Anim. Sci. 2007, 85, 3099–3109. [Google Scholar] [CrossRef]
- Cruz, A.; Hakenasen, I.M.; Skugor, A.; Mydland, L.T.; Akesson, C.P.; Hellestveit, S.S.; Sorby, R.; Press, C.M.; Overland, M. Candida utilis yeast as a protein source for weaned piglets: Effects on growth performance and digestive function. Livest. Sci. 2019, 226, 31–39. [Google Scholar] [CrossRef]
- Vohra, A.; Syal, P.; Madan, A. Probiotic yeasts in livestock sector. Anim. Feed. Sci. Technol. 2016, 219, 31–47. [Google Scholar] [CrossRef]
- Kieliszek, M.; Kot, A.M.; Bzducha-Wrobel, A.; Blazejak, S.; Gientka, I.; Kurcz, A. Biotechnological use of Candida yeasts in the food industry: A review. Fungal Biol. Rev. 2017, 31, 185–198. [Google Scholar] [CrossRef]
- Carranza-Mendez, R.C.; Chavez-Gonzalez, M.L.; Sepulveda-Torre, L.; Aguilar, C.N.; Govea-Salas, M.; Ramos-Gonzalez, R. Production of single cell protein from orange peel residues by Candida utilis. Biocatal. Agric. Biotechnol. 2022, 40, 102298. [Google Scholar] [CrossRef]
- Ouedraogo, N.; Savadogo, A.; Somda, M.K.; Zongo, C.; Traore, A.S. Effect of mineral salts and nitrogen source on yeast (Candida utilis NOY1) biomass production using tubers wastes. Afr. J. Biomed. Res. 2017, 16, 359–365. [Google Scholar] [CrossRef]
- Dygas, D.; Kregiel, D.; Berlowska, J. Sugar Beet Pulp as a Biorefinery Substrate for Designing Feed. Molecules 2023, 28, 2064. [Google Scholar] [CrossRef]
- Dos Reis, K.C.; Coimbra, J.M.; Duarte, W.F.; Schwan, R.F.; Silva, C.F. Biological treatment of vinasse with yeast and simultaneous production of single-cell protein for feed supplementation. Int. J. Environ. Sci. Technol. 2019, 16, 763–774. [Google Scholar] [CrossRef]
- Li, Q.P.; Yi, P.H.; Zhang, J.Z.; Shan, Y.D.; Lin, Y.F.; Wu, M.; Wang, K.; Tian, G.M.; Li, J.; Zhu, T.H. Bioconversion of food waste to crayfish feed using solid-state fermentation with yeast. Environ. Sci. Pollut. Res. 2023, 30, 15325–15334. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Sun, Z.H.; Zeng, Y.; Hu, P.; Sun, W.Z.; Liu, Y.B.; Hu, H.; Rao, Z.B.; Tang, Z.R. Isolation, Identification and Function of Pichia anomala AR2016 and Its Effects on the Growth and Health of Weaned Pigs. Animals 2021, 11, 1179. [Google Scholar] [CrossRef]
- Wang, C.; Song, X.J.; Li, C.Q.; He, L.P.; Wang, X.; Zeng, X.F. Mixed fermentation with Lactobacillus plantarum, Bifidobacteri mu m animalis subsp. lactis and Candida utilis improves the fermentation quality of Hong Suan Tang. Food Chem. 2023, 402, 134488. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jia, O.Y.; Xu, Y.; Chen, M.; Song, X.Y.; Yong, Q.; Yu, S.Y. Optimization of culture conditions for production of yeast biomass using bamboo wastewater by response surface methodology. Bioresour. Technol. 2009, 100, 3613–3617. [Google Scholar] [CrossRef] [PubMed]
- Kurcz, A.; Blazejak, S.; Kot, A.M.; Bzducha-Wrobel, A.; Kieliszek, M. Application of Industrial Wastes for the Production of Microbial Single-Cell Protein by Fodder Yeast Candida utilis. Waste Biomass Valorization 2018, 9, 57–64. [Google Scholar] [CrossRef]
- Bezerra, C.R.F.; Borges, K.R.A.; Alves, R.D.S.; Teles, A.M.; Rodrigues, I.V.P.; da Silva, M.; Nascimento, M.; Bezerra, G.F.D. Highly efficient antibiofilm and antifungal activity of green propolis against Candida species in dentistry materials. PLoS ONE 2020, 15, e0228828. [Google Scholar] [CrossRef]
- Kujan, P.; Prell, A.; Safar, H.; Sobotka, M.; Rezanka, T.; Holler, P. Use of the industrial yeast Candida utilis for cadmium sorption. Folia Microbiol. 2006, 51, 257–260. [Google Scholar] [CrossRef]
- Rehman, A.U.; Rasool, S.; Mukhtar, H.; Ul Haq, I. Production of an extracellular lipase by Candida utilis NRRL-Y-900 using agro-industrial byproducts. Turk. J. Biochem. 2014, 39, 140–149. [Google Scholar] [CrossRef]
- Elmaleh, S.; Defrance, M.B.; Ghommidh, C. Organic acids oxidation by Candida utilis: Application to industrial waste water treatment. Process Biochem. 2000, 35, 441–449. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhao, G.J. Yeast Cultivation for Single-Cell Protein Production Using the Carbohydrate Hydrolysate of Steam-Exploded Eucalyptus Wood. Wood Res. 2022, 67, 568–581. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, W.; Zhang, G. Production of single cell protein using waste capsicum powder produced during capsanthin extraction. Lett. Appl. Microbiol. 2010, 50, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Ahmed, S.; Hashmi, A.S. Bioconversion of Beet Pulp to Microbial Biomass Protein by Candida utilis. J. Chem. Soc. Pak. 2009, 31, 115–121. [Google Scholar]
- Broach, J.R. Nutritional Control of Growth and Development in Yeast. Genetics 2012, 192, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.C.; Ren, L.J.; Chen, S.L.; Zhang, L.; Ji, X.J.; Huang, H. The roles of different salts and a novel osmotic pressure control strategy for improvement of DHA production by Schizochytrium sp. Bioprocess. Biosyst. Eng. 2015, 38, 2129–2136. [Google Scholar] [CrossRef]
- Hansen, J.O.; Hofossaeter, M.; Sahlmann, C.; Anestad, R.; Reveco-Urzua, F.E.; Press, C.M.; Mydland, L.T.; Overland, M. Effect of Candida utilis on growth and intestinal health of Atlantic salmon (Salmo salar) parr. Aquaculture 2019, 511, 734239. [Google Scholar] [CrossRef]
- Reveco-Urzua, F.E.; Hofossaeter, M.; Kovi, M.R.; Mydland, L.T.; Anestad, R.; Sorby, R.; Press, C.M.; Lagos, L.; Overland, M. Candida utilis yeast as a functional protein source for Atlantic salmon (Salmo solar L.): Local intestinal tissue and plasma proteome responses. PLoS ONE 2019, 14, e0218360. [Google Scholar] [CrossRef]
- Overland, M.; Karlsson, A.; Mydland, L.T.; Romarheim, O.H.; Skrede, A. Evaluation of Candida utilis, Kluyveromyces marxianus and Saccharomyces cerevisiae yeasts as protein sources in diets for Atlantic salmon (Salmo salar). Aquaculture 2013, 402, 1–7. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, H.; Li, B.; Liu, G.; Huang, R.; Li, F.; Liao, P.; Zhang, Y.; Martin Nyachoti, C.; Deng, D. Dietary supplementation with yeast product improves intestinal function, and serum and ileal amino acid contents in weaned piglets. Livest. Sci. 2015, 171, 20–27. [Google Scholar] [CrossRef]
- Espinosa, C.D.; Lagos, L.V.; Stein, H.H. Effect of torula yeast on growth performance, diarrhea incidence, and blood characteristics in weanling pigs. J. Anim. Sci. 2020, 98, skaa307. [Google Scholar] [CrossRef]
- Yang, Z.G.; Wang, Y.; He, T.L.; Gifty, Z.B.; Sun, Z.H.; Sun, W.Z.; Tang, Z.R. Effects of Dietary Yucca Schidigera Extract and Oral Candida utilis on Growth Performance and Intestinal Health of Weaned Piglets. Front. Nutr. 2021, 8, 685540. [Google Scholar] [CrossRef] [PubMed]
- Jarmolowicz, S.; Zakes, Z.; Siwicki, A.; Kowalska, A.; Hopko, M.; Glabski, E.; Demska-Zakes, K.; Partyka, K. Effects of brewer’s yeast extract on growth performance and health of juvenile pikeperch Sander lucioperca (L.). Aquac. Nutr. 2012, 18, 457–464. [Google Scholar] [CrossRef]
- Azizi, T.; Daneshyar, M.; Alimehr, M.; Shalizar-Jalali, A.; Tukmechi, A.; Khalilvandi-Behroozyar, H. Effect of Lactobacillus sp. and yeast supplementation on performance and some blood attributes in deoxynivalenol-challenged broiler chickens. Res. Vet. Sci. 2023, 159, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.M.; El-Senousey, H.K. Nutritional Factors Affecting Abdominal Fat Deposition in Poultry: A Review. Asian-australas. J. Anim. Sci. 2014, 27, 1057–1068. [Google Scholar] [CrossRef]
- Pajarillo, E.A.B.; Chae, J.P.; Balolong, M.P.; Kim, H.B.; Kang, D.K. Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J. Gen. Appl. Microbiol. 2014, 60, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.K.; Xu, X.; Zhang, Q.; Li, P.; Zhao, P.F.; Li, Q.Y.; Liu, J.D.; Piao, X.S. Effects of essential oil supplementation of a low-energy diet on performance, intestinal morphology and microflora, immune properties and antioxidant activities in weaned pigs. Anim. Sci. J. 2015, 86, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- Yu, X.R.; Cui, Z.C.; Qin, S.K.; Zhang, R.Q.; Wu, Y.P.; Liu, J.S.; Yang, C.M. Effects of Bacillus licheniformis on Growth Performance, Diarrhea Incidence, Antioxidant Capacity, Immune Function, and Fecal Microflora in Weaned Piglets. Animals 2022, 12, 1609. [Google Scholar] [CrossRef]
- Wlodarska, M.; Willing, B.P.; Bravo, D.M.; Finlay, B.B. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Zhou, J.; Luo, J.; Yang, S.M.; Xiao, Q.L.; Wang, X.L.; Zhou, Z.T.; Xiao, Y.C.; Shi, D.S. Different Responses of Microbiota across Intestinal Tract to Enterococcus faecium HDRsEf1 and Their Correlation with Inflammation in Weaned Piglets. Microorganisms 2021, 9, 1767. [Google Scholar] [CrossRef]
- Collado, M.C.; Grzeskowiak, L.; Salminen, S. Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr. Microbiol. 2007, 55, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Miller, R.A.; Schmidt, T.M. Muribaculaceae Genomes Assembled from Metagenomes Suggest Genetic Drivers of Differential Response to Acarbose Treatment in Mice. Msphere 2021, 6, e0085121. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Pan, L.; Shang, Q.H.; Ma, X.K.; Long, S.F.; Xu, Y.T.; Piao, X.S. Effects of isomalto-oligosaccharides as potential prebiotics on performance, immune function and gut microbiota in weaned pigs. Anim. Feed. Sci. Technol. 2017, 230, 126–135. [Google Scholar] [CrossRef]
- Iakhno, S.; Delogu, F.; Umu, O.C.O.; Kjos, N.P.; Hakenasen, I.M.; Mydland, L.T.; Overland, M.; Sorum, H. Longitudinal analysis of the faecal microbiome in pigs fed Cyberlindnera jadinii yeast as a protein source during the weanling period followed by a rapeseed- and faba bean-based grower-finisher diet. Anim. Microbiome 2022, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Iljazovic, A.; Roy, U.; Galvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021, 14, 113–124. [Google Scholar] [CrossRef]
- Fan, P.X.; Liu, P.; Song, P.X.; Chen, X.Y.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, srep43412. [Google Scholar] [CrossRef]
- Li, J.; Li, H.Y.; Zhou, Y.; Xiang, H.W.; Lv, M.Z.; Ruan, B.; Bo, Z.Y.; Shen, H.X.; Xu, F.Z.; Huang, Y.F.; et al. Effects of Compound Probiotics on Cecal Microbiota and Metabolome of Swine. Animals 2023, 13, 1006. [Google Scholar] [CrossRef]
- Yang, M.Q.; Ye, L.L.; Liu, X.L.; Qi, X.M.; Lv, J.D.; Wang, G.; Farhan, U.K.; Waqas, N.; Chen, D.D.; Han, L.; et al. Gingerol activates noxious cold ion channel TRPA1 in gastrointestinal tract. Chin. J. Nat. Med. 2016, 14, 434–440. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Seve, B. Biological roles of tryptophan and its metabolism: Potential implications for pig feeding. Livest. Sci. 2007, 112, 23–32. [Google Scholar] [CrossRef]
- Ramirez-Perez, O.; Cruz-Ramon, V.; Chinchilla-Lopez, P.; Mendez-Sanchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16, S21–S26. [Google Scholar] [CrossRef]
- Povarnina, P.Y.; Kolyasnikova, K.N.; Nikolaev, S.V.; Antipova, T.A.; Gudasheva, T.A. Neuropeptide Cycloprolylglycine Exhibits Neuroprotective Activity after Systemic Administration to Rats with Modeled Incomplete Global Ischemia and in In Vitro Modeled Glutamate Neurotoxicity. Bull. Exp. Biol. Med. 2016, 160, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Han, S.H.B. Telbivudine: A new nucleoside analogue for the treatment of chronic hepatitis B. Expert. Opin. Investig. Drugs 2005, 14, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Mukherjee, S.; Choi, M.J.; Kang, N.H.; Pham, H.G.; Yun, J.W. Theobromine alleviates diet-induced obesity in mice via phosphodiesterase-4 inhibition. Eur. J. Nutr. 2020, 59, 3503–3516. [Google Scholar] [CrossRef] [PubMed]
- Megersa, M.; Jima, T.T.; Goro, K.K. The Use of Medicinal Plants for the Treatment of Toothache in Ethiopia. Evid. Based Complement. Alternat Med. 2019, 2019, 2645174. [Google Scholar] [CrossRef]

| Ingredients, % | CON | 2.5% CU |
|---|---|---|
| Corn | 40 | 40 |
| Storage japonica brown rice | 10 | 10 |
| Flour | 6 | 6 |
| Soybean meal (43.5% CP) | 10 | 10 |
| Fermented Soybean meal | 5 | 5 |
| Puffed soybean | 5 | 5 |
| Fish meal (65.2% CP) | 3 | 3 |
| Sucrose | 2 | 2 |
| Glucose | 1 | 1 |
| Whey powder | 10 | 10 |
| Saccharomyces cerevisiae | 2.5 | 0 |
| Candida utilis | 0 | 2.5 |
| Soybean oil | 1 | 1 |
| CaH2(PO4)2 | 0.6 | 0.6 |
| Stone powder | 0.5 | 0.5 |
| NaCl | 0.2 | 0.2 |
| Lysine Hydrochloride (Lys) (98%) | 0.63 | 0.63 |
| Methionine (Met) | 0.12 | 0.12 |
| Threonine (Thr) | 0.15 | 0.15 |
| Tryptophan (Trp) | 0.05 | 0.05 |
| Cr2O3 | 0.25 | 0.25 |
| Premix 1 | 2 | 2 |
| Total | 100 | 100 |
| Nutrition level (%) | ||
| DE (MJ/kg) | 14.01 | 14.06 |
| CP | 18.6 | 18.6 |
| Lys | 1.37 | 1.36 |
| Met | 0.40 | 0.41 |
| Thr | 0.78 | 0.78 |
| Trp | 0.24 | 0.24 |
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Concentration of glucose master liquor (%) | 6 | 8 | 10 |
| Concentration of corn steep liquor (%) | 0.5 | 1.0 | 1.5 |
| Concentration of KH2PO4 (%) | 0.05 | 0.15 | 0.25 |
| Runs | Concentration of Glucose Master Liquor (%) | Concentration of Corn Steep Liquor (%) | Concentration of KH2PO4 (%) | Optical Density Value | Number of Yeast Cells (108/mL) |
|---|---|---|---|---|---|
| 1 | 1 | −1 | 0 | 0.605 | 2.42 |
| 2 | −1 | −1 | 0 | 0.595 | 2.24 |
| 3 | 0 | 0 | 0 | 0.676 | 2.72 |
| 4 | 0 | 1 | −1 | 0.679 | 2.52 |
| 5 | 0 | −1 | −1 | 0.637 | 2.41 |
| 6 | 0 | 0 | 0 | 0.685 | 2.81 |
| 7 | 0 | 0 | 0 | 0.668 | 2.7 |
| 8 | 0 | 0 | 0 | 0.676 | 2.85 |
| 9 | 0 | −1 | 1 | 0.653 | 2.57 |
| 10 | −1 | 1 | 0 | 0.616 | 2.36 |
| 11 | −1 | 0 | −1 | 0.611 | 2.1 |
| 12 | 1 | 1 | 0 | 0.651 | 2.48 |
| 13 | 1 | 0 | −1 | 0.671 | 2.47 |
| 14 | 0 | 1 | 1 | 0.659 | 2.66 |
| 15 | 0 | 0 | 0 | 0.675 | 2.75 |
| 16 | −1 | 0 | 1 | 0.631 | 2.42 |
| 17 | 1 | 0 | 1 | 0.635 | 2.39 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.0131 | 9 | 0.0015 | 40.02 | <0.0001 | *** |
| A—Concentration of glucose master liquor | 0.0015 | 1 | 0.0015 | 40.89 | 0.0004 | *** |
| B—Concentration of corn steep liquor | 0.0017 | 1 | 0.0017 | 45.51 | 0.0003 | *** |
| C—Concentration of KH2PO4 | 0.0000 | 1 | 0.0000 | 1.38 | 0.2791 | ns |
| AB | 0.0002 | 1 | 0.0002 | 4.30 | 0.0768 | ns |
| AC | 0.0008 | 1 | 0.0008 | 21.59 | 0.0024 | ** |
| BC | 0.0003 | 1 | 0.0003 | 8.92 | 0.0203 | * |
| A2 | 0.0066 | 1 | 0.0066 | 182.02 | <0.0001 | *** |
| B2 | 0.0016 | 1 | 0.0016 | 44.65 | 0.0003 | *** |
| C2 | 0.0000 | 1 | 0.0000 | 0.0453 | 0.8375 | ns |
| Residual | 0.0003 | 7 | 0.0000 | |||
| Lack of Fit | 0.0001 | 3 | 0.0000 | 0.9886 | 0.4829 | ns |
| Pure Error | 0.0001 | 4 | 0.0000 | |||
| Cor Total | 0.0133 | 16 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.6574 | 9 | 0.0730 | 31.14 | <0.0001 | *** |
| A—Concentration of glucose master liquor | 0.0512 | 1 | 0.0512 | 21.83 | 0.0023 | ** |
| B—Concentration of corn steep liquor | 0.0181 | 1 | 0.0181 | 7.69 | 0.0275 | * |
| C—Concentration of KH2PO4 | 0.0364 | 1 | 0.0364 | 15.54 | 0.0056 | ** |
| AB | 0.0009 | 1 | 0.0009 | 0.3837 | 0.5553 | ns |
| AC | 0.0400 | 1 | 0.0400 | 17.05 | 0.0044 | ** |
| BC | 0.0001 | 1 | 0.0001 | 0.0426 | 0.8423 | ns |
| A2 | 0.3615 | 1 | 0.3615 | 154.10 | <0.0001 | *** |
| B2 | 0.0404 | 1 | 0.0404 | 17.24 | 0.0043 | ** |
| C2 | 0.0690 | 1 | 0.0690 | 29.41 | 0.0010 | *** |
| Residual | 0.0164 | 7 | 0.0023 | |||
| Lack of Fit | 0.0007 | 3 | 0.0002 | 0.0594 | 0.9786 | ns |
| Pure Error | 0.0157 | 4 | 0.0039 | |||
| Cor Total | 0.6738 | 16 |
| Items | CON | 2.5% CU | p-Value |
|---|---|---|---|
| Initial BW | 6.73 ± 1.26 | 7.09 ± 1.30 | 0.11 |
| Final BW | 14.05 ± 3.16 | 14.33 ± 3.24 | 0.62 |
| ADG, kg | 0.35 ± 0.08 | 0.34 ± 0.07 | 0.77 |
| ADFI, kg | 0.56 ± 0.13 | 0.56 ± 0.07 | 0.93 |
| FCR | 1.64 ± 0.09 | 1.69 ± 0.24 | 0.71 |
| Items | CON | 2.5% CU | p-Value |
|---|---|---|---|
| ALT, U/L | 82.22 ± 18.94 | 67.50 ± 10.19 | 0.10 |
| AST, U/L | 91.48 ± 36.41 | 78.53 ± 23.68 | 1.00 |
| ALP, U/L | 351.10 ± 100.05 | 366.10 ± 97.40 | 0.60 |
| GGT, U/L | 48.65 ± 38.78 | 47.79 ± 26.49 | 0.96 |
| T-BIL, μmol/L | 0.58 ± 0.28 | 0.50 ± 0.33 | 0.40 |
| D-BIL, μmol/L | 0.85 ± 0.67 | 1.30 ± 0.32 | 0.29 |
| TC, mmol/L | 2.23 ± 0.50 | 1.90 ± 0.22 | 0.23 |
| TG, mmol/L | 0.23 ± 0.11 | 0.14 ± 0.04 | 0.13 |
| HDL-C, mmol/L | 0.43 ± 0.10 | 0.35 ± 0.07 | 0.21 |
| LDL-C, mmol/L | 0.61 ± 0.29 | 0.46 ± 0.21 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Wang, R.; Wang, C.; Wang, R.; Shen, J.; Fang, H.; Zhang, J. Integrated Microbiome and Metabolomics Analysis of the Effects of Dietary Supplementation with Corn-Steep-Liquor-Derived Candida utilis Feed on Black Pigs. Animals 2024, 14, 306. https://doi.org/10.3390/ani14020306
Qi H, Wang R, Wang C, Wang R, Shen J, Fang H, Zhang J. Integrated Microbiome and Metabolomics Analysis of the Effects of Dietary Supplementation with Corn-Steep-Liquor-Derived Candida utilis Feed on Black Pigs. Animals. 2024; 14(2):306. https://doi.org/10.3390/ani14020306
Chicago/Turabian StyleQi, Huiyu, Ruqi Wang, Chuanqi Wang, Rui Wang, Jinglin Shen, Hengtong Fang, and Jing Zhang. 2024. "Integrated Microbiome and Metabolomics Analysis of the Effects of Dietary Supplementation with Corn-Steep-Liquor-Derived Candida utilis Feed on Black Pigs" Animals 14, no. 2: 306. https://doi.org/10.3390/ani14020306
APA StyleQi, H., Wang, R., Wang, C., Wang, R., Shen, J., Fang, H., & Zhang, J. (2024). Integrated Microbiome and Metabolomics Analysis of the Effects of Dietary Supplementation with Corn-Steep-Liquor-Derived Candida utilis Feed on Black Pigs. Animals, 14(2), 306. https://doi.org/10.3390/ani14020306






