Development of an Alternative In Vitro Rumen Fermentation Prediction Model
Abstract
Simple Summary
Abstract
1. Introduction
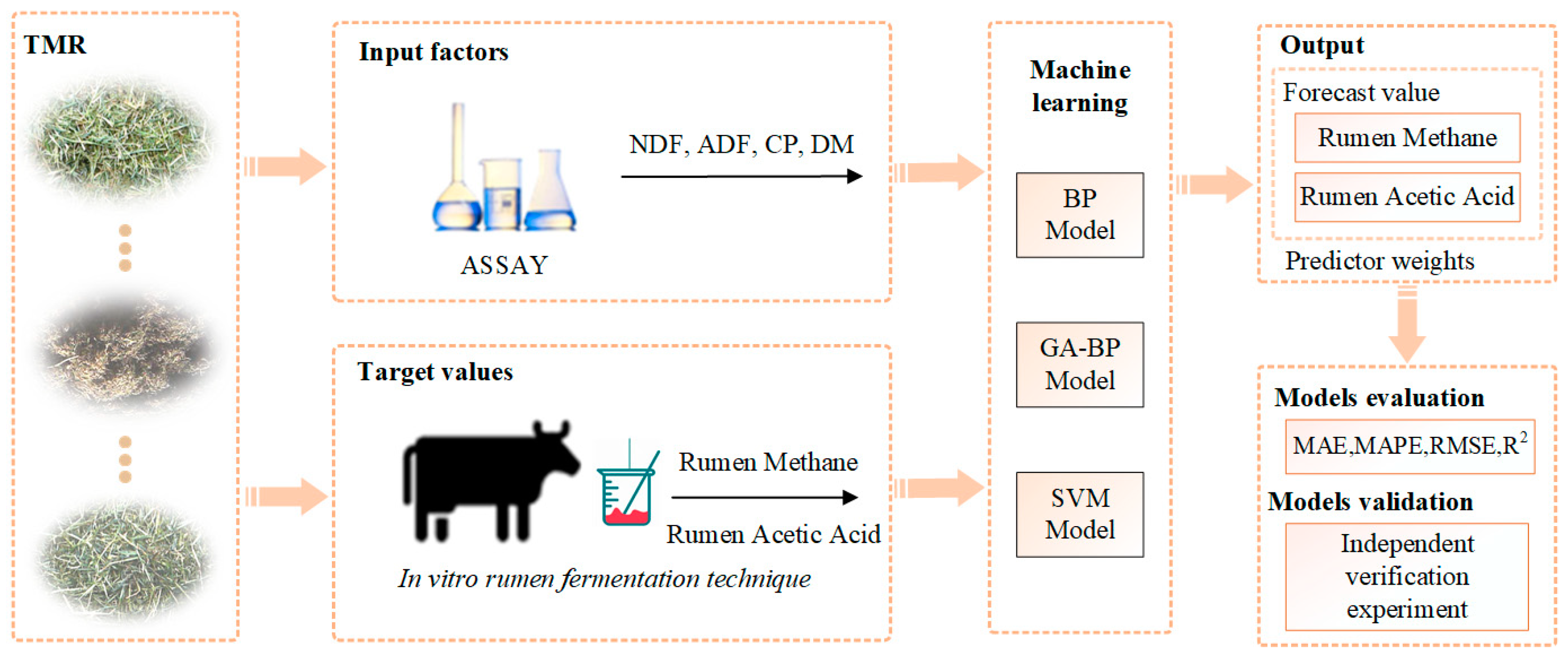
2. Materials and Methods
2.1. Experimental Design
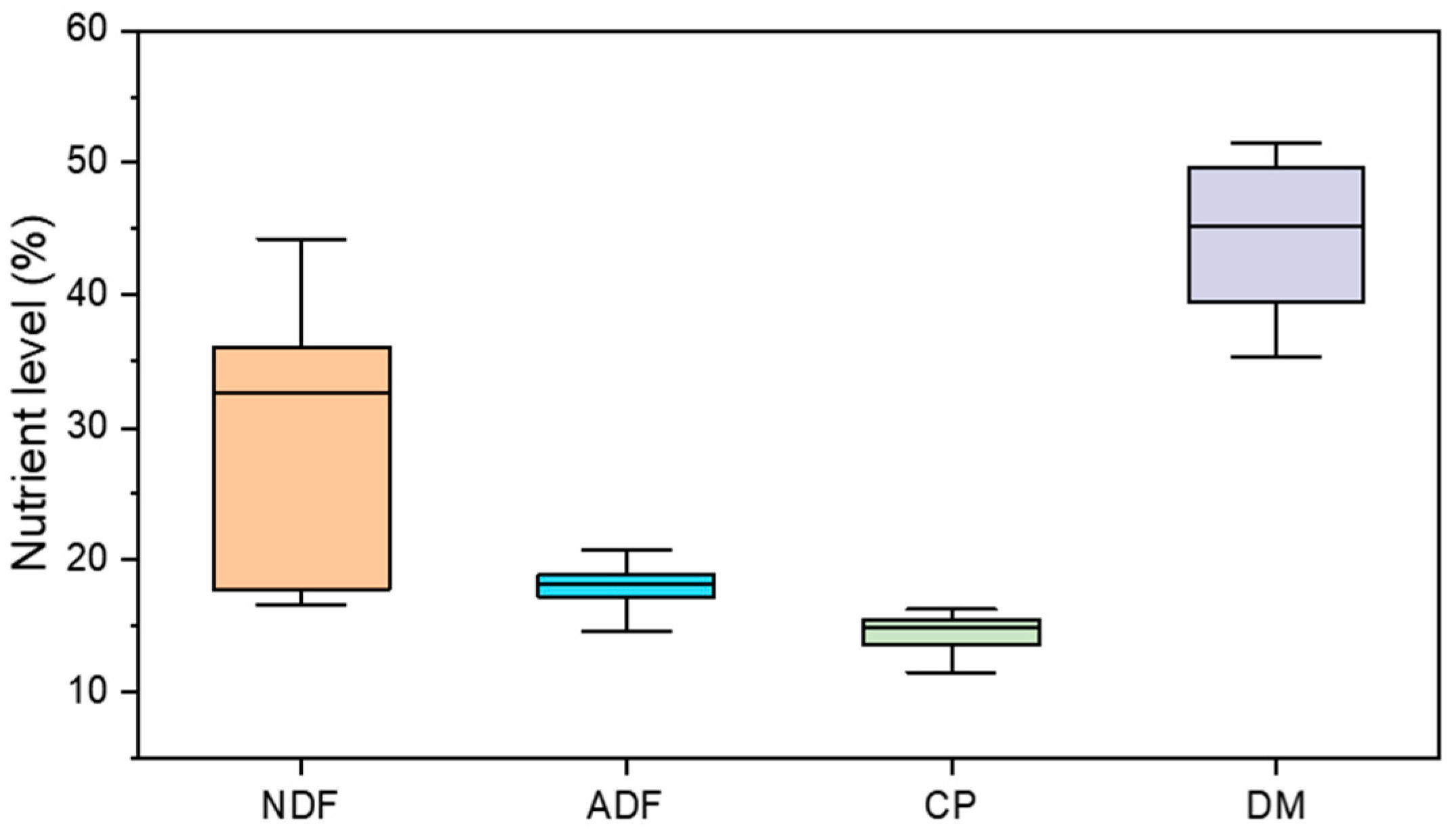
2.2. Experimental Data and Data Processing
2.3. Validation Experiment Design
2.4. Algorithm and Model
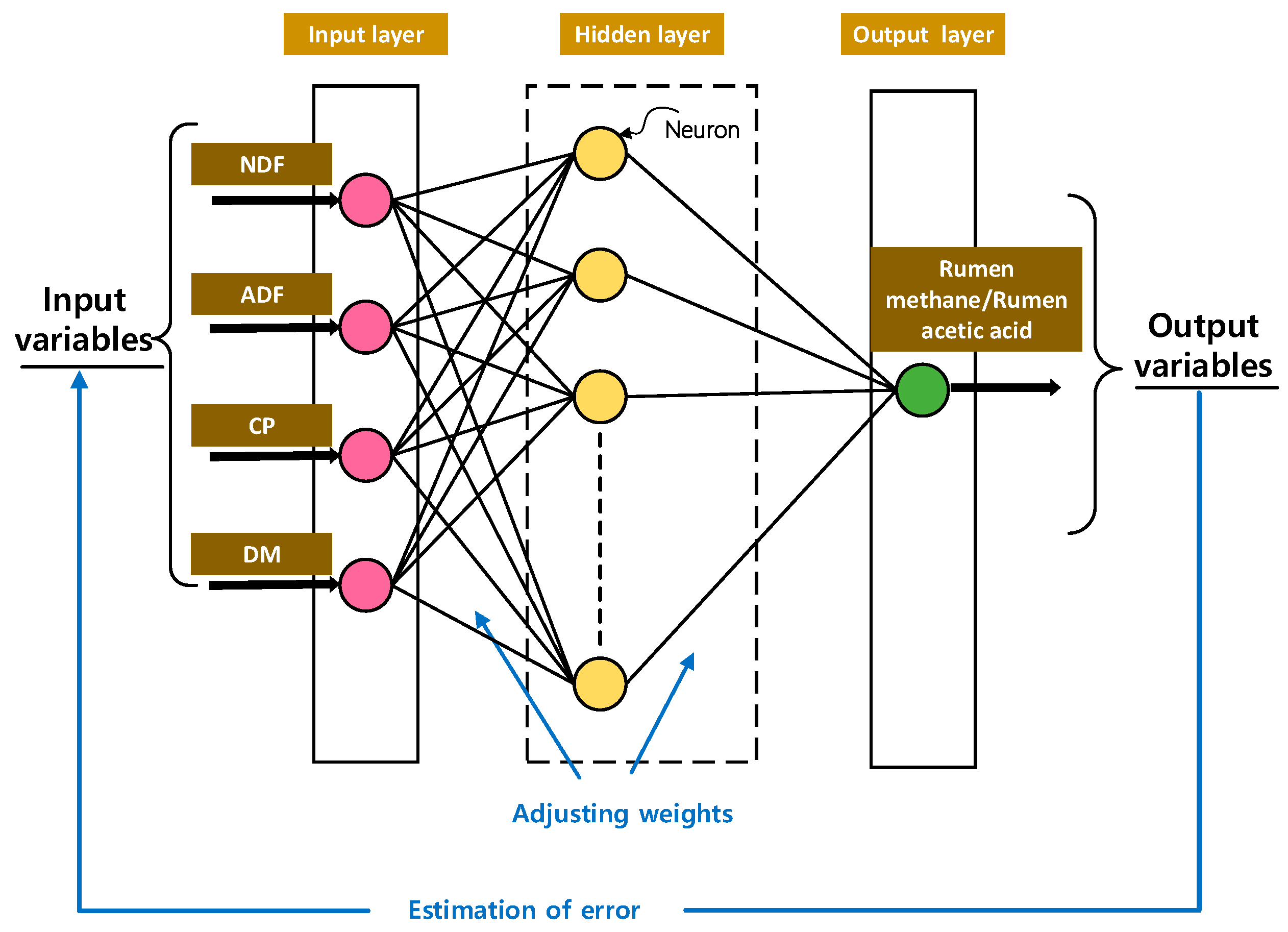
2.4.1. BP Model of In Vitro Rumen Fermentation in Dairy Cows
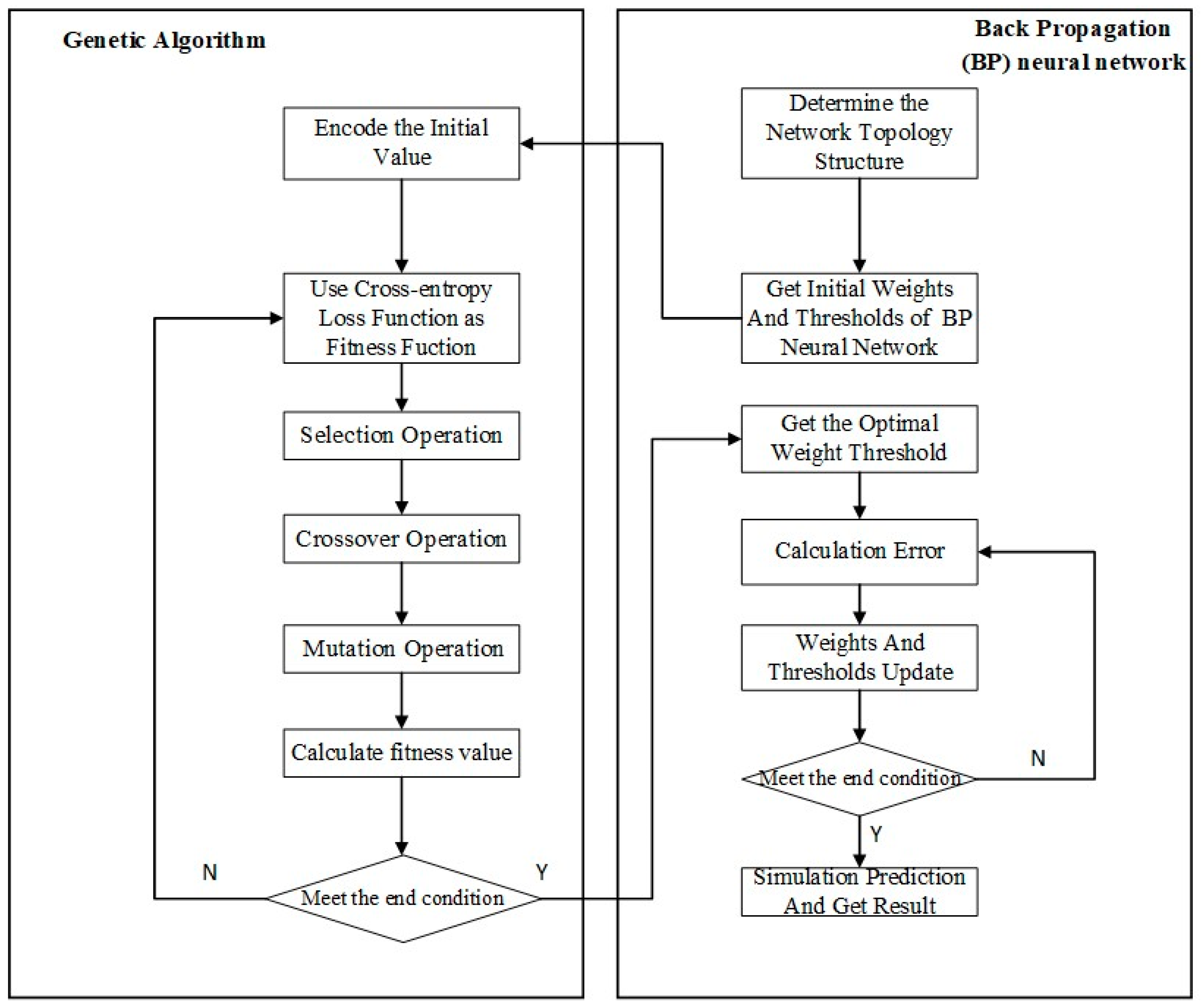
2.4.2. GA-BP Model of In Vitro Rumen Fermentation in Dairy Cows
2.4.3. Support Vector Machine Model of In Vitro Rumen Fermentation in Dairy Cows
2.5. Model Evaluation and Validation Method
3. Results and Discussion
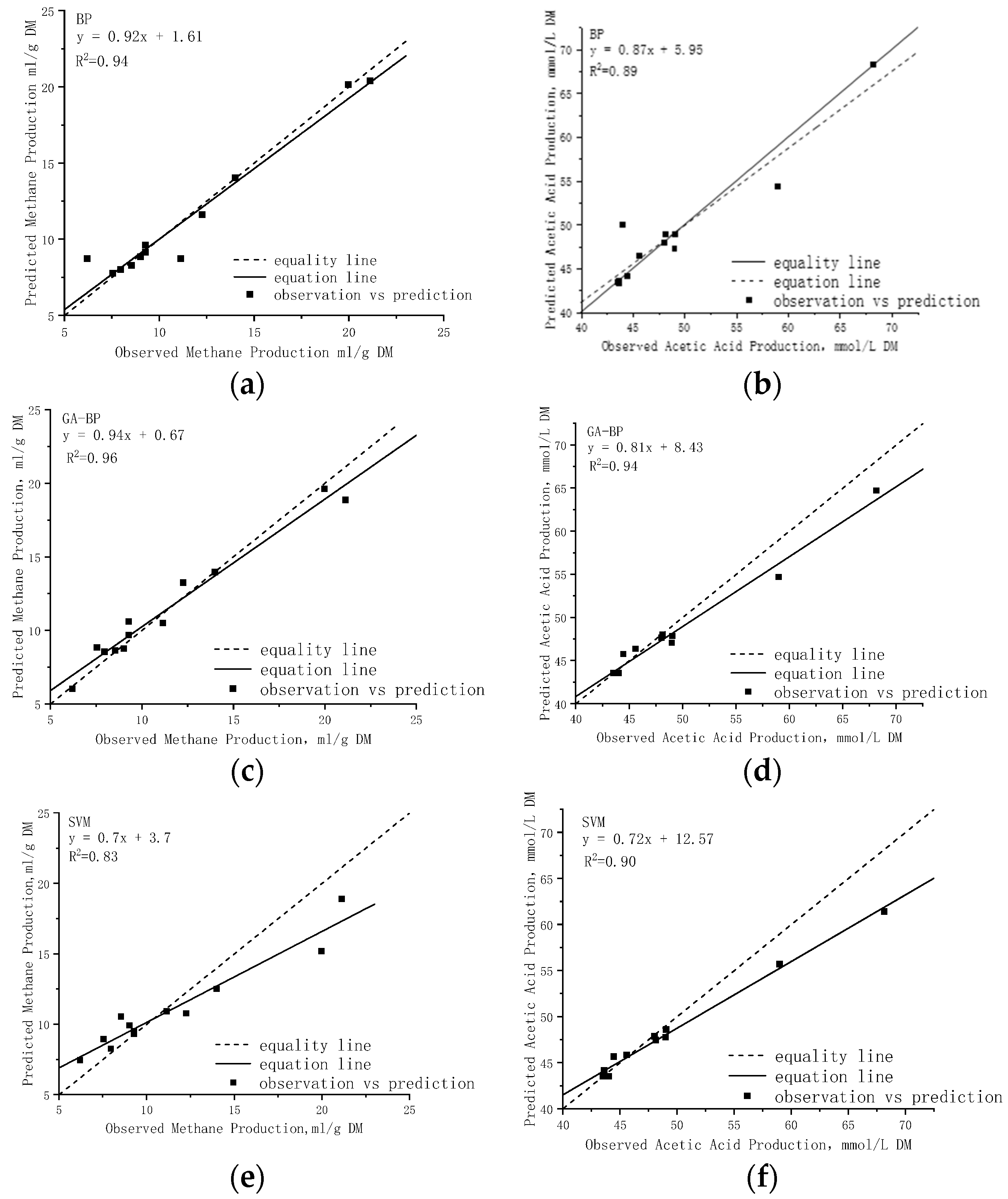
3.1. Model Evaluation
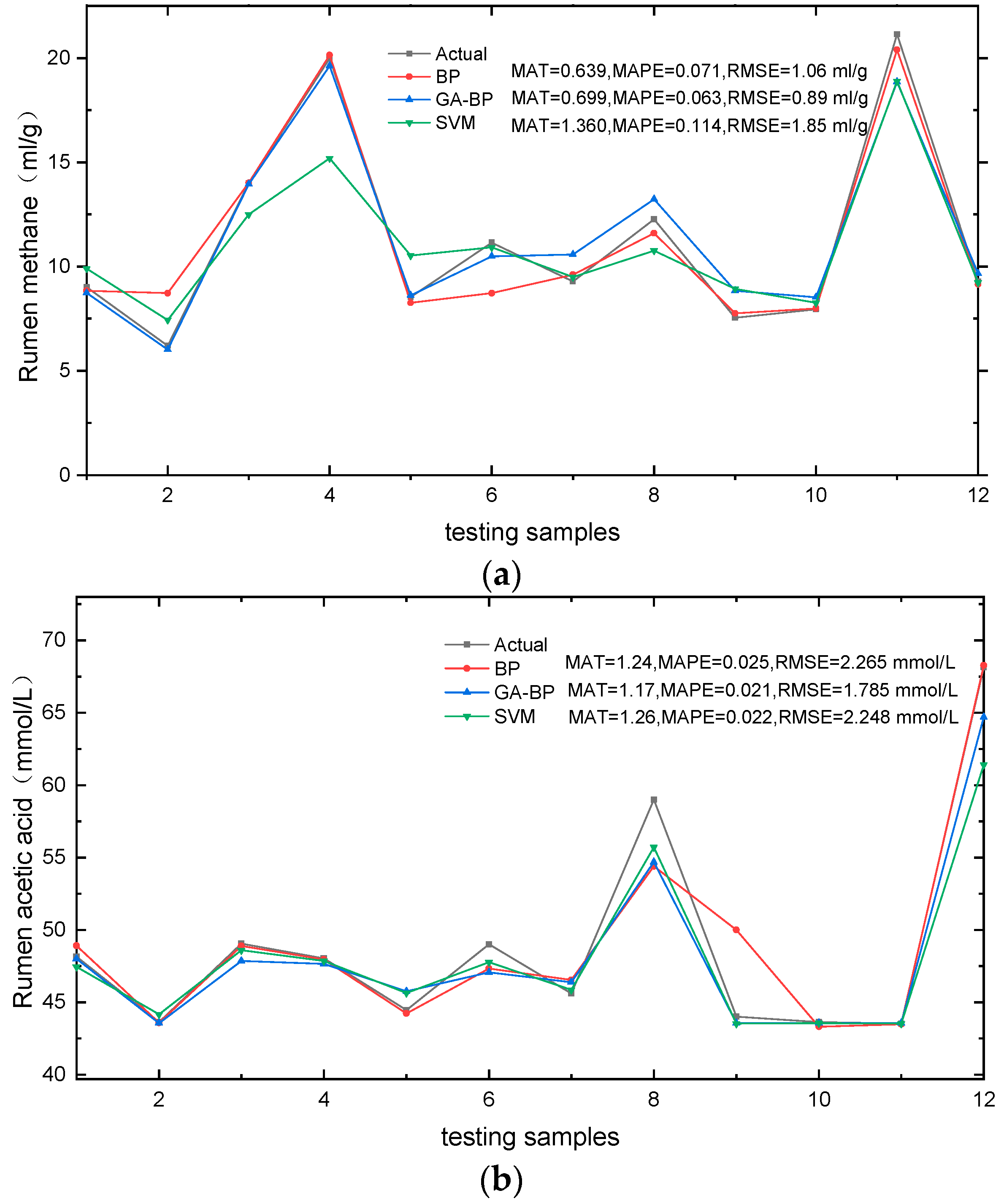
3.2. Model Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiao, H.; Yan, T.; Wills, D.A.; Carson, A.F.; McDowell, D.A. Development of prediction models for quantification of total methane emission from enteric fermentation of young Holstein cattle at various ages. Agric. Ecosyst. Environ. 2014, 183, 160–166. [Google Scholar] [CrossRef]
- Tee, T.; Goh, Y.; Zainudin, M.; Candyrine, S.; Sommart, K.; Kongphitee, K.; Sumamal, W.; Phaowphaisal, I.; Namsilee, R.; Angthong, W.; et al. Enteric methane emission models for diverse beef cattle feeding systems in South-east Asia: A meta-analysis. Anim. Feed Sci. Technol. 2022, 294, 115474. [Google Scholar] [CrossRef]
- Yan, Z.; Kaidon, D.; Lifeng, D.; Guishan, X.; Tao, M.; Qiyu, D. Intestinal methane production and emission reduction measures of ruminant livestock. J. Domest. Anim. Ecol. 2018, 39, 6–10. [Google Scholar]
- Wang, Y.; Zhou, J.; Wang, X.; Yu, Q.; Sun, Y.; Li, Y.; Zhang, Y.; Shen, W.; Wei, X. Rumen Fermentation Parameters Prediction Model for Dairy Cows Using a Stacking Ensemble Learning Method. Animals 2023, 13, 678. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lv, J.; Wang, J.; Zhou, S.; Zhang, G.; Wei, B.; Sun, Y.; Lan, Y.; Dou, X.; Zhang, Y. Changes in Carbohydrate Composition in Fermented Total Mixed Ration and Its Effects on in vitro Methane Production and Microbiome. Front. Microbiol. 2021, 12, 738334. [Google Scholar] [CrossRef] [PubMed]
- Van Gastelen, S.; Schumacher, F.; Cone, J.; Dijkstra, J.; Pellikaan, W. In dairy cattle, the stomach tube method is not a feasible alternative to the rumen cannulation method to examine in vitro gas and methane production. Anim. Feed Sci. Technol. 2019, 256, 114259. [Google Scholar] [CrossRef]
- Liu, Q.-H.; Li, X.-Y.; Desta, S.T.; Zhang, J.-G.; Shao, T. Effects of Lactobacillus plantarum and fibrolytic enzyme on the fermentation quality and in vitro digestibility of total mixed rations silage including rape straw. J. Integr. Agric. 2016, 15, 2087–2096. [Google Scholar] [CrossRef]
- Hosoda, K.; Ohmori, H.; Nakamura, Y.-N.; Kamiya, M. Effect of inclusion rate of corn silage in ensiled total mixed ration on dry matter intake, nutrient digestibility, and ruminal fermentation in Japanese Wagyu steer. Livest. Sci. 2019, 229, 126–130. [Google Scholar] [CrossRef]
- Ramin, M.; Huhtanen, P. Development of an in vitro method for determination of methane production kinetics using a fully automated in vitro gas system—A modelling approach. Anim. Feed Sci. Technol. 2012, 174, 190–200. [Google Scholar] [CrossRef]
- Pérez-Ruchel, A.; Britos, A.; Alvarado, A.; Fernández-Ciganda, S.; Gadeyne, F.; Bustos, M.; Zunino, P.; Cajarville, C. Impact of adding tannins or medium-chain fatty acids in a dairy cow diet on variables of in vitro fermentation using a rumen simulation technique (RUSITEC) system. Anim. Feed Sci. Technol. 2023, 305, 115763. [Google Scholar] [CrossRef]
- Li, Y.; Jia, M.; Han, X.; Bai, X. Towards a comprehensive optimization of engine efficiency and emissions by coupling artificial neural network (ANN) with genetic algorithm (GA). Energy 2021, 225, 120331. [Google Scholar] [CrossRef]
- Yang, J.; Kuizhong, Y.; Xin, L.; Jita, N.; Guangning, Z.; Yan, L.; Yonggen, Z. Prediction of performance of dairy cows by simulated rumen fermentation in vitro. China Anim. Husb. Vet. Med. 2018, 45, 2167–2174. [Google Scholar]
- Fu, Q.; Shen, W.; Wei, X.; Zheng, P.; Xin, H.; Zhao, C. Prediction of the diet nutrients digestibility of dairy cows using Gaussian process regression. Inf. Process. Agric. 2019, 6, 396–406. [Google Scholar] [CrossRef]
- Shen, W.; Li, G.; Wei, X.; Fu, Q.; Zhang, Y.; Qu, T.; Chen, C.; Wang, R. Assessment of dairy cow feed intake based on BP neural network with polynomial decay learning rate. Inf. Process. Agric. 2022, 9, 266–275. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, K.; Zhang, H.; Deng, G. Dynamic Nonlinear Soft Sensor Modelling Method Using Linear Slow Feature Analysis and Least Squares Support Vector Regression for Batch Processes. Can. J. Chem. Eng, 2023; early view. [Google Scholar] [CrossRef]
- Alabi, J.O.; Okedoyin, D.O.; Anotaenwere, C.C.; Wuaku, M.; Gray, D.; Adelusi, O.; Ike, K.; Olagunju, L.K.; Dele, P.; Anele, U. Essential Oil Blends with or without Fumaric Acid Influenced In Vitro Rumen Fermentation, Greenhouse Gas Emission, and Volatile Fatty Acids Production of a Total Mixed Ration. Ruminants 2023, 3, 31. [Google Scholar] [CrossRef]
- Han, X.; Gao, M.; Shen, W.; Liu, H.; Dai, B.; He, Y.; Liu, H. A framework for generating anomaly analysis comments in DHI interpretation report. Comput. Electron. Agric. 2023, 214, 108331. [Google Scholar] [CrossRef]
- Pintelon, R.; Schoukens, J.; Vandersteen, G. Model Selection through a Statistical Analysis of the Global Minimum of a Weighted Nonlinear Least Squares Cost Function. IEEE Trans. Signal Process. 1997, 45, 281–285. [Google Scholar] [CrossRef]
- Poczynek, M.; Toledo, A.F.; Silva, A.P.; Silva, M.D.; Oliveira, G.B.; Coelho, M.G.; Virginio, G.F.; Polizel, D.; Costa, J.H.; Bittar, C.M. Partial corn replacement by soybean hull, or hay supplementation: Effects of increased NDF in diet on performance, metabolism and behavior of pre-weaned calves. Livest. Sci. 2020, 231, 103858. [Google Scholar] [CrossRef]
- De Carvalho, N.M.; Oliveira, D.L.; Saleh, M.A.D.; Pintado, M.E.; Madureira, A.R. Importance of gastrointestinal in vitro models for the poultry industry and feed formulations. Anim. Feed Sci. Technol. 2021, 271, 114730. [Google Scholar] [CrossRef]
- Dong, L.-F.; Jia, P.; Li, B.-C.; Wang, B.; Yang, C.-L.; Liu, Z.-H.; Diao, Q.-Y. Quantification and prediction of enteric methane emissions from Chinese lactating Holstein dairy cows fed diets with different dietary neutral detergent fiber/non-fibrous carbohydrate (NDF/NFC) ratios. J. Integr. Agric. 2022, 21, 797–811. [Google Scholar] [CrossRef]
- Guo, W.; Guo, X.J.; Xu, L.N.; Shao, L.W.; Zhu, B.C.; Liu, H.; Wang, Y.J.; Gao, K.Y. Effect of whole-plant corn silage treated with lignocellulose-degrading bacteria on growth performance, rumen fermentation, and rumen microflora in sheep. Animal 2022, 16, 100576. [Google Scholar] [CrossRef] [PubMed]
- Tayyab, U.; Sinclair, L.A.; Wilkinson, R.G.; Humphries, D.J.; Reynolds, C.K. Milk production, rumen function, and digestion in dairy cows fed diets differing in predominant forage and concentrate type. Anim. Feed Sci. Technol. 2022, 284, 115151. [Google Scholar] [CrossRef]
- Weiby, K.V.; Krizsan, S.J.; Eknæs, M.; Schwarm, A.; Whist, A.C.; Schei, I.; Steinshamn, H.; Lund, P.; Beauchemin, K.A.; Dønnem, I. Associations among nutrient concentration, silage fermentation products, in vivo organic matter digestibility, rumen fermentation and in vitro methane yield in 78 grass silages. Anim. Feed Sci. Technol. 2022, 285, 115249. [Google Scholar] [CrossRef]
- Li, J.; Narayanan, V.; Kebreab, E.; Dikmen, S.; Fadel, J.G. A mechanistic thermal balance model of dairy cattle. Biosyst. Eng. 2021, 209, 256–270. [Google Scholar] [CrossRef]
- Li, M.M.; Sengupta, S.; Hanigan, M.D. Using artificial neural networks to predict pH, ammonia, and volatile fatty acid concentrations in the rumen. J. Dairy Sci. 2019, 102, 8850–8861. [Google Scholar] [CrossRef]
- Sun, Y.K.; Yan, X.G.; Ban, Z.B.; Yang, H.M.; Hegarty, R.S.; Zhao, Y.M. The effect of cysteamine hydrochloride and nitrate supplementation on in-vitro and in-vivo methane production and productivity of cattle. Anim. Feed Sci. Technol. 2017, 232, 49–56. [Google Scholar] [CrossRef]
- Qian, K.; Hou, Z.; Sun, D. Sound Quality Estimation of Electric Vehicles Based on GA-BP Artificial Neural Networks. Appl. Sci. 2020, 10, 5567. [Google Scholar] [CrossRef]
- Qian, L.; Zhao, J.; Ma, Y. Option Pricing Based on GA-BP neural network. Procedia Comput. Sci. 2022, 199, 1340–1354. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Kang, H.; Kang, D.-Y.; Park, J. Genetic Algorithm Based Deep Learning Neural Network Structure and Hyperparameter Optimization. Appl. Sci. 2021, 11, 744. [Google Scholar] [CrossRef]
- Fu, Q.; Shen, W.; Wei, X.; Zhang, Y.; Xin, H.; Su, Z.; Zhao, C. Prediction of the diet energy digestion using kernel extreme learning machine: A case study with Holstein dry cows. Comput. Electron. Agric. 2020, 169, 105231. [Google Scholar] [CrossRef]
- Kumar, S.; Dagar, S.S.; Puniya, A.K.; Upadhyay, R.C. Changes in methane emission, rumen fermentation in response to diet and microbial interactions. Res. Vet. Sci. 2013, 94, 263–268. [Google Scholar] [CrossRef] [PubMed]
| Index | NDF % | ADF % | CP % | DM % | Methane mL/g | Acetic Acid mmol/L |
|---|---|---|---|---|---|---|
| 1 | 35.22 | 15.19 | 15.44 | 37.84 | 9.02 | 48.15 |
| 2 | 36.67 | 18.03 | 12.92 | 36.31 | 6.2 | 43.61 |
| 3 | 35.2 | 16.83 | 14.65 | 35.6 | 14 | 49.05 |
| 4 | 38.05 | 22.67 | 15.64 | 43.72 | 19.98 | 55.57 |
| 5 | 34.3 | 18.43 | 11.58 | 48.87 | 8.54 | 44.45 |
| 6 | 35.59 | 17.7 | 14.53 | 49.4 | 11.15 | 49 |
| 7 | 33.43 | 18.64 | 15.6 | 51.44 | 9.28 | 45.6 |
| 8 | 32.67 | 19.9 | 15.43 | 49.78 | 12.26 | 59 |
| 9 | 39.65 | 18.34 | 12.08 | 41.19 | 7.55 | 44.01 |
| 10 | 36.15 | 17.39 | 13.57 | 45.02 | 7.96 | 43.62 |
| 11 | 34.43 | 17.09 | 16.23 | 46.98 | 21.13 | 43.52 |
| 12 | 42.93 | 20.78 | 12.55 | 46.58 | 9.28 | 68.15 |
| Composition | T1 Ingredient, % | T2 Ingredient, % |
|---|---|---|
| DM (dry matter) | 47.26 | 47.35 |
| CP (crude protein) | 16.75 | 17.62 |
| NPN (non-protein nitrogen) | 7.23 | 9.41 |
| SP (soluble protein) | 7.92 | 10.74 |
| NDICP (neutral detergent insoluble crude protein) | 3.92 | 3.55 |
| ADICP (acid detergent insoluble crude protein) | 0.88 | 0.80 |
| EE (ether extract) | 3.45 | 4.53 |
| Ash | 8.12 | 8.08 |
| NDF (neutral detergent fiber) | 41.24 | 33.61 |
| ADF (acid detergent fiber) | 27.52 | 20.03 |
| Lignin | 10.84 | 8.56 |
| Starch | 20.26 | 24.86 |
| NFC (non-fibrous carbohydrate) | 30.44 | 36.20 |
| CHO (carbohydrate) | 71.67 | 69.80 |
| Models | T1-Obs | T1-Sim | T1-Re | T2-Obs | T2-Sim | T2-Re |
|---|---|---|---|---|---|---|
| Rumen methane mL/g | ||||||
| BP | 23.11 | 16.34 | 0.292 | 21.94 | 14.51 | 0.339 |
| GA-BP | 23.11 | 20.86 | 0.097 | 21.94 | 16.81 | 0.234 |
| SVM | 23.11 | 13.93 | 0.397 | 21.94 | 12.78 | 0.418 |
| Rumen acetic acid mmol/L | ||||||
| BP | 38.16 | 50.22 | 0.316 | 41.55 | 48.73 | 0.173 |
| GA-BP | 38.16 | 44.49 | 0.165 | 41.55 | 46.04 | 0.108 |
| SVM | 38.16 | 48.40 | 0.269 | 41.55 | 48.45 | 0.167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhou, J.; Jiang, R.; Wang, Y.; Zhang, Y.; Wu, R.; A, X.; Du, H.; Tian, J.; Wei, X.; et al. Development of an Alternative In Vitro Rumen Fermentation Prediction Model. Animals 2024, 14, 289. https://doi.org/10.3390/ani14020289
Wang X, Zhou J, Jiang R, Wang Y, Zhang Y, Wu R, A X, Du H, Tian J, Wei X, et al. Development of an Alternative In Vitro Rumen Fermentation Prediction Model. Animals. 2024; 14(2):289. https://doi.org/10.3390/ani14020289
Chicago/Turabian StyleWang, Xinjie, Jianzhao Zhou, Runjie Jiang, Yuxuan Wang, Yonggen Zhang, Renbiao Wu, Xiaohui A, Haitao Du, Jiaxu Tian, Xiaoli Wei, and et al. 2024. "Development of an Alternative In Vitro Rumen Fermentation Prediction Model" Animals 14, no. 2: 289. https://doi.org/10.3390/ani14020289
APA StyleWang, X., Zhou, J., Jiang, R., Wang, Y., Zhang, Y., Wu, R., A, X., Du, H., Tian, J., Wei, X., & Shen, W. (2024). Development of an Alternative In Vitro Rumen Fermentation Prediction Model. Animals, 14(2), 289. https://doi.org/10.3390/ani14020289







